Advances in cervical cancer: current insights and future directions
Miaochun Xu and Canhui Cao contributed equally to this work.
Abstract
In alignment with the World Health Organization's strategy to eliminate cervical cancer, substantial progress has been made in the treatment of this malignancy. Cervical cancer, largely driven by human papillomavirus (HPV) infection, is considered preventable and manageable because of its well-established etiology. Advancements in precision screening technologies, such as DNA methylation triage, HPV integration detection, liquid biopsies, and artificial intelligence-assisted diagnostics, have augmented traditional screening methods such as HPV nucleic acid testing and cytology. Therapeutic strategies aimed at eradicating HPV and reversing precancerous lesions have been refined as pivotal measures for disease prevention. The controversy surrounding surgery for early-stage cervical cancer revolves around identifying optimal candidates for minimally invasive and conservative procedures without compromising oncological outcomes. Recent clinical trials have yielded promising results for the development of systemic therapies for advanced cervical cancer. Immunotherapies, such as immune checkpoint inhibitors (ICIs), antibody-drug conjugates (ADCs), and targeted therapy have demonstrated significant effectiveness, marking a substantial advancement in cervical cancer management. Various combination therapies have been validated, and ongoing trials aim to enhance outcomes through the development of novel drugs and optimized combination regimens. The prospect of eradicating cervical cancer as the first malignancy to be eliminated is now within reach. In this review, we provide a comprehensive overview of the latest scientific insights, with a particular focus on precision managements for various stages of cervical disease, and explore future research directions in cervical cancer.
List of Abbreviations
-
- AIDS
-
- acquired immunodeficiency syndrome
-
- aCC
-
- advanced cervical cancer
-
- ACT
-
- adoptive cell therapy
-
- ALA-PDT
-
- 5-aminolevulinic acid PDT
-
- ADC
-
- antibody-drug conjugate
-
- AI
-
- artificial intelligence
-
- AUC
-
- area under the curve
-
- BP-cSEs
-
- breakpoint-induced cellular super enhancer
-
- CAR-T
-
- chimeric antigen receptor T-cell therapy
-
- CCRT
-
- concurrent chemoradiotherapy
-
- CRT
-
- chemoradiotherapy
-
- CIN
-
- cervical intraepithelial neoplasia
-
- CPS
-
- combined positive score
-
- CTC
-
- circulating tumor cell
-
- ctDNA
-
- circulating tumor DNA
-
- CRR
-
- complete response rates
-
- CR
-
- completion rate
-
- CCRT
-
- concurrent chemoradiotherapy
-
- CTLA-4
-
- cytotoxic T lymphocyte-associated antigen-4
-
- DCR
-
- disease control rate
-
- DFS
-
- disease-free survival
-
- ddPCR
-
- droplet digital PCR
-
- ESGO
-
- European Society of Gynaecological Oncology
-
- ESTRO
-
- European Society for Radiotherapy and Oncology
-
- ESP
-
- European Society of Pathology
-
- esCC
-
- early-stage cervical cancer
-
- EMT
-
- epithelial-mesenchymal transition
-
- EP
-
- etoposide combined with platinum
-
- FIGO
-
- International Federation of Gynecology and Obstetrics
-
- FDA
-
- Food and Drug Administration
-
- GCA
-
- gastric-type cervical adenocarcinoma
-
- GCIG
-
- Gynecologic Cancer Inter Group
-
- GWAS
-
- genome-wide association studies
-
- HACA
-
- HPV-associated cervical adenocarcinomas
-
- HICs
-
- high-income countries
-
- HRD
-
- homologous recombination deficiency
-
- HSIL
-
- high-grade squamous intraepithelial lesion
-
- Hi-C
-
- high-throughput chromosomal conformation capture
-
- HAT
-
- histone acetylase
-
- HDAC
-
- histone deacetylase
-
- hrHPV
-
- high-risk human papillomavirus
-
- ICI
-
- immune checkpoint inhibitor
-
- ITT
-
- intent-to-treat
-
- IECC
-
- International Endocervical Adenocarcinoma Criteria and Classification
-
- LMIC
-
- low- and middle-income country
-
- laCC
-
- local advanced cervical cancer
-
- LB
-
- liquid biopsies
-
- LCR
-
- long control region
-
- LEEP
-
- loop electrosurgical excision procedures
-
- LVSI
-
- lympho-vascular space invasion
-
- ML
-
- machine learning
-
- mOS
-
- median overall survivalS
-
- mPFS
-
- median progression-free survival
-
- MIS
-
- minimally invasive surgery
-
- MSI
-
- microsatellite stability
-
- NACT
-
- neoadjuvant chemotherapy
-
- NCCN
-
- National Comprehensive Cancer Network
-
- NCDB
-
- National Cancer Database
-
- NECC
-
- neuroendocrine cervical carcinoma
-
- circRNA
-
- circular RNA
-
- miRNA
-
- microRNA
-
- lncRNA
-
- long non-coding RNAs
-
- ncRNA
-
- non-coding RNA
-
- NGS
-
- Next-generation sequencing
-
- NHACA
-
- non-HPV-associated cervical adenocarcinoma
-
- TAD
-
- topologically associated domain
-
- ORR
-
- objective response rate
-
- OS
-
- overall survival
-
- pCR
-
- pathological complete response
-
- PD
-
- progressive disease
-
- PDT
-
- photodynamic therapy
-
- PD-1
-
- programmed cell death protein 1
-
- PD-L1
-
- programmed death-ligand 1
-
- PFS
-
- progression-free survival
-
- p/r/mCC
-
- persistent, recurrent or metastatic cervical cancer
-
- r/mCC
-
- recurrent or metastatic cervical cancer
-
- PJS
-
- Peutz-Jeghers syndrome
-
- SLN
-
- sentinel lymph node
-
- SNP
-
- single-nucleotide polymorphism
-
- SCNCC
-
- small cell neuroendocrine cervical carcinoma
-
- SCLC
-
- small cell lung cancer
-
- SE
-
- super enhancer
-
- SEER
-
- Surveillance, Epidemiology, and End Results
-
- Sp1
-
- specificity protein 1
-
- TCR
-
- T-cell receptor
-
- 3D
-
- three-dimensional
-
- TC
-
- paclitaxel and carboplatin
-
- TF
-
- tissue factor
-
- TP
-
- paclitaxel and cisplatin
-
- TPB
-
- topotecan, paclitaxel, and bevacizumab
-
- TILs
-
- tumor-infiltrating lymphocytes
-
- TV
-
- tisotumab vedotin
-
- TALEN
-
- transcription activator-like effector nucleases
-
- TME
-
- tumor microenvironment
-
- VEGF
-
- vascular endothelial growth factor
-
- WHO
-
- World Health Organization
-
- ZFN
-
- zinc-finger nucleases
-
- 1/2/3L
-
- first/second/third line
-
- HIV
-
- human immunodeficiency virus.
1 EPIDEMIOLOGY OF CERVICAL CANCER
Cervical cancer is the fourth most frequently diagnosed malignancy among women worldwide, with approximately 660,000 new cases reported in 2022 [1]. According to the World Health Organization's (WHO) global strategy for the elimination of cervical cancer, defined as an incidence rate of less than four per 100,000 women annually [2], an estimated 350,000 women worldwide [3], including 44,750 women in China [4], could potentially avoid death each year. In China, cervical cancer was estimated to have dropped out of the top five causes of cancer-related deaths between 1973 and 2016 [5]. Although the incidence of cervical cancer has significantly declined over the past three decades, it remains a global public health concern with notable regional disparities in morbidity and mortality. In Europe, there are approximately 58,000 new diagnoses (10.7 per 100,000 women) and 26,000 deaths (3.76 per 100,000 women) due to cervical cancer annually [6]. In the United States, the age-adjusted and hysterectomy-corrected incidence rate of cervical cancer was 11.5 per 100,000 person-years among women aged 15-75 years between 2000 and 2018, with an estimated 13,820 new cases and 4,360 deaths projected to occur in 2024 [1]. In contrast, the age-standardized incidence rate in Canada has remained largely unchanged at approximately 7.1 per 100,000 women since the mid-2000s [7]. Australia reported 942 new diagnoses (7.1 per 100,000 women) and 222 deaths in 2022 [8]. Improved public health infrastructure, high uptake of human papillomavirus (HPV) vaccines, and high-quality screening enable high-income countries (HICs) to eliminate cervical cancer sooner [1]. It is announced to be on track for near elimination in Australia, Canada, England, and the United States by 2028, 2034, 2040, and 2038-46, respectively [7, 9].
Nevertheless, due to limited resources and ineffective interventions, low- and middle-income countries (LMICs) bear the majority of the disease burden, accounting for 80% of cervical cancer cases [10]. Regions, such as Africa, Melanesia, South America, Southeast Asia, and South-Central Asia, exhibit the highest incidence and mortality rates, with age-standardized incidence rates ranging from 15.3 to 40.1 per 100,000 women and mortality rates from 7.8 to 28.6 per 100,000 women [11, 12]. Africa, in particular, bears approximately 20% of the global cervical cancer burden, with an estimated 120,000 new cases annually (24.6 per 100,000 women) [13]. Within sub-Saharan Africa, which also represents the global epicenter of the human immunodeficiency virus (HIV) pandemic [14], cervical cancer is the leading cause of cancer-related mortality among women [15].
Although the incidence in Asia (12.7 per 100,000 women) is only half that in Africa, it remains nearly twice that in Europe and the United States [16]. An upward trend in the number of cases was noted in both sub-Saharan Africa and East Asia [17]. Despite the “Two Cancers” screening program [18, 19] and the health plan “Accelerating the Elimination of Cervical Cancer by 2030” [20] launched by the Chinese government, the morbidity and mortality of cervical cancer in China are still on the rise. The incidence rate increased from the seventh to the sixth leading cause of cancer-related deaths among adult women and to the third leading cause among women aged 20-39 between 2005 and 2020, with an average annual increase of 2.72% in the number of deaths across all age groups [4].
2 BIOLOGY AND MOLECULAR MECHANISMS OF CERVICAL CANCER
2.1 High-risk HPV (hrHPV) infection
Persistent HPV infection is the primary cause of cervical cancer [21]. Among over 200 identified HPV types, 40 infect the genital tract and 13 are classified as carcinogenic hrHPV, including types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68 [22]. HPV genotypes are evolutionarily linked to a single branch of the alpha genus, of which alpha-9, -7, -5, and -6 species contain carcinogenic hrHPV genotypes [23]. Over 90% of cervical cancer cases are caused by HPV infection [21], with more than 85% attributed to hrHPV [24], predominantly types 16 and 18, which together account for over 70% of all cases [25]. Almost 90% of these infections are transient and can naturally clear within 1-2 years [26]. However, persistent infection occurs in almost 10% of women, significantly reducing the probability of clearance [27]. Among these, 1%-4% may eventually progress to cervical intraepithelial neoplasia (CIN) or cancer, typically over five years [2, 28]. The persistence and progression of HPV are strongly associated with HPV type and immunodeficiency conditions, such as those found in HIV-positive women, acquired immunodeficiency syndrome (AIDS) patients, and transplant recipients [29]. Additionally, factors such as reproductive history [21], oral contraceptive use, smoking, obesity, chlamydia trachomatis infection [30, 31], and severe mental illness [32] have also been implicated in the persistence and progression of HPV infections.
2.2 Carcinogenesis of HPV integration
HPV integration is a key molecular driver of cervical carcinogenesis [33]. While transient HPV DNA remains episomal, persistent HPV DNA often integrates into the host genome, increasing the risk of high-grade cervical lesions and potentially leading to malignant transformation [34, 35]. Integration rates increased from 53.8% in patients with CIN [36, 37] to 91.6% in patients with cervical cancer [38], varying among HPV and pathological subtypes [38-41]. Initially, HPV integration was classified into type 1 (single complete viral genome) and type 2 (multiple tandem repeats) [42]. Advances in sequencing technologies have revealed more types of HPV integration. Long-read sequencing has conclusively identified four types of HPV integration in cervical cancer cases, including truncated HPV genomes lacking the E6/E7 genes [43]. Multiomics and single-cell data have revealed silent and productive HPV integration, contributing to cervical cancer pathophysiology [38]. Various classifications have provided insights into HPV integration and its role in cervical cancer progression.
HPV integration primarily disrupts the E2 gene, leading to upregulation of E6/E7 oncogenes [44]. HPV E1, another common breakpoint, initiates viral DNA replication along with E2 [36, 45, 46]. Loss of control over E6 and E7 expression inactivates p53 and members of the pRb family, leading to uncontrolled cell apoptosis, infinite cell proliferation, and malignant transformation [35]. Integration sites are often located in microhomologous regions (1-10 bp) between viral and host genes, particularly in microRNA (miRNA) regions, with 54% of the integration events occurring in functional genes [47]. Host genomic instability in chromosomes and gene alterations, particularly the dysregulation of cancer suppressor genes/oncogenes, chromatin reorganization, and chromosomal rearrangements, are associated with HPV integration [48]. Tumor suppressor genes often suffer direct disruption of HPV integration, resulting in a loss of function. For example, RAD51B, a component of the DNA double-strand break repair pathway, shows 28-fold amplification of a 24 kb segment, including parts of the viral genome and nonfunctional RAD51B transcripts [49]. Similarly, integrations ETS2, FHIT, and LRP1B have been described [36]. Conversely, overexpression of host oncogenes is associated with cellular flanking gene amplification. For instance, HPV integration upstream of the NR4A2 oncogene results in 284-fold amplification and overexpression of NR4A2 [50]. Amplifications of MYC, HMGA2, ERBB2, and STARD3 loci have also been observed in cervical cancer [36, 39]. These disruptions and activations drive cervical carcinogenesis. HPV integration often occurs at non-random, fragile sites in the host genome and is prone to fractures and rearrangements, leading to genomic instability and chromosomal aberrations such as deletions and amplifications [51, 52]. Multiple clonal integration events can involve interchromosomal translocations and the formation of extrachromosomal virus-human hybrid structures, thereby promoting malignant progression [43]. Moreover, HPV integration facilitates immune evasion by downregulating viral antigen expression or interfering with host immune responses, allowing HPV-infected cells to evade detection and clearance [38]. E6/E7 oncogenes interfere with the host's innate antiviral responses [53]. Targeting mediators of HPV-host interactions, such as specificity protein 1 (Sp1), has been shown to enhance immune antigen presentation and immune pathways [54], providing new insight into the effect of HPV integration on host immune evasion. The pathological progression of HPV infection leading to the development of cervical cancer and the carcinogenic processes associated with HPV integration is depicted in Figure 1A.
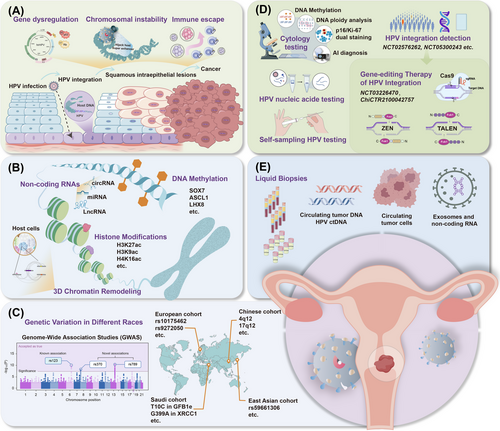
2.3 Epigenetic alterations
Epigenetic mechanisms enable precise and long-lasting gene regulation. Epigenetic alterations contribute to the incidence and development of cervical lesions and influence processes such as cancer occurrence, progression, invasion, and metastasis [55]. Some epigenetic changes occur before histological alterations, suggesting their potential use as strategies for early diagnosis and risk assessment.
2.3.1 DNA methylation
In cervical cancer, researchers have primarily focused on the hypermethylation of promoter CpG islands and tumor suppressor genes. High CpG island methylation in the SOX17 promoter region is closely associated with cervical carcinogenesis and serves as a critical biomarker for malignant transformation process [56]. Tumor suppressor genes, such as PAX1, ASCL1, LHX8, and ST6GALNAC5, exhibit increased DNA methylation in cervical cancer, with higher levels correlating with disease progression [57, 58]. DNA methylation typically occurs in the pre-cancer stage and peaks after hrHPV-induced carcinogenesis [59, 60]. As an early event in tumorigenesis, abnormal DNA methylation of both the host and HPV genomes during persistent hrHPV infection may facilitate HPV DNA integration, leading to the dysfunction of tumor suppressor genes and the promotion of cervical cancer progression [55]. HPV integration alters host methylation patterns, with significant differences between integrated and non-integrated samples in genes such as tumor suppressors BARX2 and IRX4 and oncogenes SIM2 and CTSE [46]. Further research is needed to understand the relationship between HPV integration and host gene methylation in order to develop new screening strategies.
2.3.2 Histone modifications
Histone modifications regulated by histone acetylases (HAT) and histone deacetylases (HDAC) are dynamic and reversible [55]. In cervical cancer cells, the HAT-active factor p300 enhances the acetylation of H3K27, H3K9, and H4K16, whereas Tip60 enhances H3K27 and H4K5 acetylation, thereby influencing the expression of HPV E6/E7 by affecting RNA polymerase II recruitment to the HPV18 long control region (LCR) [61]. These activities underscore the dual role of acetylation in promoting or inhibiting cervical cancer progression. Furthermore, acetylation of histones H3 and H4 is associated with activation of HPV16 gene expression in cervical cancer cells [62]. Research has also indicated that HDAC1 collaborates with the nuclear matrix protein SMAR1 to suppress E6 transcription by inhibiting the recruitment of the tumor promoter c-Fos to the E6 promoter, thereby hindering cervical cancer development [63]. Emerging evidence highlights the importance of super-enhancers (SE) in HPV transcription and cervical cancer induction [64], with the SE marker H3K27ac detected as highly enriched in cervical cancer cells with tandem HPV integration [65], driving the expression of cellular oncogenes. Tandem integration of the HPV genome can form SE-like elements that drive E6/E7 transcription and promote cervical cancer, which are crucial for cervical cancer progression [65].
2.3.3 Non-coding RNAs (ncRNAs)
Numerous studies have demonstrated that aberrant expression of miRNAs is associated with the progression of cervical cancer. For instance, miR-34a inhibits cell proliferation and induces apoptosis by targeting the oncogene SIRT1, whereas the miR-200 family reduces tumor invasion and metastasis by inhibiting genes associated with epithelial-mesenchymal transition (EMT) [66]. Circular RNAs (circRNAs) play a crucial role in cervical cancer progression by acting as “miRNA sponges” and sequestering miRNAs to regulate their target mRNAs [67]. They also contribute to oncogenesis by modulating pathways, such as the N6-methyladenosine-dependent IGF2BP2/FOXM1 pathway and MAPK pathways [55]. Long non-coding RNAs (LncRNAs) regulate the malignant transformation of cervical epithelial cells. For instance, HOTAIR interacts with polycomb repressive complexes to increase H3K27me3 modifications and silence tumor suppressor genes [68]. Conversely, DINO, another lncRNA, can reduce cervical cancer cell activity by activating TP53 via the ATM/CHK2 signaling pathway [69].
2.3.4 Three-dimensional (3D) chromatin remodeling
The 3D chromatin genome structure plays a key role in cell function and gene regulation [70]. High-throughput chromosomal conformation capture (Hi-C) techniques provide genome-wide chromatin interactions and facilitate the identification of dynamic changes in genome architecture in cellular biological functions [70]. Combining Hi-C technology with RNA sequencing (RNA-seq) revealed significant changes in the number and boundaries of topologically associated domains (TADs) near HPV16 integration sites and chromosomes containing these sites, supporting the looping-based model of HPV integration [48, 71]. The loop span distribution varied between normal and cervical cancer samples, with a higher percentage of cis-element interactions (promoters or enhancers) in cervical carcinoma [54]. HPV-CCDC106 integration has recently been shown to alter local chromosomal structure by dividing one TAD into two smaller TADs and hijacking an enhancer from PEG3 to CCDC106 via 3D genome structure remodeling [72]. Additionally, another study conducted a multi-omics analysis and identified seven HPV breakpoint-induced cellular SEs (BP-cSEs) generated by HPV integration within HPV-human hybrid extrachromosomal DNAs, leading to the intra- and inter-chromosomal regulation of genes associated with tumorigenic pathways [73]. The TADs around each BP-cSE changed noticeably, potentially affecting local intra-chromosomal interactions and the transcriptional activities of related genes. These results support the hypothesis that HPV genome interactions with chromatin regulatory factors alter the chromatin structure and promote tumorigenesis. The epigenetic alterations in cervical cancer are shown in Figure 1B.
2.4 Genetic variations
Cervical cancer exhibits familial clustering, with a pattern of reduced familial relative risk correlated with biological relatedness, suggesting that genetic factors are the primary cause of familial aggregation [74]. Genome-wide association studies (GWAS) in different populations have identified genetic susceptibility loci associated with cervical cancer development. A GWAS in 16,484 Chinese individuals identified 11 single-nucleotide polymorphisms (SNPs) in patients with cervical cancer, confirming previously reported susceptibility loci at 6p21.32 and identifying new loci at 4q12 and 17q12 [75]. Susceptibility loci in the EXOC1, GSDMB, and HLA-DP alleles suggest a role for T cell-mediated immune responses or tumor cell proliferation in determining cervical cancer risk. Another GWAS in European individuals identified strong associations at SNPs rs10175462 in PAX8, rs27069 in CLPTM1L, and rs9272050 in HLA-DQA, suggesting disruptions in apoptotic and immune function pathways [76]. SNPs T10C in GFB1e and G399A in XRCC1 in Saudi populations [77], and SNP rs59661306 in ARRDC3 in East Asian populations [78] were also associated with increased cervical cancer risk. Additionally, four DNA repair genes (FANCA, EXO1, CYBA, and XRCC1) and two immune response genes (IRF3 and TLR2) are significantly associated with CIN3 or cervical cancer [79]. Analyses of cervical cancer and HLA haplotypes show strong correlations between risk and protective HLA haplotypes, with protective haplotypes determined by amino acids at positions 13 and 71 of HLA-DRB1 and position 156 of HLA-B [80, 81]. Genetic variations in the GWAS for different ethnic populations are shown in Figure 1C.
3 ADVANCES IN DISEASE SCREENING METHODS
The management of HPV-infected lesions remains a significant global challenge. Benefiting from the extended period between HPV infection and cancer development, effective screening and treatment of cervical precancerous lesions can reduce the risk of the disease by less than 0.5% [82]. This highlights the potential for highly effective treatments through early detection using preventive strategies, timely diagnosis, and suitable therapies [83]. Abstention from smoking and the use of barrier contraception methods may contribute to the prevention of HPV infection [83]. Global efforts to eliminate cervical cancer have focused on increasing access to HPV vaccination and cervical cancer screening [84].
A significant reduction in cervical lesions has been observed since the implementation of the HPV vaccination program. International randomized controlled trials involving female adolescents and women aged 15-26 years have demonstrated a vaccine efficacy of at least 96% in preventing cervical precancers attributable to vaccine-targeted HPV types in per-protocol populations who had no evidence of infection with or exposure to a given HPV type at the time of vaccination [26]. In addition to preventing cervical lesions, HPV vaccination can decrease the rates of lower genital tract dysplasia and genital warts [85]. A multi-center retrospective study showed that non-avalent HPV vaccination could protect against lower genital tract dysplasia, including anal, vulvar, and vaginal intraepithelial neoplasia, after hysterectomy for high-grade squamous intraepithelial lesions (HSIL) and early-stage cervical cancer [86]. However, by 2022, only 125 countries (64%) had introduced HPV vaccination into their national immunization programs, with most HICs and less than 25% of LMICs [87]. Most of the global population remains unprotected owing to low vaccination coverage. Additionally, the immunogenicity and protective efficacy of the prophylactic vaccine for women over age 25 are significantly reduced, decreasing by 20%-30% [88]. Furthermore, for women previously infected with HPV, particularly those with persistent infections of subtypes 16/18, prophylactic vaccines cannot eradicate the virus [89]. Consequently, routine disease screening remains essential for the prevention and management of cervical cancer in women over 25 years of age, ensuring effective prevention regardless of the HPV immunization status. Disease screening methods are shown in Figure 1D-E.
3.1 Cytologic and HPV testing
Since the introduction of the Papanicolaou (Pap) cytologic test in the 1940s and the development of the ThinPrep cytologic test, visual inspection with acetic acid, and excision of CIN, the global incidence and mortality rates of cervical cancer have decreased by 60%-80% [90]. Given the heterogeneity of cytological and histological detection, HPV nucleic acid testing (DNA or mRNA) improves screening accuracy by identifying the HPV-infection status and HPV subtypes [91]. The sensitivity of HPV nucleic acid testing for CIN exceeds 90% compared to 50%-70% for cytological tests [23, 92]. Interestingly, co-testing with HPV DNA and cytology increases the sensitivity for detecting CIN2+ and CIN3+ but reduces specificity, leading to more colposcopy referrals [88]. The WHO's updated guidelines recommend HPV-DNA testing as the preferred initial screening method, although other guidelines still recommend three screening strategies (cytology every three years, co-testing every five years, or HPV testing every five years) owing to the lack of facilities in some regions to enable standalone HPV testing [2]. Compared to HPV DNA testing, HPV E6/E7-mRNA shows similar sensitivity but greater specificity for detecting HSIL [88]. HPV E6/E7-mRNA specifically detects viral RNA fragments, providing information about active viral replication and carcinogenic potential, thus better targeting high-risk populations [93]. p16/Ki-67 dual staining [94-96] and DNA ploidy analysis [97, 98] also allow for the earlier detection of abnormalities, enhancing screening sensitivity. For women who experience sampling embarrassment or reside in regions with a shortage of trained medical staff, self-sampling for HPV testing may be a viable alternative to increase screening rates, showing good consistency with physician-collected samples in detecting HPV and sufficient performance in detecting HSIL [99]. Due to its convenience and high acceptability, it is widely practiced abroad and serves as an essential tool for enhancing cervical cancer screening rates [100, 101].
3.2 DNA methylation triage
Multiple single-gene and multi-gene DNA methylation assays have been investigated for the early detection of cervical cancer and have demonstrated considerable sensitivity and specificity. For instance, a multi-gene methylation panel (ASTN1, DLX1, ITGA4, RXFP3, SOX17, and ZNF671) exhibited a sensitivity and specificity of 85% and 85.4%, respectively, in diagnosing HSIL and cervical cancer [102]. Furthermore, studies have indicated that the combined screening method for HPV 16/18 and PAX1/JAM3 methylation detection has a specificity of 96.1% in CIN3+ [103], while the PAX1/ST6GALNAC5 methylation combined test demonstrated a detection sensitivity close to 100% for cervical cancer in HPV-positive women [104]. Recently, an innovative triage technique based on DNA methylation, the WID-qCIN test, was introduced to evaluate the predictive performance when combined with HPV16/18 genotyping and cytological classification [105]. The study found that combining WID-qCIN with HPV16/18 could detect 93.4% of CIN3 and 100% of invasive cervical cancers and significantly reduced colposcopic referrals for CIN2+ patients over 6 years, with cytology and WID-qCIN/HPV16/18 triage requiring 4.1 and 2.4 colposcopic referrals, respectively. These findings support the use of DNA methylation combined with HPV16/18 testing as a reference for further colposcopy, improving the accuracy of cervical lesion screening and avoiding repeated colposcopy and unnecessary anxiety among women.
3.3 HPV integration detection
Based on an in-depth understanding of the mechanism of HPV integration in cervical cancer, detection of HPV integration has been introduced into clinical practice. Combined HPV integration and HPV subtype detection have superior diagnostic performance compared to HPV detection alone, suggesting that HPV integration can serve as a molecular marker for distinguishing patients with cervical cancer from normal populations [37]. Compared with cytology, HPV integration detection has shown higher specificity and equivalent sensitivity for diagnosing CIN3+ in HPV-positive women, especially in HPV16/18-positive women [106]. The progression rate in HPV integration-positive women was higher than that in HPV integration-negative women within 1-year of follow-up. Multiple prospective clinical trials are underway to accurately screen for cervical cancer using hrHPV integration detection, comparing the risks associated with current screening methods and cervical disease progression (NCT02576262, NCT05300243, NCT05570331, and NCT05510830). More follow-up data may support the use of the HPV integration test as a precise triage tool for HPV-positive women to avoid the excessive use of colposcopy.
3.4 Artificial intelligence (AI) diagnosis
Pathologists’ subjective biases contribute to a false-negative rate exceeding 10% for conventional diagnoses, particularly in developing countries compared to developed ones [107]. Using machine learning (ML) algorithms or deep neural networks, AI-assisted slide reading has shown the capability to reduce false positives and false negatives, increase cytologists’ diagnostic accuracy to 99.8%, achieve 100% sensitivity, and improve screening efficiency by more than fourfold [108]. The incorporating of AI and ML algorithms into clinical decision-making processes can enhance the precision and efficiency of patient stratification and treatment selection. However, this technology has limitations, including a lack of quality control for electronic cytology images, potential system biases from different digital pathology scanners, and insufficient training sets for AI learning. Consequently, AI-assisted pathological slide reading and AI electronic colposcopy diagnostic systems can assist cytologists in focusing on diagnosis and significantly improving work efficiency but cannot fully replace manual work at present.
3.5 Liquid biopsies (LB)
LB offers a noninvasive alternative to cervical lesion screening, which is traditionally performed using tissue biopsy. Advances in NGS, droplet digital PCR (ddPCR), and other high-throughput technologies have enabled the detection of RNA and DNA in tumor cells in bodily fluids [109]. Plasma is the most commonly used liquid biopsy sample and contains circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), circulating noncoding RNA, and exosomes [110]. ctDNA analysis can identify somatic mutations, sequence polymorphisms, and gene fusions, which are useful for diagnosis, monitoring therapeutic responses, and assessing disease recurrence risk [111]. In ctDNA, PIK3CA, ZFHX3, KMT2C, and KMT2D were the most frequent mutations, reflecting the cervical cancer lesions in some cases [40]. However, single-gene ctDNA methods are often impractical because of tumor mutation heterogeneity, necessitating the use of optimal ctDNA mutation panels. Epigenetic changes detected in liquid biopsies may serve as sensitive biomarkers. Studies have shown that methylation analysis of CADM1/MAL combined markers can distinguish CIN and cervical cancer, with methylation positivity rates of 90% (CIN2), 85.7% (CIN3), and 83.3% (cervical cancer) [112]. The presence of HPV integration in cervical cancer implies that HPV DNA is released as ctDNA [113]. Most studies have used primers targeting the L1, E6, and E7 regions of HPV16/18, with positivity rates ranging from 11% to 90% [109]. However, ctHPV DNA is often found in advanced disease, but is typically negative for precancerous lesions [113]. Although ctHPV DNA alone may not be a reasonable screening tool, it can complement traditional cytological examinations. It is crucial to clarify whether ctHPV DNA can be detected in precursor lesions, with ongoing trials investigating ctHPV DNA under precancerous conditions (NCT04274465). In addition to early screening, ctDNA shows promise as a detection marker, aiding clinical decision-making, monitoring early chemotherapy effects, and tracking acquired resistance mechanisms. Longitudinal ctDNA analysis, including HPV ctDNA, has provided earlier indications of disease progression after chemotherapy or chemoradiotherapy (CRT) compared to radiological imaging [114, 115]. Compared to ctDNA, CTCs specifically indicate the presence of circulating live cells, offering greater stability because they can originate from necrotic or apoptotic cells [110]. CTCs are recognized as predictive biomarkers for assessing treatment efficacy and early recurrence in patients with advanced cervical cancer undergoing radiotherapy [116], CRT [117], or bevacizumab treatment [118]. Furthermore, ncRNAs and exosomes in the plasma, including lncRNAs (PVT1, AL592284.1, and XLOC_000303) [119], microRNA (miR-25, miR-29a, and miR-486-5p) [120], and exosomal miRNAs (let-7d-3p and miR-30d-5p) [121], have been reported as noninvasive biomarkers for cervical cancer. In addition to plasma, urine HPV DNA testing has demonstrated sensitivities and specificities comparable to cervical samples, detecting 79%-80% of CIN2+ lesions [122]. DNA methylation analysis of urine (FAM19A4, GHSR, PHACTR3, PRDM14, SST, and ZIC1) moderately correlated with cervical scrape methylation (r = 0.508-0.717) and distinguished cancer samples from normal samples (area under the curve [AUC] = 0.744-0.887) [123]. However, external factors can affect the accuracy and cannot replace cervical sampling. Therefore, larger clinical trials are required to validate the efficacy of these drugs. Noninvasive tests hold promise for identifying patients at a high risk of recurrence, but their application needs to be expanded.
4 NOVEL PERSPECTIVES IN DISEASE MANAGEMENT
4.1 Elimination and reversal of precancerous lesions
Patients with HSIL or CIN 2/3 have a high risk of progression to cervical cancer if left untreated [124]. Treatment aims to destroy the lesions and the entire squamocolumnar junction. The preferred methods include loop electrosurgical excision procedures (LEEP), large loop excision of the transformation zone, and sometimes cold knife conization, which is the focus of tertiary prevention [83]. The WHO guidelines also accept ablative therapies like cryotherapy and thermal ablation [125, 126]. However, the risk of CIN3 recurrence after excision and ablation is 1.6% and 2.9% at six months, rising to 3.2% and 7.2% at twelve months [127, 128]. HPV persistence and viral load after conization have been suggested as risk factors for developing persistent or recurrent cervical dysplasia, in which HPV persistence is the most impacting risk factor [129, 130]. It was reported that the persistence of HPV at twelve months and the presence of positive endocervical margins are associated with an increased risk of disease recurrence after primary conization [129, 131]. The post-excision surveillance of persistent HPV is recommended from 6 months to 12 months [129, 132]. In addition, adverse pregnancy outcomes in young women undergoing conization cannot be underestimated, and the postponement of treatment for cervical dysplastic lesions identified during pregnancy until the postpartum period is a safe and well-established practice for both maternal and neonatal health [83]. Future therapeutic strategies should focus on noninvasive treatments and long-term virus elimination.
4.1.1 Photodynamic therapy (PDT)
PDT uses a photosensitizer and specific light wavelengths to produce reactive oxygen species that kill tumor cells and is effective in treating various grades of CIN with high remission and low recurrence rates [133-136]. 5-aminolevulinic acid PDT (ALA-PDT), a second-generation photosensitizer, has shown complete response rates of 93.9% for CIN1, 92.4% for CIN2, and 82.4% for CIN3, with 12-month clearance rates of 86.4%, 85.5%, and 80.4%, respectively [137]. These results suggest that PDT can be recommended for women of childbearing age or for those seeking minimally invasive treatment.
4.1.2 Therapeutic HPV vaccines
Therapeutic vaccine strategies aim to generate T-cell-mediated immunity by specifically targeting HPV E6/E7, which is continuously expressed in infected and cancer cells [138]. VGX-3100, a DNA plasmid-based vaccine, has shown efficacy in eliminating HPV-16/18-associated HSIL and long-term viral clearance [139, 140]. This dual benefit of lesion reduction and viral elimination using VGX-3100 makes it a promising treatment option for HPV-related cervical lesions. PepCan, a peptide-based vaccine, is currently being evaluated in a phase II trial for HSIL (NCT02481414). Larger trials will provide clinical evidence for the use of therapeutic vaccines as noninvasive treatments for cervical precancerous lesions. Notably, an important consideration for therapeutic vaccines is their dependence on an intact immune system, posing potential issues for patients with immunosuppressive diseases, such as organ transplant recipients and HIV+ patients [141].
4.1.3 Gene-editing therapy of HPV integration
With the advent of gene-editing technologies, identifying optimal targets within viral integration sequences, and reducing potential oncogenic risks can reduce the HPV infection load and reverse cervical lesions [142]. Tools such as CRISPR/Cas9 [143-147], transcription activator-like effector nucleases (TALEN) [148], and zinc-finger nucleases (ZFN) [149] offer precise targeting and cleavage of integrated viral sequences, showing promise in preclinical studies for inhibiting malignant phenotypes in HPV-driven cervical carcinogenesis. Furthermore, clinical trials of gene-editing technology, such as TALEN-T512 (NCT03226470, ChiCTR2100042757), are ongoing to evaluate the efficacy and safety of gene-editing techniques for the treatment of HSIL. These findings highlight the potential of gene editing as a targeted therapeutic approach to address persistent HPV infections and associated lesions and represent a significant advancement in HPV-related cancer treatment. Further research is essential to validate these approaches for widespread clinical use.
4.2 Surgical controversies in early-stage cervical cancer (esCC)
Surgery remains the initial therapy for esCC (International Federation of Gynecology and Obstetrics [FIGO] stage IA1-IB2, IIA1), achieving a cure rate of 90% with appropriate surgical plans [150, 151]. Currently, precise and personalized surgical approaches for patients with esCC, supported by a large number of real-world studies, have gradually improved clinical cure rates while simultaneously enhancing patients’ quality of life and preserving fertility (Figure 2A).
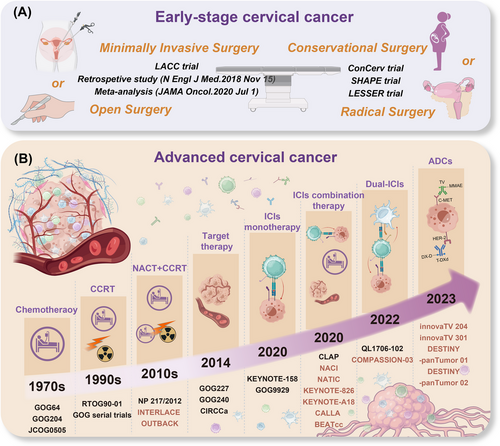
4.2.1 Minimally invasive surgery (MIS) or open surgery?
In the era of MIS, laparoscopic procedures have gained popularity among gynecologic oncologists and patients. However, the results of several major studies cast doubt on the indications for MIS in cervical cancer. In 2018, the initial results of Laparoscopic Approach to Cervical Cancer (LACC), a global multicenter prospective phase III randomized controlled trial, indicated that compared to open surgery for radical hysterectomy, MIS was associated with a lower rate of overall survival (OS) and a lower disease-free survival (DFS) (3-year OS, 93.8% vs. 99.0%; 4.5-year DFS, 86.0% vs. 96.5%) [152]. Recently, after 4.5 years of follow-up, the final results of this trial were released, showing consistent findings with the initial results (4.5-year OS was 90.6% in the MIS group vs. 96.2% in the open surgery group, respectively) and a higher local recurrence risk in the MIS group (hazard ratio [HR] = 4.70) [153]. This landmark trial resulted in a global sensation. Similarly, findings from a population-based study using the National Cancer Database (NCDB) [154] and a large meta-analysis [155] were consistent with the LACC trial findings of worse oncological outcomes with MIS. These results have led to revised guidelines favoring open surgery, and real-world studies have shown a decline in laparoscopic surgery for cervical cancer since 2018 [156].
Patients often prefer MIS owing to its rapid recovery and reduced complications. A study found that MIS for early-stage cervical cancer is linked to better perioperative outcomes and a lower incidence of postoperative lymphadenitis [157]. However, quality-of-life assessments showed no difference compared to open surgery in the LACC trial [158]. In addition, no statistical difference in complications was reported at 90 days after surgery between the MIS and open surgery groups, although there was a greater proportion in the latter after LACC trial [159]. This evidence aligns with the SUCCOR database analysis [160], which indicates that open surgery does not increase heighten the occurrence of postoperative complications.
Despite the findings of the LACC trial, which should be duly considered owing to its level I evidence, many gynecologic oncologists continue to endorse MIS for specific cases of cervical cancer. Numerous real-world studies have indicated that MIS is safe and effective for early-stage low-risk cervical cancer, particularly for tumors with a diameter of ≤ 2 cm [161-167]. The 2023 guidelines issued by the European Society of Gynaecological Oncology (ESGO), European Society for Radiotherapy and Oncology (ESTRO), and the European Society of Pathology (ESP), supported MIS as a potential option for low-risk tumors (with a diameter of less than 2 cm and negative margins after conization), considering it acceptable for lymph node staging [168]. However, these recommendations are mostly based on patients with cervical squamous cell carcinoma, leaving it uncertain whether MIS is available for adenocarcinoma due to its distinct biological background [169]. Although a study has shown no significant differences in DFS and OS when comparing MIS to open surgery for patients with FIGO 2009 stage IB1-IIA2 cervical adenocarcinoma [157], future studies are required to further explore the role of MIS in managing early-stage cervical adenocarcinoma.
Rather than abandoning MIS, surgeons have refined laparoscopic techniques in accordance with tumor-free principles to mitigate poor prognoses. These modifications include avoiding the use of uterine manipulators and vaginal incisions under pneumoperitoneum and thoroughly irrigating the pelvic cavity and vaginal stump before suturing to prevent peritoneal contamination and tumor seeding [170-176]. However, the evidence presented by the LACC trial should not be ignored in favor of earlier level II-III data from smaller retrospective studies when guiding clinical decision making.
In China, where the disease burden is substantial and surgeons are highly skilled in performing laparoscopic procedures, reversion to open surgery may not be optimal. The necessity for additional research to elucidate the mechanisms underlying these contemporary findings and to identify patient subgroups that could benefit from MIS remains critical. Prospective trials focusing on East Asian populations involving large cohorts should be prioritized to provide robust data. Currently, several clinical trials are underway to compare the efficacy of MIS and open radical hysterectomy for cervical cancer, intending to identify patient populations that would derive the greatest benefit from MIS [161, 177–180]. These efforts will be instrumental in refining the surgical guidelines and improving patient outcomes.
4.2.2 Radical surgery or conservational surgery?
The concept of “less is more” in treating esCC prioritizes the enhancement of quality of life while ensuring effective tumor control [181]. Radical trachelectomy with pelvic lymph node dissection has been recognized as a viable and safe option for patients with esCC (Stage IB1-IB2) desiring fertility preservation, especially for tumors smaller than 2 cm [182, 183]. By 2023, there will be a significant shift towards less extensive surgical interventions for low-risk esCC patients, challenging traditional radical surgical treatments. The ConCerv trial, a prospective, single-arm, multicenter study on conservative surgery for low-risk esCC, demonstrated that patients meeting specific criteria could opt for conization [184]. In this trial, low-risk esCC was defined as including no lympho-vascular space invasion (LVSI), negative conization margins, squamous cell carcinoma (any grade) or usual-type adenocarcinoma (grade 1 or 2 only), tumor size ≤ 2 cm, invasion depth ≤ 10 mm, and no metastatic lesions on imaging. Based on these promising results, the 2023 National Comprehensive Cancer Network (NCCN) guidelines recommend conservative surgery for patients meeting the ConCerv criteria. Specifically, cone biopsy with negative margins plus pelvic lymphadenectomy or sentinel lymph node (SLN) mapping is suggested for those wishing to preserve fertility, whereas extrafascial hysterectomy plus pelvic lymph node dissection or SLN mapping is recommended for those without fertility requirements. Additionally, the SHAPE trial results demonstrated that simple hysterectomy had similar prognostic outcomes to radical hysterectomy, with a lower incidence of postoperative urinary complications in low-risk esCC patients, who were defined similarly to the ConCerv criteria [185]. The LESSER trial in Brazil further indicated the efficacy and safety of simple hysterectomy in esCC patients with tumor diameters of less than 2 cm [186]. Based on these findings, discussions with specialists have advocated conservative surgery for esCC, suggesting that, with strict patient evaluation and selection, conservative surgery, including conization and simple hysterectomy, can be a viable option without compromising tumor outcomes [187-190]. Further large-scale clinical trials are necessary to support the broader implementation of conservative surgery for esCC and ensure that this approach can be safely and effectively integrated into clinical practice.
4.3 Major advances in combination therapies for advanced cervical cancer (aCC)
The landscape of cervical cancer treatment is evolving rapidly, particularly for the treatment of aCC, encompassing both locally advanced cervical cancer (laCC), defined as FIGO 2018 stage IB3, IIA2-IVA, and recurrent/metastatic cervical cancer (r/mCC). While the 5-year OS rate for laCC ranges from 40%-70%, the median OS (mOS) for r/mCC with current therapy is only 14.3-18.3 months [191]. Concurrent chemoradiotherapy (CCRT) remains the standard treatment for laCC, whereas systemic therapy is the primary approach for r/mCC [192]. Evolving evidence is reshaping the standards of care for aCC and posing challenges for clinicians to determine the optimal treatment sequence and type (Figure 2B). Moreover, unequal access to therapeutic strategies continues to complicate aCC treatment.
4.3.1 Chemotherapy combined with CCRT
Several decades of development have firmly established the significant role of CCRT in the treatment of laCC through several key trials, from cisplatin monotherapy (GOG64 trial) [193] and the cisplatin-paclitaxel combination chemotherapy (GOG204 trial) [194], to cisplatin-based CCRT (RTOG90-01 trial) [195]. In recent years, research on induction chemotherapy before CCRT has emerged, and its efficacy has been further verified, potentially altering the standard of care for laCCs. The Gynecologic Cancer Inter Group (GCIG) INTERLACE trial, a phase III multicenter randomized controlled trial, found that dose-dense weekly paclitaxel and carboplatin induction chemotherapy followed by CCRT reduced the recurrence risk by 35% and mortality by 39% compared to CCRT alone, with acceptable toxicity of induction chemotherapy and high compliance with radiotherapy [196]. Another study indicated that neoadjuvant chemotherapy (NACT) plus CCRT increased complete response rates (CRR) and reduced distant metastasis rates in laCC patients with tumors larger than 4 cm [197]. Similar findings have been previously reported [198, 199]. These results support the use of induction chemotherapy followed by CRT as a new standard of care. Notably, all these trials used paclitaxel and carboplatin/cisplatin (TC/TP) as NACT regimens. However, a study using cisplatin-gemcitabine in NACT did not show the same benefits and reported challenges in continuing chemotherapy during CCRT, leading to delays in initiating CCRT [200], which may have contributed to the observed inferior outcomes in the NACT group. These results highlight the importance of carefully considering drug toxicity and patient tolerance when selecting NACT regimens, and the combination of paclitaxel and platinum may offer a more suitable option owing to its manageable side effect profile [196-198]. Furthermore, the dose-dense weekly schedule demonstrated in the INTERLACE trial could be well-tolerated by patients and accommodate a short interval between NACT and CCRT, with only a 7-day gap, thus optimizing treatment efficacy and patient compliance [196]. Furthermore, findings from the OUTBACK trial, a multicenter, open-label, randomized phase III trial, revealed that there was no significant difference in the 5-year OS rates between CCRT alone and CCRT followed by adjuvant chemotherapy (71% vs. 72%), but increased short-term toxicity was reported in the CCRT followed by adjuvant chemotherapy group [201]. These findings suggest that adjuvant chemotherapy may not effectively eliminate residual lesions after CCRT in patients with laCC. Together, these findings suggest that using an appropriate chemotherapy regimen, with careful consideration of the interval between chemotherapy and CCRT, as well as the treatment sequence, can enhance the effectiveness of CCRT in patients with laCC. This approach may be particularly suitable for many patients with laCC and can help alleviate the economic burden on LMICs with limited radiotherapy resources. Future research should focus on determining the optimal sequence of chemotherapy and CCRT, and identifying patient subgroups that would benefit the most from specific sequencing strategies.
4.3.2 NACT followed by radical surgery
In the context of NACT combined with surgery for aCC, the current consensus leans against its effectiveness. Although some postoperative improvements have been reported, NACT has not demonstrated significant benefits for OS or PFS. Both the EORTC-55994 study [202] and the Tata Memorial Center study (NCT00193739) [203] for patients with stage IB2-IIB concluded that NACT followed by radical surgery was not superior to CCRT. Recent research suggests that chemotherapy may beneficially remodel the anti-tumor microenvironment (anti-TME) in responsive patients [204], indicating that combining immunotherapy with NACT could be more advantageous before radical surgery. Two recent clinical trials have focused on the clinical response rate to NACT combined with immune checkpoint inhibitors (ICIs) in patients with laCC. The NACI phase II trial demonstrated that adding the anti-PD-1 (anti-programmed cell death protein 1) antibody camrelizumab to platinum-based NACT improved the pathological complete response (pCR) rate in untreated patients with laCC (stage IB3, IIA2, or IIB/IIIC1r), with an objective response rate (ORR) of 98% and a pCR of 38% in the overall population [205]. Preliminary multiomic analysis of samples from patients with pCR demonstrated that NACT induces a state transition to epithelial-immune system, which may contribute to the response to ICIs [206]. Based on these results, a phase III clinical trial comparing neoadjuvant chemo-immunotherapy followed by radical surgery with CCRT is currently underway (NCT04516616). Similarly, another phase II trial, NATIC (ChiCTR2200065392), also supported that the combination of the anti-PD-1 antibody Tislelizumab with NACT is safe and effective in laCC (stage IB3-IIA2), resulting in a pCR of 60.9% and an ORR of 73.9% and reduced rates of postoperative adjuvant therapy (26.1%) and adjuvant radiotherapy (17.4%) [207]. These studies suggest that NACT combined with ICIs could become the standard treatment for resectable laCC, although a longer follow-up is needed to support long-term survival outcomes. Overall, while NACT followed by surgery does not significantly improve survival outcomes for patients with laCC, long-term data are necessary for a comprehensive evaluation of the combined approach of ICIs with NACT.
4.3.3 Target therapy
Bevacizumab is a representative drug that targets vascular endothelial growth factor (VEGF), a key factor in tumor growth and metastasis, and has proven effective in treating cervical cancer. The GOG227 trial in 2009 supported the confirmed tolerability and activity of bevacizumab in second- and third-line (2/3L) treatment for patients with r/mCC [191]. In 2014, another trial, GOG240, established the role of bevacizumab in cervical cancer and was the first phase III clinical study of an anti-angiogenesis targeted therapy for cervical cancer treatment [208]. The preliminary results showed that patients with r/mCC receiving chemotherapy plus bevacizumab had a higher mOS (17.0 months vs. 13.3 months) and an improved ORR (48% vs. 36%) compared to those receiving chemotherapy alone. The results confirmed the benefits of combining bevacizumab and demonstrated the long-term efficacy and tolerability of anti-angiogenic therapy in r/Mcc [208]. The combination of chemotherapy and bevacizumab has become the preferred first-line (1L) treatment option for r/mCC, with single-agent bevacizumab serving as a second-line (2L) alternative [209]. Subsequent clinical trials confirmed the efficacy of bevacizumab combined with platinum-based chemotherapy for aCC [210] and standard pelvic CCRT in laCC [211]. The toxicity and decreased quality of life caused by bevacizumab cannot be ignored, although some studies indicate that patients who discontinue treatment because of toxicity still have longer survival times than those with progressive disease (PD) [212]. Future trials should include supportive care interventions. In addition to bevacizumab, other VEGF/VEGFR inhibitors have been evaluated in patients with aCC in clinical trials. The CIRCCa trial, a randomized, double-blind, placebo-controlled phase II trial, demonstrated significant efficacy when cediranib (a potent tyrosine kinase inhibitor of VEGFR1, 2, and 3) was added to TC chemotherapy, showing a median progression-free survival (mPFS) of 8.1 months versus 6.7 months in the treatment of r/mCC [213]. However, there was no difference in OS between the cediranib and placebo groups, and the treatment was accompanied by an increase in toxic effects. Although cediranib did not benefit treatment-naïve patients, it may be used as an alternative for patients who experience PD after receiving bevacizumab in a previous trial. Further clinical trials focusing on this population of patients treated with cediranib can be developed.
Non-VEGF-dependent angiogenesis inhibitors, such as the angiopoietin axis inhibitors apatinib, trebananib, and anlotinib, as well as vascular-disrupting agents targeting the existing tumor vasculature, are being explored [214]. Apatinib, a novel tyrosine kinase inhibitor targeting tumor angiogenesis, has been indicated as a 2L treatment for patients with aCC, with an ORR of 15.0% [215]. EGFR is one of the most common therapeutic targets for cervical cancer. A phase II trial, MITO CERV-2, evaluated the efficacy of cetuximab (an anti-EGFR monoclonal antibody) combined with TC chemotherapy in the treatment of patients with r/mCC [216]. Although no increased OS was found in all participants, CR was observed in 27% of patients without PIK3CA mutations but in none of the patients with one or more PIK3CA mutations, suggesting a correlation between these mutations and cetuximab resistance. Dual-drug therapy targeting the PI3K/Akt/mTOR and RAS/RAF/MEK/ERK pathways was tested in a phase II trial in recurrent cervical cancer, but the trial was terminated due to discontinuation of drug development, and no survival data have been reported [217]. The feasibility of targeted therapies focusing on these signal transduction pathways relevant to cervical carcinogenesis, as well as homologous recombination deficiency (HRD) and the Notch binary cell fate decision pathway, requires further clinical evidence [218].
4.3.4 Immunotherapy
A pivotal advancement in addressing the therapeutic challenges associated with aCC has been the advent of immunotherapies. The year 2023 witnessed the publication of numerous clinical trials, heralding a new era in aCC treatment. Immunotherapy has been recognized as a groundbreaking modality in contemporary oncology, offering promising avenues for tumor control and, in certain cases, a potential cure [219]. Current immunotherapeutic strategies include ICIs, antibody-drug conjugates (ADCs), and adoptive cell therapy (ACT) [220, 221]. Although pembrolizumab (an ICI) and Tisotumab Vedotin (TV, an ADC) are currently the only immunotherapies approved by the Food and Drug Administration (FDA) for cervical cancer, ongoing research is vigorously exploring additional immunotherapeutic agents and methodologies to enhance the clinical efficacy of immunotherapy in cervical cancer management.
ICIs
Immune checkpoints are critical regulatory pathways exploited by neoplastic cells to evade immune surveillance and subsequent immune-mediated destruction [222]. Increased activity of these checkpoints within the TME plays a pivotal role in facilitating immune escape mechanisms. ICIs have emerged as a transformative therapeutic approach, enhancing the cytotoxic efficacy of T cells by targeting inhibitory receptors, such as PD-1 and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4).
ICIs monotherapy
Given that the majority of cervical cancers are etiologically linked to HPV infection and exhibit high programmed death-ligand 1 (PD-L1) expression rates (70%-90%), PD-1/PD-L1 inhibitors represent particularly promising therapeutic targets [223]. Several PD-1/PD-L1 inhibitors have been evaluated as ≥2L treatments for patients with aCC [224-228]. The KEYNOTE-158 trial demonstrated that pembrolizumab monotherapy in patients with r/mCC resulted in an mPFS of 2.1 months, mOS of 11 months, and ORR of 12.2%, with 14.6% in patients with positive PD-L1 [226]. Similarly, other PD-1 inhibitors have exhibited limited efficacy when used as a monotherapy for aCC, with ORR of 27.6% for zimberelimab [229], 26.3% for nivolumab (20% in patients with positive PD-L1) [227], and 15% for balstilimab (20% in patients with positive PD-L1) [228], resulting in an average response rate of approximately 20%. In addition to PD-1 and PD-L1, CTLA-4 has emerged as an extensively studied immune checkpoint molecule. Ipilimumab, a monoclonal antibody targeting CTLA-4, has shown potential efficacy and safety in laCC patients with positive nodes after CCRT, as evidenced by the GOG9929 trial [230]. ICIs may be particularly beneficial for patients with specific biomarkers such as positive PD-L1, mismatch repair deficiency (MMR), and high tumor mutational burden (TMB-H) [231]. However, the efficacy of monotherapy in patients lacking these biomarkers is limited, underscoring the necessity for combination strategies.
Dual-ICIs
PD-1/CTLA-4 bispecific antibodies, such as cadonilimab and epalizumab, have recently demonstrated effectiveness in r/mCC within Chinese cohorts and are currently under evaluation by the National Medical Products Administration (NMPA) for potential approval in patients with r/mCC when combined with platinum-based chemotherapy [232-234]. Additionally, more dual-ICIs, such as PD-1/TIM-3 inhibitor (NCT06238635) and PD-1/TIGIT inhibitor (NCT06241235), are being evaluated for their feasibility and efficacy in patients with aCC.
ICIs combined with chemotherapy/radiotherapy
The KEYNOTE-826 study corroborated that the combination of pembrolizumab with paclitaxel and platinum-based chemotherapy substantially improved PFS of 10.4 months and 1-year OS of 53.3% for patients with persistent, recurrent, or metastatic cervical cancer (p/r/mCC) and PD-L1 combined positive score (CPS) ≥ 1 [235]. Subsequent data further substantiated these findings, reporting an mOS of 28.6 months, thereby reinforcing the clinical value of chemo-immunotherapy regimens [236]. Consequently, the NCCN guidelines endorse a 1L treatment strategy for p/r/mCC involving a regimen of TP/TC chemotherapy with or without bevacizumab in conjunction with pembrolizumab for PD-L1 positive populations [224]. A phase II clinical trial evaluating the safety and efficacy of sintilimab (a PD-1 inhibitor) combined with albumin-bound paclitaxel in patients with aCC who failed 1L platinum-based therapy demonstrated an ORR of 44.4%, with an mPFS of 5.2 months and mOS of 13.1 months [237]. Another study reported an ORR of 57.1% with the same combination treatment in PD-L1 positive patients with aCC [238], indicating that the combination of sintilimab and nab-paclitaxel is a viable and well-tolerated 2L treatment option. As mentioned above, chemo-immunotherapy was also demonstrated to be feasible as a neoadjuvant treatment before radical surgery in NACI and NATIC trials (see “4.3.2 NACT followed by radical surgery”). However, it is imperative to note that these results are based on single-arm studies. Future trials with larger sample sizes and comparative analyses with existing guideline-recommended chemo-immunotherapy treatments are essential for further validation.
The efficacy of immunotherapy combined with CCRT has also been shown to be promising for the treatment of laCCs. ENGOT-cx11/GOG-3047/KEYNOTE-A18, a randomized phase III clinical trial, demonstrated that pembrolizumab combined with CCRT exhibited superior efficacy compared to CCRT alone in previously untreated, high-risk laCC patients [239]. The study reported a 24-month PFS rate of 67.8% in the pembrolizumab plus CCRT group and 57.3% in the placebo plus CCRT group. Conversely, the CALLA trial, a randomized, double-blind, phase III study, showed no significant improvement in PFS for patients with laCC treated with a combination of durvalumab and CCRT compared with those receiving placebo and CCRT [240]. This discrepancy may be attributed to the varying levels of PD-L1 expression among the patients in each study, a factor that was not explicitly accounted for in either trial. Given the significant influence of PD-L1 expression levels on the efficacy of ICIs, further investigation of the use of immunotherapy combined with CCRT in laCC patients with high PD-L1 expression is warranted.
ICIs combined with target therapy
ICIs combined with targeted therapies have demonstrated significant potential in oncological treatment by harnessing the immune system to recognize and eliminate neoplastic cells. The CLAP trial investigated the combination of camrelizumab (a PD-1 inhibitor) and apatinib (a VEGFR2 inhibitor) as a 2L therapy in patients with aCC undergoing PD after 1L platinum-based chemotherapy and showed encouraging antitumor activity (55.6% ORR) and manageable safety profiles [241]. A multicohort phase II study (NCT03827837) assessed the combination of camrelizumab and famitinib (a multikinase inhibitor) in patients with advanced solid tumors. This regimen showed durable antitumor activity in previously treated r/mCC patients, with an mPFS of 10.3 months and a 12-month OS rate of 77.7% [242]. Comparable outcomes were observed in another open-label, randomized phase II clinical trial (NCT04680988), particularly in patients with PD-L1 positive tumors [243]. Furthermore, the TORCH-2 study (NCT04337463) demonstrated the efficacy of combining toripalimab (a PD-1 inhibitor) and onatasertib (an mTORC1/2 inhibitor), presenting a novel therapeutic strategy that concurrently targets immune checkpoints and mTOR signaling pathways in advanced solid tumors, including aCC [244]. These findings support the potential of PD-1/PD-L1 inhibitors combined with targeted drugs as a 2L therapy for aCC and identify specific biological targets and patient subgroups that would derive the greatest benefit from these dual-drug regimens to maximize therapeutic efficacy.
ICIs, VEGF inhibitors, and chemotherapy
A promising treatment modality as combination of ICI, VEGR inhibitor, and chemotherapy has emerged for patients with previously untreated r/mCC. The BEATcc trial evaluated the efficacy of integrating atezolizumab (a PD-L1 inhibitor) with the standard treatment protocol established in the GOG240 trial, which consisted of bevacizumab and chemotherapy [245]. This trial met its primary endpoints and demonstrated substantial and clinically significant enhancements in both PFS and OS. Specifically, the trial reported an mPFS of 13.7 months and an mOS of 32.1 months. These outcomes exceeded those observed in the GOG240 trial (mPFS of 8.2 months and mOS of 17.0 months) and the KEYNOTE-826 trial (mPFS of 10.4 months). In light of these combined findings, the current 1L treatment guidelines for patients with untreated r/mCC should incorporate a regimen of chemotherapy plus bevacizumab in conjunction with PD-1/PD-L1 inhibitors in patients eligible for bevacizumab [235]. A combination of chemotherapy and PD-1/PD-L1 inhibitors is recommended for patients in whom bevacizumab is contraindications. Future research should prioritize evaluating the safety and side-effect profiles of these triplet combination therapies, optimizing the sequence of administration, and identifying specific patient populations that would most benefit from this treatment strategy.
Despite several encouraging clinical trials, limitations remain in ICI treatment of patients with aCC, including the lack of predictive biomarkers, variability in patient PD-L1 expression, and differences in the TME that affect treatment outcomes. Future studies should focus on identifying robust predictive biomarkers and refining combination therapeutic strategies to enhance treatment efficacy and safety. Additionally, it is imperative to conduct larger randomized controlled trials to validate these findings and establish standardized treatment guidelines.
ADCs
ADCs, often referred to as “magic bullets,” represent a groundbreaking class of therapeutics designed to deliver chemotherapy directly to solid tumors [246]. By conjugating monoclonal antibodies to chemotherapeutic agents, ADCs enhance treatment efficacy while minimizing off-target effects, thereby improving therapeutic outcomes.
Tissue factor (TF)-ADCs
TV, which combines a TF-directed antibody with a microtubule inhibitor, was approved by the FDA in 2021 for patients with advanced or recurrent cervical cancer. This approval was based on phase I/II innovaTV 201 trials and phase II innovaTV 204 trials, which demonstrated promising outcomes, including a 24% ORR and a disease control rate (DCR) of 72% [247, 248]. Furthermore, 79% of patients experienced a reduction in tumor size from baseline. Initially recommended by the NCCN as ≥2L therapy for patients with aCC, TV was classified as a “preferred” treatment option in 2023. The latest phase III innovaTV 301 trial reported a 30% reduction in mortality risk among patients with rCC who received TV instead of chemotherapy, supporting TV as a 2/3L therapy for these patients [249]. Additionally, interim results from ongoing innovaTV 205 trial, which investigated combinations of TV with PD-1 inhibitors or platinum/bevacizumab as 1/2/3L treatment for patients with r/mCC, revealed encouraging ORRs of 54.5% with 1L TV + carboplatin, 40.6% with 1L TV + pembrolizumab, and 35.3% with 2L/3L TV + pembrolizumab [250]. These findings suggest that TV could become the new standard of care for patients with r/mCC and experiencing PD after systemic therapy, enhancing both survival and quality of life.
HER2-ADCs
Trastuzumab deruxtecan (T-DXd), an ADC composed of the humanized anti-HER2 antibody trastuzumab, has been approved by the FDA for the treatment of advanced stages HER2-positive breast cancer [251]. The prevalence of HER2-positive tumors in cervical cancer varies widely from 0%-87% [252]. The phase II DESTINY-PanTumor02 basket trial, which included 40 patients with cervical cancer, showed benefits of T-DXd in HER2-positive advanced solid tumors [253]. Another phase II trial, DESTINY-PanTumor01, targeted tumors with activating HER2 mutations and observed an ORR in 66.7% of patients with aCC [254]. In 2024, the NCCN recommended T-DXd for HER2 IHC 2+ or 3+ as a ≥2L systemic treatment for recurrent cervical cancer. Disitamab vedotin (RC48), another HER2-targeting ADC, demonstrated an ORR of 36.4% in patients with HER2-expressing (IHC 1+, 2+, 3+) r/mCC in the RC48-C018 study [255]. Despite variability in HER2 expression, its predictive role in aCC remains unclear.
Other ADCs
Sacituzumab govitecan (SG), an ADC targeting Trop-2 and carrying the potent payload SN-38 (the active metabolite of irinotecan), has shown promising anti-tumor activity in Chinese patients with r/mCC [256], as evidenced by a phase II EVER-132-003 study [257], which reported an ORR of 50%. Benefits were also observed in patients previously treated with ICIs, consistent with the overall study population. Additionally, ADC 9MW2821, targeting Nectin-4, demonstrated preliminary clinical efficacy in cervical cancer, with an ORR of 40.5% and a DCR of 89.2% in r/mCC patients with PD during or after platinum-based chemotherapy with or without bevacizumab [258]. Ongoing trials are aimed at confirming the survival benefits of 9MW2821 in cervical cancer.
ADCs offer a promising alternative to traditional cytotoxic drugs for the treatment of aCC following platinum-based chemotherapy with or without bevacizumab and ICIs. However, caution is warranted in the current applications. Among the available options, TV is preferred over T-DXd in patients previously exposed to ICIs because of the level I evidence of survival benefit and efficacy independent of biomarker expression. With accumulating evidence supporting a unique combination of potent cytotoxicity and precise targeting of ADCs, future research should explore the potential of ADCs to replace chemotherapy as primary drugs in gynecologic oncology and investigate their combination with other targeted therapies.
ACT
ACT involves the ex vivo expansion of autologous or allogeneic tumor-specific T cells, which are subsequently reinfused into the patient to target and eliminate tumor cells [259]. ACT can be categorized into three primary types: tumor-infiltrating lymphocytes (TILs), TCR-engineered T cells (TCR-T), and chimeric antigen receptor T cells (CAR-T) [260]. Although ACT has demonstrated limited efficacy in solid tumors, it has the potential to induce complete clinical remission in patients with B-cell malignancies and metastatic melanoma [261]. Current research is exploring its application in cervical cancer, focusing on the selection of TILs that are primarily reactive to HPV E6 and E7 antigens, with recent evidence suggesting additional targetable neoepitopes. TILs have shown superior tumor regression capabilities compared to lymphocytes generated through vaccine therapy, indicating their effectiveness in overcoming immune evasion mechanisms [262]. In a clinical trial involving nine patients with recurrent cervical cancer receiving TIL infusions, complete remission was observed in two patients and partial remission in one, highlighting the need for further clinical investigation of this highly personalized treatment approach [263]. TCR-T therapy is an emerging anti-tumor strategy for HPV-related epithelial cancers including cervical, oropharyngeal, vulvar, and vaginal cancers. A phase I/II clinical trial demonstrated significant anti-tumor effects using genetically engineered T-cells to treat metastatic HPV-related epithelial cancers [264]. Twelve patients with HPV-16 positive metastatic tumors, including six with cervical cancer, received HPV-16 E6 TCR-T cells, with most patients experiencing tumor regression, although the depth and duration of the response varied. Research has identified TCR (10F04) specific to HPV18 E784-98 and HLA-DRA/DRB1*09:01, demonstrating potent anti-tumor activity both in vitro and in vivo [265]. HPV-specific cytotoxic T lymphocytes (HPV-rejT) derived from induced pluripotent stem cells, which have shown efficacy in inhibiting cervical cancer, have been further developed into HLA-A24 & HLA-E double-integrated HPV-rejT, effectively suppressing recipient immune rejection while maintaining enhanced cytotoxicity compared with the original cytotoxic T lymphocytes [266]. These advancements underscore the potential clinical applications of TCR-T therapy in HPV-positive patients, indicating promising therapeutic avenues for this subset of patients with cervical cancer.
In summary, the role of CCRT in patients with laCC remains steadfast. For certain high-risk patients with laCC, combining pembrolizumab with CCRT offers survival benefits and is poised to revolutionize clinical diagnostic and therapeutic approaches. The treatment paradigm for r/mCC is evolving towards quadruple therapy. The combination of TP/TC ± bevacizumab + pembrolizumab is the recommended 1L regimen for PD-L1 positive populations, while TP/TC ± bevacizumab for PD-L1 negative ones. In 2L treatment, pembrolizumab or nivolumab is the preferred option for PD-L1 positive patients, with other recommendations including 2L chemotherapy and single-agent bevacizumab. With the increasing application of ICIs, future challenges include evaluating the feasibility of retreatment with PD-1/PD-L1 inhibitors for patients with cervical cancer who have experienced progression or recurrence after initial ICI therapy, and exploring whether additional interventions during ICI treatment can lead to enhanced disease control, a concept already validated in other solid tumors [267-269]. Current clinical studies are investigating the application of 2L therapies for r/mCC. Assessing ADCs, passive immunotherapies (including PD-1/PD-L1 inhibitors and bispecific antibodies), and active immunotherapies such as ACT as initial salvage therapies will be indispensable in shaping future practices. Ongoing trials will facilitate the incorporation of additional drug regimens for advanced recurrent metastatic cervical cancer into the guideline recommendations, thereby further enhancing patient outcomes and prolonging survival (Table 1).
| ClincalTrials.gov ID | Phase | Description | Enrollment | Disease status of participationsa | Interventions | Therapy-line | Status |
|---|---|---|---|---|---|---|---|
| PD-1/PD-L1 inhibitors combined with chemotherapy/radiotherapy/CCRT | |||||||
| NCT05179239 | III | Multi-center, Randomized, Double-blind, Controlled | 32 | p/r/mCC | CT+SHR-170 | 1L | Recruiting |
| NCT03912415 | III | International, Randomized, Double-blind | 316 | aCC |
Experimental: CT + BCD-100 ± Bevacizumab Control: CT + Placebo ± Bevacizumab |
1L | Recruiting |
| NCT06093438 | I/II | Single-arm | 20 | laCC | CT + Tislelizumab | NA | Recruiting |
| NCT05511623 | II | Multicenter, Randomized | 112 | laCC (stage IIIC2) |
Experimental: CCRT + Tislelizumab Controal: CCRT |
NA | Recruiting |
| NCT05084677 | II | Open-label, Single-arm | 96 | laCC | CCRT + Toripalimab | NA | Recruiting |
| NCT05311566 | II | Single-center, Open-label, Single-arm | 92 | laCC (stage IB2-IIIB) | CCRT + Camrelizumab | NA | Recruiting |
| NCT06237257 | II | Open-label, Single-arm | 50 | laCC | CCRT + SHR-1316 | NA | Recruiting |
| NCT04884906 | II |
Single-center, Open-label, Single-arm |
40 | r/mCC | CCRT + Camrelizumab | NA | Recruiting |
| NCT03614949 | II | Open Label, Single-arm | 26 | p/r/mCC |
Experimental: Atezolizumab + RT Control: Atezolizumab |
NA | Recruiting |
| NCT05588219 | II | Single-center, Single-arm | 30 | aCC (stage IIIA, IIIB, IVA) | CCRT + Tislelizumab | NA | Recruiting |
| NCT05437692 | II | Single-arm | 19 | laCC (stage IIB-IVA) | CCRT + Zimberelimab | NA | Recruiting |
| NCT04918628 | II | Open Label, Single Group | 50 | aCC (stage IIIC2-IVB) | NACT + CCRT + Sintilimab | NA | Recruiting |
| NCT06055738 | II | Single-arm | 20 | laCC (stage IIB3, IIA2) | NACT + Zimberelimab | NA | Recruiting |
| NCT05554276 | II | Open-label, Single-arm | 36 | laCC (stage IIB-IVA) | NACT + PD-1 antibody + RT | NA | Recruiting |
| PD-1/PD-L1 inhibitors combined with VEGF/VEGFR inhibitor | |||||||
| NCT04680988 | II | Randomized, Open-label, Multi-center | 194 | r/mCC |
Experimental-1: Camrelizumab + Famitinib Experimental-2: Camrelizumab Control: CT |
NA | Active, not recruiting |
| NCT04974944 | II | Open-label, Randomized | 172 | p/r/mCC |
Experimental: Camrelizumab + Apatinib Control: CT + Bevacizumab |
1L | Recruiting |
| NCT05247619 | II | Open-label | 49 | p/r/mCC | CT + Tislelizumab + Bevacizumab | 1L | Recruiting |
| NCT04865887 | II | 35 | aCC | Pembrolizumab + Lenvatinib | ≥2L | Recruiting | |
| NCT06266338 | II | Single-center, Open-label | 30 | r/mCC | Pembrolizumab + Lenvatinib | ≥3L | Recruiting |
| PD-1 inhibitor +PARP inhibitor | |||||||
| NCT04068753 | II | 66 | r/mCC | Niraparib + Dostarlimab | ≥2L | Recruiting | |
| PD-1/CTLA-4 Dual immune checkpoint inhibitor (ICIs) | |||||||
| NCT05446883 | III | Multicenter, Randomized, Double-blind, Placebo-controlled | 55 | p/r/mCC |
Experimental: QL1706 + CT ± Bevacizumab Control: Placebo + CT± Bevacizumab |
1L | Recruiting |
| NCT04982237 | III | Double-blind, Randomized, Placebo-controlled | 440 | p/r/mCC |
Experimental: AK104 + CT ± bevacizumab Control: Placebo + CT ± bevacizumab |
1L | Recruiting |
| NCT05557565 | II | Multicenter, Open-label, Single-arm | 150 | r/mCC | QL1706 | ≥2L | Recruiting |
| NCT06251388 | II | Multicenter, Single-arm | 50 | laCC (stage III-IVA) | Cadonilimab + CCRT | 1L | Recruiting |
| NCT05606263 | II | Multicenter, Single-arm | 55 | r/mCC | Caldonirimab + Nimotuzumab | 2L | Recruiting |
| NCT05817214 | II | Open-label, Single-arm | 35 | p/r/mCC | Cadonilimab + Anlotinib | 2L | Recruiting |
| NCT05687851 | II | Multicenter, Single-arm | 33 | laCC | Candonilimab + RT | 1L | Recruiting |
| NCT05475171 | II | Open-label, Single-arm | 40 | p/r/mCC | MGD019 | NA | Recruiting |
| NCT05235516 | III | Randomized, Double-blind, Placebo-controlled | 636 | laCC(stage IIIA-IVA) |
Experimental: AK104 + CRT Control:Placebo + CRT |
NA | Recruiting |
| NCT05492123 | II | Open-label, Two-arm | 112 | laCC(stage IIB-IVA) |
Experimental: Nivolumab-Ipilimumab + Nivolumab + CCRT Control: CCRT |
NA | Recruiting |
| Other dual immune checkpoint inhibitors (ICIs) | |||||||
| NCT05990803 | II | Open-label, Single-arm | 98 | r/mCC |
Cohort 1: BL-B01D1 Monotherapy Cohort2: SI-B003 Monotherapy Cohort3: BL-B01D1 + SI-B003 (PD-1/CTLA-4 inhibitor + EGFR/HER2 inhibitor) |
1/2L | Recruiting |
| NCT06238635 | II | Non-randomized, Open-label, Two-arm | 66 | r/mCC |
Dostarlimab+Cobolimab (PD-1 inhibitor + TIM-3 inhibitor) |
≥2L | Recruiting |
| NCT06241235 | I/II | Multicenter, Open-label | 48 | aCC |
ZG005 +CT ± Bevacizumab (PD1/Tigit inhibitor) |
≥2L | Recruiting |
| Antibody-drug conjugates (ADCs) | |||||||
| NCT05838521 | II | Open-label, Single-arm | 20 | p/rCC | Sacituzumab Govitecan | ≥2L | Recruiting |
| NCT06155396 | II | Multicenter, Open- label, Single-arm | 116 | r/mCC, HER2(+) | Disitamab Vedotin + Zimberelimab | 2L | Recruiting |
| NCT06063018 | II | Single Center, Single-arm | 30 | rCC, HER2(+) | Disitamab vedotin + Tislelizumab | 2L | Recruiting |
| Adoptive cell therapy (ACT) and Vaccine | |||||||
| NCT03108495 | II | Interventional, Multicenter, Open-label, Single-arm | 189 | r/mCC |
Cohort1: TILs + Pembrolizumab Cohort2: TILs |
≥2L | Recruiting |
| NCT05686226 | II | Open-label, Single-arm | 20 | HPV-Associated Cancers | E7 TCR-T cells | NA | Recruiting |
| NCT05357027 | I/II | Open-label, Single-arm | 18 | r/mCC, HPV16(+) | TC-E202 Targeting HPV16 E6 | ≥2L | Recruiting |
- Abbreviations: aCC, advanced cervical cancer; CT, chemotherapy; CCRT, concurrent chemoradiotherapy; laCC, local advanced cervical cancer; NA, not applicable; NACT, neoadjuvant chemotherapy; p/r/mCC, persistent, recurrent or metastatic cervical cancer; RT, radiotherapy; r/mCC, recurrent or metastatic cervical cancer; TILs, tumor-infiltrating lymphocytes; TCR, T-cell receptors.
- a The clinical stages are according to FIGO 2018.
4.4 Management of specific pathological subtypes
Despite recent advancements in treatment, significant unmet clinical needs persist in patients with rare or uncommon aggressive histological subtypes of cervical cancer, such as neuroendocrine cervical carcinoma (NECC) and gastric-type cervical adenocarcinoma (GCA). The rarity of these histological subtypes poses substantial challenges for the design of prospective clinical trials, resulting in a lack of mature and standardized treatment strategies. Consequently, these patients are often excluded or underrepresented in large randomized clinical trials. To address these knowledge gaps, the gynecological oncology community must rely on case series and registry studies to retrospectively evaluate the effectiveness of new therapies in these specific patient subgroups.
4.4.1 Small cell neuroendocrine cervical carcinoma (SCNCC)
NECC, particularly SCNCC, is a rare but highly aggressive subtype of cervical cancer, accounting for less than 5% of cases [270]. Unlike the local spread observed in cervical squamous cell carcinoma and adenocarcinoma, SCNCC tends to spread early, both locally and distantly, leading to high mortality and poor prognosis. The 5-year DFS rates were 36.8% for FIGO stages I-IIA, 9.8% for stages IIB-IVA, and virtually zero for stage IVB [271, 272]. Currently, specific treatment recommendations for SCNCC are not defined separately in the FIGO and ESGO guidelines. A comprehensive treatment approach involving surgery, chemotherapy, and radiotherapy is recommended [273-275]. However, there is no standard initial treatment for SCNCC at different stages. According to the NCCN guidelines, for early-stage SCNCC (stage IA-IB2), the initial treatment options include surgery, chemoradiation brachytherapy, or NACT depending on the tumor size. Non-surgical methods are primarily recommended as the 1L treatment for locally advanced tumors (stage IB3–IVA).
Although small-sample retrospective studies comparing the prognosis of radical surgery and direct chemoradiation in stage I-II patients have produced inconsistent results [276, 277], a recent large retrospective study involving 1,288 participants from the Surveillance, Epidemiology, and End Results (SEER) registry cohort and a Chinese multi-institutional registry found that surgery is a protective factor in patients with locally advanced SCNCC (stage IB3-IIA2) [278]. This study represents the largest retrospective analysis to date examining the prognostic differences between various treatment regimens in patients with SCNCC. In the absence of high-quality prospective trials, surgery followed by systemic therapy is currently considered the preferred treatment for resectable early-stage SCNCC, including locally advanced disease (IB3-IIA2).
Studies have demonstrated that chemotherapy is crucial in patients with SCNCC. All patients were advised to undergo chemotherapy, including postoperative adjuvant chemotherapy for early-stage disease, NACT for locally advanced disease, and systemic therapy for advanced disease [270]. Etoposide combined with platinum (EP) is the 1L treatment for at least five cycles [279], referenced from small cell lung cancer (SCLC) treatments. In addition to the EP regimen, irinotecan combined with cisplatin (IP) is recommended as 1L treatment for locally advanced SCNCC [271]. Immunotherapy and targeted therapies may also offer benefits, particularly for patients with recurrent or metastatic SCNCC; however, there are no approved targeted therapies for SCNCC. A retrospective analysis showed that the combined topotecan, paclitaxel, and bevacizumab (TPB) regimen in recurrent SCNCC resulted in an mPFS of 7.8 months, compared to 4.0 months in the TB group (P = 0.001), with 23% of patients in the TPB group achieving a CR [280]. Based on this evidence, the NCCN guidelines now recommend the TPB regimen as the 1L treatment for recurrent or metastatic SCNCC. Several other small retrospective studies have also supported a significant improvement in PFS with VEGF inhibitors as 1L or 2L therapy [281, 282]. In the context of SCLC treatment, several key targets in the HR pathway, such as PARP [283], ATR [284], Chk1 [285], and Wee1 [286], have shown promise. These targets have not been reported in SCNCC. However, the prevalence of PARP expression in SCNCC suggests its potential as a therapeutic target [287]. An ongoing phase II clinical trial (NCT04701307) is currently investigating the effectiveness of the PARP inhibitor niraparib in combination with the PD-1 inhibitor dostarlimab for the treatment of recurrent SCLC and other high-grade neuroendocrine carcinomas, including SCNCC. This trial may provide further insights. Upregulation of phosphorylated ATR, Chk1/2, and BRCA1 in the HR pathway has been observed in hrHPV-infected cells [270]. Additionally, SCNCC exhibits three HPV integration modes that activate host oncogenes, which may offer valuable molecular standards for clinical care [288].
Nearly all SCNCC cases are PD-L1 negative and exhibit microsatellite stability (MSI) [287], which requires careful consideration when using ICIs. However, recent studies have suggested that the effectiveness of ICIs is not solely dependent on PD-L1 expression, particularly when used in combination therapies. Results from two phase III clinical trials in patients with SCLC indicated that the addition of PD-1 inhibitors such as atezolizumab and durvalumab to 1L chemotherapy significantly improved OS and PFS [289, 290]. As a result, the NCCN guidelines also recommend combining EP with atezolizumab/durvalumab as 1L therapy for patients with recurrent/metastatic SCNCC. Small preliminary studies and case reports on previously treated patients with SCNCC have shown encouraging results with nivolumab combined therapy or monotherapy, as well as ACT, whereas pembrolizumab monotherapy exhibited limited antitumor activity [291-295]. Further trials are required to validate the efficacy of combination therapies in a larger patient population.
4.4.2 GCA
The International Endocervical Adenocarcinoma Criteria and Classification (IECC), introduced in 2018, categorized cervical adenocarcinomas into two main groups based on HPV status and morphology: HPV-associated cervical adenocarcinomas (HACA) and non-HPV-associated cervical adenocarcinomas (NHACA) [296]. Notably, NHACA displays unique morphological and molecular traits, resulting in poor prognosis and presenting different potential targets for future therapies. This subtype is frequently detected at an advanced stage, partly because of its reliance on HPV testing for cervical cancer screening, leading to higher rates of lymph node metastasis and reduced prognosis. Currently, treatment strategies for cervical cancer are not differentiated based on HPV status, and there is a paucity of evidence supporting distinct therapeutic approaches for NHACA. Clinicians generally adhere to the treatment protocols for stage-matched cervical adenocarcinomas.
GCA is the most prevalent subtype of NHACA and the second most common cervical adenocarcinoma, accounting for approximately 10% [297]. NGS analyses have identified frequent TP53 and STK11 mutations in GCA [298, 299]. Notably, STK11 mutations are closely associated with Peutz-Jeghers syndrome (PJS), an autosomal dominant disorder characterized by mucocutaneous pigmentation and gastrointestinal polyposis [298]. Studies have indicated that 11%-17% of female patients with PJS develop GCA, and approximately 50% of patients with GCA also present with PJS [300, 301]. This underscores the significant role of genetic susceptibility in GCA pathogenesis. At diagnosis, 40% to 100% of GCA patients are in stages II to IV, with 75% demonstrating distant metastases [297, 302]. Even at stage I, GCA is associated with a poor prognosis, as evidenced by a 5-year survival rate of only 42%, in stark contrast to the 91% observed in usual-type cervical adenocarcinoma [297]. GCA has exhibited primary resistance to both chemotherapy and radiotherapy and locally advanced GCA may not respond optimally to CCRT, highlighting the imperative need for novel therapeutic strategies [303, 304]. Molecular genetic analyses revealed that 14.7% of GCA cases exhibited ERBB2 amplification, suggesting that HER2-targeted therapies may be beneficial [305, 306]. Clinical outcomes for patients with ERBB2 alterations treated with trastuzumab have been promising, paralleling experiences with HER2-positive endometrial, breast, and gastric cancers [307, 308]. Moreover, Claudin-18.2 (CLDN18.2) in GCA can be as high as 65%, indicating its potential as a therapeutic target [308]. Zolbetuximab, a pioneering monoclonal antibody targeting CLDN18.2, has been investigated for 1L treatment in patients with CLDN18.2-positive, HER2-negative locally advanced unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma [309, 310]. Although specific therapeutic outcomes for cervical cancer have not been published, it is plausible to anticipate that zolbetuximab could offer substantial clinical benefits to patients with CLDN18.2-positive GCA.
5 PROMISING EXPLORATION AND FUTURE EXPECTATION
In the age of precision medicine, personalized diagnosis and treatment of cervical cancer relies on the identification of innovative biomarkers and molecular targets. However, not all identified biomarkers are universally applicable, which poses a primary challenge for patient selection in precision medicine. Recent advancements in high-throughput sequencing technologies and bioinformatic tools have enabled the discovery and validation of predictive biomarkers for early lesions, treatment responses, and prognosis. Integrating multi-omics approaches, such as genomics, transcriptomics, and proteomics, allows for thorough molecular analysis of tumors to inform personalized treatment strategies.
The effectiveness of ICIs in the treatment of aCC is well established, although some patients experience no response. The advent of single-cell sequencing technology has enabled the exploration of the TME in cervical cancer, which has helped in identifying specific subpopulations related to the immune response to tumors and in optimizing treatment approaches. Studies have utilized single-cell RNA-seq (scRNA-seq) analysis of patients with aCC to discover neuro-like progenitor cells associated with radiotherapy efficacy [311], suggesting their potential as predictive biomarkers for radiotherapy response. Additionally, a separate study identified CCL20+ and APOE+ macrophages, PODXL+ endothelial cells (ECs), CD16+ NK cells, and MHC+ ECs, all of which were correlated with patient survival [312]. Furthermore, clonally expanded T cells and BCL6+ NEIL1+ germinal center B cells, and tertiary lymphoid structures have been identified as potential predictive biomarkers of immunotherapy response in patients with cervical cancer [313]. In addition, research has demonstrated that CRT can induce innate immune activation and elevate MHC-II expression in cervical cancer, indicating a comprehensive immune response in epithelial cells [314]. This encompasses the recruitment of CD16+ NK cells with heightened cytotoxic gene expression and FCN1+ M-MDSCs with augmented pro-inflammatory characteristics, providing valuable insights into the potential synergy of CRT and immunotherapy. Different single-cell HPV infection statuses have been proposed as personalized treatment strategies for HPV-positive squamous cell carcinoma, HACA, and NHACA. Moreover, promising new immunotherapeutic approaches targeting anti-CD96 and anti-TIGIT have been validated for cervical cancer, laying a strong foundation for further preclinical investigations [315]. Other studies have identified specific cellular interactions and elucidated the potential mechanisms of immune evasion in cervical cancer. The collaborative role of IDO1-expressing DC_LAMP3 cells and reactive T cells has been observed in shaping the immunosuppressive microenvironment of cervical cancer, suggesting that a combination in IDO1 and ICB therapies (e.g., anti-PD1/anti-CTLA4 drugs) could improve the outcomes of immunotherapy for IDO1-positive cervical cancer patients [316].
To fully leverage the potential of precision medicine in oncology, it is crucial to conduct thorough research on emerging biomarkers and molecular targets of cervical cancer. By delving into the intricate molecular makeup of this disease, researchers can identify new diagnostic indicators and therapeutic targets, offering the promise of personalized treatment approaches. As our understanding of cervical cancer biology deepens, along with the successful construction of innovative preclinical research models such as organoids [317, 318], integrating biomarker-guided diagnostics and targeted therapies into clinical practice presents new opportunities to enhance patient outcomes and mitigate the global burden of this disease.
6 CONCLUSIONS
Advances in our understanding of the pathobiology and immune microenvironment of cervical cancer are driving a paradigm shift in the treatment landscape. Future research is shifting toward individualized and precision medicine. With well-defined etiology, early and precise screening of high-risk populations is pivotal for the fight against this cancer. In addition to current screening technologies, ongoing research is exploring more accurate and efficient screening methods such as HPV integration screening, epigenetic markers, and liquid biopsies. In the early stages of the disease, surgical treatment evolves away from traditional radical approaches and increasingly embraces a more conservative stance focused on quality of life and fertility preservation. For advanced cervical cancer, new therapies offer hope for addressing treatment challenges, with combination therapies holding promise for overcoming these hurdles. Nevertheless, considerable variability exists in treatment efficacy, underscoring the need for personalized treatment to shape the future of therapy. Although several of these treatments are still in their early stages, their specific advantages and risks warrant further investigation. Looking ahead, the treatment of cervical cancer will increasingly hinge on interdisciplinary collaboration, integrating fields such as molecular biology, immunology, pharmacology, and precision engineering. This integrated approach aims to develop safer, more effective, and personalized treatment methods to enhance patient survival rates and quality of life.
AUTHOR CONTRIBUTIONS
Ding Ma, Xiaoyuan Huang, and Miaochun Xu conceived the manuscript. Miaochun Xu and Canhui Cao collected relevant references and drafted the manuscript. Miaochun Xu, Canhui Cao, and Peng Wu finished the table and figures. Xiaoyuan Huang and Peng Wu offered crucial content revision and language polishing. Ding Ma and Xiaoyuan Huang completed the final manuscript. All authors contributed actively to the manuscript and approved the final version.
ACKNOWLEDGEMENTS
We would like to thank BioRender for providing the tools necessary to create the figures in this manuscript. This study was supported by the National Key R&D Program of China (2021YFC2701201), the Natural Science Foundation of China (82072895, 82372929, 82141106, and 82203453) and the Foundation of Tongji Hospital of HUST, China (2023CXZH014 and 24-2KYC13057-08).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




