Partial hepatectomy versus interventional treatment in patients with hepatitis B virus-related hepatocellular carcinoma and clinically significant portal hypertension: a randomized comparative clinical trial
Yichuan Yuan, Hong Peng, Wei He, and Yun Zheng contributed equally to this work.
Trial Registration: ClinicalTrials.gov identifier (NCT01642446).
Abstract
Background
The widely accepted view that portal hypertension (PHT) is a contraindication to hepatectomy for patients with hepatocellular carcinoma (HCC) is being increasingly challenged. The long-term survival outcomes and safety of partial hepatectomy versus interventional treatment using ablation with or without pre-ablation transarterial chemoembolization (TACE) in patients with HBV-related HCC within the Milan criteria and with clinically significant PHT were compared in this study.
Methods
This open-label randomized clinical trial was conducted on consecutive patients with clinically PHT and hepatitis B virus (HBV)-related HCC with tumors which were within the Milan criteria. These patients were randomized 1:1 to receive either partial hepatectomy or interventional treatment between December 2012 and June 2018. The primary endpoint was overall survival (OS); secondary endpoints included recurrence-free survival (RFS) and therapeutic safety.
Results
Each of the 2 groups had 80 patients. The 1-, 3- and 5-year OS rates in the partial hepatectomy group and the interventional treatment group were 95.0%, 86.2%, 69.5% versus 93.8%, 77.5%, 64.9%, respectively (P = 0.325). The corresponding RFS rates were 78.8%, 55.0%, 46.2% versus 71.3%, 52.5%, 45.0%, respectively (P = 0.783). The partial hepatectomy group had a higher complication rate compared to the interventional group (67.5% vs. 20%, P < 0.001). However, the differences were mainly in Clavien-Dindo Grade I complications (P < 0.001), while not significant in Grade II/III/IV/V (All P > 0.05).
Conclusions
This study shows that partial hepatectomy treatment did not meet prespecified significance for improved OS and RFS compared to interventional treatment for patients with HBV-related HCC within the Milan criteria and with clinically significant PHT. However, partial hepatectomy is still a safe procedure and should be considered as a treatment option rather than a contraindication.
Abbreviations
-
- AFP
-
- alpha fetoprotein
-
- BCLC
-
- barcelona clinic liver cancer
-
- CT
-
- computed tomography
-
- EASL
-
- European association for study of liver
-
- HBV
-
- hepatitis B virus
-
- HCC
-
- hepatocellular carcinoma
-
- HCV
-
- hepatitis C virus
-
- HR
-
- hazard ratio
-
- HVPG
-
- hepatic venous pressure gradient
-
- KPS
-
- karnofsky performance status
-
- MRI
-
- magnetic resonance imaging
-
- OS
-
- overall survival
-
- PHT
-
- portal hypertension
-
- RCT
-
- randomized comparative trial
-
- RFS
-
- recurrence free survival
-
- TACE
-
- transarterial chemoembolization
1 BACKGROUND
Hepatocellular carcinoma (HCC) is the sixth most common cancer and it ranks as the third leading cause of cancer-related mortality in the world [1]. Over 80% of HCC are associated with liver cirrhosis [2], which contributes to the development of portal hypertension (PHT).
PHT have been regarded by the European Association for Study of Liver (EASL) since 2001 to be a contraindication to liver resection for HCC patients. This recommendation is based on the findings of the two studies conducted in 1996 [3] and 1999 [4] which showed that PHT, as defined by a hepatic venous pressure gradient (HVPG) ≥ 10 mmHg, to be a strong predictor of postoperative liver decompensation and poor survival after hepatectomy. The current Barcelona Clinic Liver Cancer (BCLC) staging system also recommends transarterial chemoembolization (TACE), ablation, or radioembolization to treat HCC patients with PHT instead of hepatectomy, with the exception of using liver transplantation in indicated patients when a donor liver is available [5]. However, it is important to note that no specific portal pressure cut-off value may be given for such a decision and no robust recommendation can be made. Furthermore, the measurement of HVPG is not commonly used in clinical practice, with clinical diagnosis methods for PHT being more commonly employed [5]. Nonetheless, these recommendations have sparked numerous controversies due to the fact that many patients diagnosed with PHT are deemed to be surgical candidates and can tolerate hepatectomy [6-8]. In addition, the recommendations by the BCLC group are based on results obtained in western patient cohorts who predominantly have hepatitis C virus (HCV)-related HCC and PHT, thus leaving the impact at hepatitis B virus (HBV)-related HCC and PHT on eastern patient cohorts uncovered. And previous studies which compared survival and safety outcomes after hepatectomy versus ablation treatment for patients with HCC and PHT reported either equivocal or controversial outcomes between these two treatment strategies [9-12]. In addition, most of these studies are retrospective studies with inherent selection biases and small sample sizes. To our knowledge, no prospective randomized clinical trial has been carried out to examine the efficacy of partial hepatectomy versus non-resection treatment for HCC patients with clinically significant PHT.
The present study was conducted to compare the long-term survival and safety outcomes of partial hepatectomy versus interventional treatment (ablation with or without pre-ablation TACE) in HBV-related HCC patients with clinically significant PHT.
2 MATERIALS AND METHODS
2.1 Study design
This is a multi-center, prospective, randomized comparative trial (RCT). Patients were recruited at Sun Yat-Sen University Cancer Center (SYSUCC; Guangzhou, Guangdong, China) and the First Affiliated Hospital of Sun Yat-Sen University (SYSU-FAH; Guangzhou, Guangdong, China) between December, 2012 to June, 2018. Prior to randomization, detailed information regarding the clinical protocol was explained to all participants, and written informed consent was obtained. This trial was approved by the Institutional Review Board of SYSUCC and registered in clinicaltrials.gov (NCT01642446). The research was conducted in accordance with the ethical standards of the Declaration of Helsinki.
2.2 Participants
From December 2012 to June 2018, consecutive patients were screened for enrollment. HCC was diagnosed based on the criteria used by the EASL [2]. Both liver surgeons and interventional radiologists reviewed radiographic images of all the patients and confirmed that they were treatable using both surgery and ablation.
The inclusion criteria comprised: (1) HCC within the Milan criteria, defined as a single nodule < 5 cm in diameter or three or fewer nodules, each < 3 cm in diameter; (2) chronic HBV infection; (3) clinically significant portal hypertension; (4) Karnofsky Performance Status (KPS) ≥ 70; (5) Child-Pugh A or B; (6) sufficient residual liver volume. Due to the conventional view that surgery entails greater trauma and heightened risks than ablation, stringent exclusion criteria have been implemented to ensure safety. This aims to prevent patients from randomly entering the surgery group and encountering sever postoperative liver dysfunction and bleeding. Exclusion criteria encompassed: (1) HCV infection; (2) prior treatment for HCC; (3) previous or concurrent malignancies; (4) patients with hepatic encephalopathy, uncontrolled ascites, history of gastrointestinal bleeding, and hepatorenal syndrome; (5) inadequate hematologic function (platelet count < 30 × 109/L, hemoglobin < 90 g/L, prothrombin time > 18 s, total bilirubin > 50 µmol/L).
The gold standard criteria for PHT is a HVPG ≥ 10 mmHg [3]. However, due to its invasiveness, this measurement is not routinely performed in daily clinical practice. Instead, PHT is commonly diagnosed indirectly by incorporating the BCLC criteria and the Italian Program on Liver Cirrhosis [13-15]. Specifically, clinically significant PHT was diagnosed if two or more of the following criteria were met: (1) platelet count < 100 × 109/L and/or white blood cell count < 4 × 109/L on two times in succession; (2) splenomegaly (spleen thickness > 4.5 cm or major diameter > 10 cm); (3) portal vein width > 14 mm or splenic vein width > 10 mm by ultrasound; (4) esophageal and gastric varices detected by endoscopy or computed tomography/magnetic resonance imaging (CT/MRI).
2.3 Sample size
Sample size was determined based on our previous retrospective study [12], the 5-year OS rates for patients with HCC and clinically significant PHT who met the Milan criteria were 63.9% and 42.6% in the surgical and ablation groups, respectively. It required 70 patients per group (a = 0.05, power = 0.8). We increased the sample size by 10% to compensate for withdrawal/dropout, leading to a total of 160 patients. Based on randomized block design, the block number was 8.
2.4 Randomization and blinding
Patients were randomized to receive either partial hepatectomy or interventional treatment in a 1:1 ratio. A computer-based random sequence stratified by centers (SYSUCC and SYSU-FAH) was generated by a third party from the data center who was not involved in this study. After randomization, the randomly assigned sequence was placed inside sequentially numbered, opaque, sealed envelopes. The randomly assigned sequence was generated by the randomization table method. After written informed consent was obtained from an eligible patient, a nurse who was not involved in this study opened a sealed envelope and informed the investigators of the assigned treatment group of the patient. The allocation was not blinded to the patients and their clinicians, but the statisticians who finally analyzed the data remained blinded.
2.5 Interventions
Patients received partial hepatectomy or interventional treatment within 1 week after randomization. Partial hepatectomy and interventional treatment was carried out as described in our previous studies[12, 16-18].
2.5.1 Partial hepatectomy
Preoperative imaging and liver function as evaluated by the Child-Pugh grading, blood biochemistry, ICG-R15 level were used to determine the tumor extent and to develop the surgical plan. Intraoperative ultrasound was used to guide tumor resection and minimize major vascular injury. To reduce intraoperative blood loss, intermittent Pringle maneuver was adopted for the majority of hepatic resections and the central venous pressure was routinely maintained at approximately 2-4 mmHg. In this study, all the tumors were completely resected macroscopically and had a microscopically tumor-free margin as determined histopathological (R0 resection).
2.5.2 The interventional treatment
In this study the interventional treatment comprised of ablation using microwave ablation/radiofrequency ablation (MWA/RFA) with or without pre-ablation TACE. Lesion which was assessed to have less than full guarantee for complete ablation e.g. large tumor size or close proximity to major vessels, were initially treated with TACE to facilitate subsequent complete tumor ablation.
TACE was performed using 30 mg/m2 of epirubicin, 200 mg/m2 of carboplatin, and 4 mg/m2 of MMC, mixed with 2-5 mL lipiodol, followed by up to 10 mL of lipiodol into the tumor-feeding artery until stasis of blood flow in the targeted artery was achieved. After 1 month of the initial TACE, CT/MRI was performed to assess treatment efficacy.
Ablation was performed under intravenous conscious sedation. Either a radiofrequency system (RF2000; Radio Therapeutics, Mountain View, USA) or a microwave system (ECO-100C; ECO Microwave Electronic Institute, Nanjing, China) was used. All procedures were guided and monitored by ultrasonography and the aim of ablation was to create a hyperechoic area which overlapped the tumor with a surrounding 1cm treatment margin. At the end of the procedure, the needle track was ablated to prevent bleeding and tumor seeding.
2.6 Follow-up and study endpoints
Complications within the first 30 days of treatment were recorded and the Clavien-Dindo classification was used to grade the severity of complications [19]. All patients were followed-up one month after treatment, and then once every 3-4 months thereafter. At each follow-up visit, history taking, physical examination, blood tests (including serum alpha fetoprotein (AFP), liver function, complete blood count and coagulation parameters) and at least one abdominal imaging scan (enhanced CT or MRI) were performed.
The primary endpoint was overall survival (OS), defined as the time from randomization to death from any cause or with censoring at the last follow-up for patients who were still alive. The secondary endpoints were recurrence free survival (RFS), defined as the time from randomization to disease recurrence or death from any cause, and treatment safety.
2.7 Statistical analysis
Data were presented as medians and range for continuous variables and number/prevalence for categorical variables. Continuous variables were compared by the independent samples t-test. Binary variables were compared using the Chi-squared test, and ordinal categorical variables were compared by the Kruskal-Wallis test. OS and RFS were analyzed with the Kaplan-Meier curves and compared with the log-rank test. The Cox proportional hazards model was used in multivariable analysis to explore independent prognostic factors of OS and RFS. Variables identified as significant on univariable and other important clinical characteristics analysis (treatment options, main tumor size, tumor number, PLT < 100 × 109/L and/or WBC < 4 × 109/L, splenomegaly, PV > 14 mm or SV > 10 mm, esophageal and gastric varices, Grade III/IV/V Complications) were entered into the multivariable Cox proportional hazard regression analysis to identify independent prognostic factors. The proportional hazards assumption was verified by the Schoenfeld residual test and plots. All analyses were two-sided, and a P < 0.05 was considered significant. All statistical analyses were performed using R version 3.5.0 and SPSS 24.0.
3 RESULTS
3.1 Patient characteristics and treatment
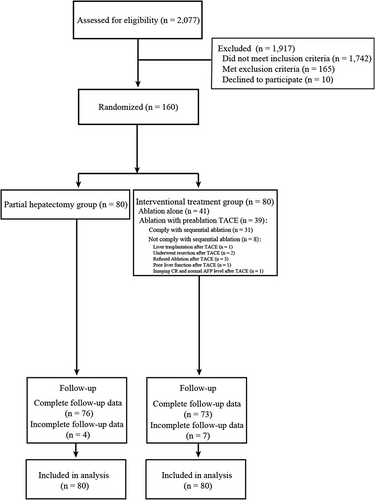
Among 2,077 patients who were assessed for eligibility between December 2012 and June 2018, 160 patients (132 [82.5%] men; median age, 54.5 [range, 31.2-81.1] years) were enrolled and randomized in a 1:1 ratio, with 80 patients each in the partial hepatectomy and interventional treatment groups (Figure 1). The baseline characteristics for the two groups are presented in Table 1. A total of 105 patients presented with esophageal and gastric varices. Seventy patients had white blood cell counts (WBC) below 4 × 109/L, with the lowest count at 2 × 109/L, and 111 patients had platelet counts (PLT) below 100 × 109/L, with the lowest count at 31 × 109/L. No significant differences were found in the Child-Pugh classifications (P = 0.620), tumor sizes (P = 0.218), number of tumors (P = 0.251), and AFP levels (P = 0.454).
| Treatment group | |||
|---|---|---|---|
| Characteristic | Partial hepatectomy (n = 80) | Interventional treatment (n = 80) | P value |
| Gender (male vs. female), n (%) | 61 (76.2) vs. 19 (23.8) | 71 (88.7) vs. 9 (11.3) | 0.060 |
| Age (y), 54.2 ± 9.5 | 52.8 ± 10.2 | 55.4 ± 8.8 | 0.089 |
| ALT (U/L, >40 vs. ≤40), n (%) | 23 (28.7) vs. 57 (71.3) | 30 (37.5) vs. 50 (62.5) | 0.314 |
| AST (U/L, >40 vs. ≤40), n (%) | 27 (33.7) vs. 53 (66.3) | 35 (43.7) vs. 45 (56.3) | 0.256 |
| WBC (109/L, ≥4 vs. <4), n (%) | 50 (62.5) vs. 30 (37.5) | 40 (50.0) vs. 40 (50.0) | 0.151 |
| PLT (109/L, ≥100 vs. <100), n (%) | 25 (31.2) vs. 55 (68.8) | 24 (30.0) vs. 56 (70.0) | 1.000 |
| Hemoglobin (g/L), 137.7 ± 20.8 | 139.8 ± 18.4 | 135.9 ± 22.5 | 0.232 |
| Creatinine (µmol/L), 76.4 ± 15.7 | 77.2 ± 16.8 | 74.6 ± 16.3 | 0.324 |
| BUN (mmol/L), 5.2 ± 1.4 | 5.4 ± 1.5 | 4.9 ± 1.4 | 0.057 |
| PT (s), 12.7 ± 1.3 | 12.4 ± 1.1 | 13.0 ± 1.3 | 0.067 |
| APTT (s), 31.4 ± 5.0 | 30.5 ± 5.0 | 32.2 ± 4.7 | 0.060 |
| INR, 1.1 ± 0.1 | 1.1 ± 0.1 | 1.1 ± 0.1 | 0.066 |
| AFP (ng/ mL, >400 vs. ≤400), n (%) | 21 (26.2) vs. 59 (73.8) | 16 (20.0) vs. 64 (80.0) | 0.454 |
| ALBI score, n (%) | |||
| 1 | 27 (33.7) | 28 (35.0) | 1.000 |
| 2 | 50 (62.5) | 49 (61.2) | 1.000 |
| 3 | 3 (3.8) | 3 (3.8) | 1.000 |
| Child-Pugh classification (A vs. B), n (%) | 79 (98.7) vs. 1 (1.3) | 77 (96.2) vs. 3 (3.8) | 0.620 |
| Tumor number (solitary vs. multiple), n (%) | 66 (82.5) vs. 14 (17.5) | 59 (73.7) vs. 21 (26.3) | 0.251 |
| Size of the tumor (cm), 2.6 ± 0.9 | 2.7 ± 0.9 | 2.5 ± 0.9 | 0.218 |
| Splenomegaly (pre vs. abs), n (%) | 75 (93.8) vs. 5 (6.2) | 77 (96.3) vs. 3 (3.7) | 0.719 |
| PV > 14 mm or SV > 10 mm (pre vs. abs), n (%) | 16 (20.0) vs. 64 (80.0) | 16 (20.0) vs. 64 (80.0) | 1.000 |
| Esophageal and gastric varices (pre vs. abs), n (%) | 52 (65.0) vs. 28 (35.0) | 53 (66.3) vs. 27 (33.7) | 1.000 |
| Grade III/IV/V complications (pre vs. abs), n (%) | 8 (10.0) vs. 72 (90.0) | 2 (2.5) vs. 78 (97.5) | 0.098 |
- Continuous variables are reported in mean and standard deviation.
- Abbreviations: abs, absence; AFP, alpha-fetoprotein; ALT, alanine aminotransferase; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; BUN, blood urea nitrogen; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; INR, international normalized ratio; PHT, portal hypertension; PLT, platelet; pre, presence; PT, prothrombin time; WBC, white blood cell.
In the partial hepatectomy group, all patients underwent R0 resection, with 68 patients undergoing non-anatomical resection and 12 patients undergoing anatomical resection. The mean occlusion time with the Pringle maneuver was 13.5 ± 8.8 min (range, 0-40.0). In the interventional treatment group, 41 patients underwent ablation only and 31 patients underwent TACE and subsequent ablation. Eight patients did not receive ablation as planned after TACE, including 3 patients refused the sequential ablation treatment, 2 patients underwent liver resection, 1 patient underwent liver transplantation, 1 patient discontinued with ablation because of poor liver function, and 1 patient was assessed to have CR on medical imaging with AFP returning to normal after TACE so that sequential ablation was not performed. Baseline characteristics of the two different treatment subgroups of patients in the interventional group are shown in Supplementary Table S1. The mean ablation time was 12.0 ± 8.2 min (range, 3.0-36.0) among the patients who underwent ablation.
The study was censored on January 1, 2023. The median follow-up was 79.6 months for all the patients, 80.3 months for the partial hepatectomy group and 78.5 months for the interventional group. Four of 80 (5.0%) patients in the partial hepatectomy group and 7 of 80 (8.8%) in the interventional group were lost to follow-up; 76 of 80 (95.0%) patients in the partial hepatectomy group and 73 of 80 (91.3%) patients in the interventional group completed the study (Figure 1).
3.2 Outcomes
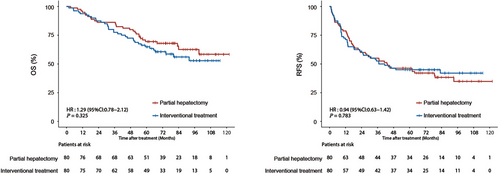
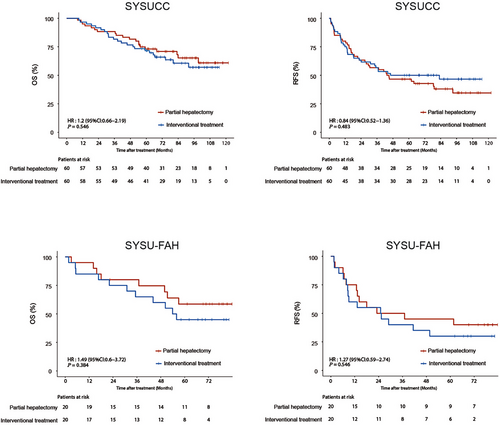
In the entire cohort, the median OS and RFS were not reached in the two groups. During follow-up, 28 (35.0%) patients in the partial hepatectomy group and 34 (42.5%) patients in the interventional treatment group died. There were no deaths in the partial hepatectomy group within 90 days of treatment, however in the interventional group, 1 patient died at 1.7 months after treatment, thus the 90-day mortality rate in the interventional group was 1.3%, and there was no statistical difference between the two treatment groups in terms of 90-day mortality. The partial hepatectomy group had a mean hospital stay of 13.8 days (range: 7.0-29.0), significantly longer than the 8.8 days (range: 4.0-15.0) for the interventional group (P < 0.001). The 1-, 3-, and 5-year OS rates in the partial hepatectomy group were 95.0%, 86.2%, and 69.5%, respectively, while those in the interventional treatment group were 93.8%, 77.5%, and 64.9%, respectively (hazard ratio [HR] = 1.29; 95% confidence interval [CI] = 0.78-2.12, P = 0.325) (Figure 2). Tumor recurrence was observed in 49 (61.3%) patients in the partial hepatectomy group and 45 (56.3%) patients in the interventional treatment group. The 1-, 3- and 5-year RFS rates in the partial hepatectomy group were 78.8%, 55.0%, and 46.2%, respectively, while those in the interventional treatment group were 71.3%, 52.5%, and 45.0%, respectively (HR = 0.94; 95% CI = 0.63-1.42, P = 0.783) (Figure 2). Additionally, Supplementary Table S2 provides data on tumor recurrence locations across different treatment groups, while Supplementary Table S3 details the treatment methods administered to the two groups of patients after recurrence. Comparison of OS and RFS in the partial hepatectomy group and interventional treatment group were separately analyzed in the SYSUCC cohort and the SYSU-FAH cohort. The results showed that there were no significant differences in OS and RFS between the partial hepatectomy group and the interventional group in both the SYSUCC cohort and the SYSU-FAH cohort (Figure 3). Subgroup analyses within the interventional treatment group were conducted by dividing the patients into subgroup 1 (Ablation alone) and subgroup 2 (TACE plus Ablation), with the specific results shown in Supplementary Figure S1 and the corresponding annotation. The OS and RFS of the partial hepatectomy group were also compared with those of the two subgroups in the interventional treatment group, as detailed in Supplementary Figure S2 and its annotation. Furthermore, the results comparing OS and RFS between partial hepatectomy and interventional treatment for HCC patients with clinically significant PHT as treated are presented in Supplementary Figure S3 and its annotation.
3.3 Prognostic factors for OS and RFS
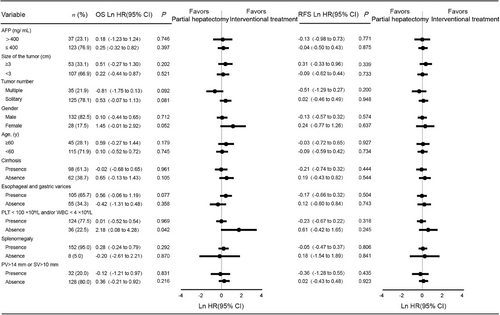
The predictors for OS and RFS on univariable and multivariable analyses are shown in Table 2 and Table 3. Univariable analysis indicated that PLT < 100 × 109/L and/or WBC < 4 × 109/L was the only factor that was significantly associated with both OS and RFS (P = 0.020 for OS; P = 0.030 for RFS). After controlling for treatment and other important clinical characteristics in the multivariable model, size of tumor and PLT < 100 × 109/L and/or WBC < 4 × 109/L were independent prognostic factors for OS (HR = 1.83, 95% CI = 1.07-3.13 and P = 0.027 for tumor size; HR = 2.51, 95% CI = 1.19-5.31 and P = 0.015 for PLT < 100 × 109/L and/or WBC < 4 × 109/L) and RFS (HR = 1.94, 95% CI = 1.26-2.97 and P = 0.002 for tumor size; HR = 1.77, 95% CI = 1.01-3.08 and P = 0.044 for PLT < 100 × 109/L and/or WBC < 4 × 109/L). Additionally, tumor number was identified as an independent prognostic factor for RFS (HR = 1.89, 95% CI = 1.18-3.05 and P = 0.008). Furthermore, subgroup analysis was conducted to investigate whether there were any survival differences among patients with different baseline characteristics in the partial hepatectomy and the intervention treatment groups. The OS and RFS of patients undergoing the partial hepatectomy or the intervention treatment in different subgroups are similar. The partial hepatectomy was associated with worse OS vs. the intervention treatment with absence of PLT > 100 × 109/L and WBC > 4 × 109/L (Ln HR = 2.18, 95% CI = 0.08-4.28, P = 0.042) (Figure 4).
| Case No. | Univariable Cox regression | Multivariable Cox regression | ||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Study group | HR | 95%CI | P value | HR | 95%CI | P value | |
| Treatment | Interventional treatment | 80 | 1.28 | 0.77-2.12 | 0.326 | 1.33 | 0.79-2.24 | 0.284 |
| Partial hepatectomy | 80 | ref | ref | ref | N/A | N/A | N/A | |
| Gender | Female | 28 | 0.89 | 0.45-1.76 | 0.751 | N/A | N/A | N/A |
| Male | 132 | ref | ref | ref | N/A | N/A | N/A | |
| Age (y) | ≥60 | 45 | 1.33 | 0.79-2.24 | 0.282 | N/A | N/A | N/A |
| <60 | 115 | ref | ref | ref | N/A | N/A | N/A | |
| Child-Pugh classification | A | 156 | 0.59 | 0.14-2.43 | 0.468 | N/A | N/A | N/A |
| B | 4 | ref | ref | ref | N/A | N/A | N/A | |
| AFP (ng/mL) | >400 | 37 | 0.98 | 0.54-1.79 | 0.969 | N/A | N/A | N/A |
| ≤400 | 123 | ref | ref | ref | N/A | N/A | N/A | |
| ALBI score | 1 | 55 | 0.90 | 0.21-3.86 | 0.888 | N/A | N/A | N/A |
| 2 | 99 | 1.01 | 0.24-4.22 | 0.979 | N/A | N/A | N/A | |
| 3 | 6 | ref | ref | ref | N/A | N/A | N/A | |
| Main tumor size(cm) | ≥3 | 53 | 1.55 | 0.93-2.57 | 0.090 | 1.83 | 1.07-3.13 | 0.027 |
| <3 | 107 | ref | ref | ref | ref | ref | ref | |
| Tumor number | Multiple | 35 | 1.45 | 0.83-2.51 | 0.182 | 1.65 | 0.93-2.94 | 0.085 |
| Solitary | 125 | ref | ref | ref | ref | ref | ref | |
| PLT < 100 × 109/L and/or WBC < 4 × 109/L | Presence | 124 | 2.41 | 1.14-5.07 | 0.020 | 2.51 | 1.19-5.31 | 0.015 |
| Absence | 36 | ref | ref | ref | ref | ref | ref | |
| Splenomegaly | Presence | 152 | 0.91 | 0.28-2.89 | 0.868 | 0.92 | 0.28-3.00 | 0.892 |
| Absence | 8 | ref | ref | ref | ref | ref | ref | |
| PV > 14 mm or SV > 10 mm | Presence | 32 | 1.06 | 0.57-1.96 | 0.842 | 1.01 | 0.54-1.89 | 0.956 |
| Absence | 128 | ref | ref | ref | ref | ref | ref | |
| Esophageal and gastric varices | Presence | 105 | 1.25 | 0.73-2.13 | 0.412 | 1.33 | 0.77-2.30 | 0.296 |
| Absence | 55 | ref | ref | ref | ref | ref | ref | |
| Grade III/IV/V complications | Presence | 10 | 1.31 | 0.48-3.63 | 0.598 | 3.08 | 1.11-8.15 | 0.030 |
| Absence | 150 | ref | ref | ref | ref | ref | ref | |
- Abbreviations: AFP, alpha-fetoprotein; CI, confidence interval; HR, hazard ratio; OS, overall survival; PLT, platelet; PV, portal vein; SV, spleen vein; WBC, white blood cell.
| Case No. | Univariable Cox regression | Multivariable Cox regression | ||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Study group | HR | 95%CI | P value | HR | 95%CI | P value | |
| Treatment | Interventional treatment | 80 | 0.94 | 0.63-1.41 | 0.783 | 0.95 | 0.62-1.46 | 0.825 |
| Hepatectomy | 80 | ref | ref | ref | N/A | N/A | N/A | |
| Gender | Female | 28 | 0.97 | 0.57-1.67 | 0.936 | N/A | N/A | N/A |
| Male | 132 | ref | ref | ref | N/A | N/A | N/A | |
| Age (y) | ≥60 | 45 | 1.45 | 0.95-2.23 | 0.081 | N/A | N/A | N/A |
| <60 | 115 | ref | ref | ref | N/A | N/A | N/A | |
| Child-Pugh classification | A | 156 | 1.20 | 0.29-4.89 | 0.795 | N/A | N/A | N/A |
| B | 4 | ref | ref | ref | N/A | N/A | N/A | |
| ALBI score | 1 | 55 | 0.72 | 0.25-2.03 | 0.533 | N/A | N/A | N/A |
| 2 | 99 | 0.64 | 0.23-1.75 | 0.381 | N/A | N/A | N/A | |
| 3 | 6 | ref | ref | ref | N/A | N/A | N/A | |
|
AFP (ng/mL) |
>400 | 37 | 1.03 | 0.64-1.67 | 0.887 | N/A | N/A | N/A |
| ≤400 | 123 | ref | ref | ref | N/A | N/A | N/A | |
| Main tumor size(cm) | ≥3 | 53 | 1.87 | 1.24-2.83 | 0.003 | 1.94 | 1.26-2.97 | 0.002 |
| <3 | 107 | ref | ref | ref | ref | ref | ref | |
| Tumor number | Multiple | 35 | 1.58 | 1.00-2.49 | 0.046 | 1.89 | 1.18-3.05 | 0.008 |
| Solitary | 125 | ref | ref | ref | ref | ref | ref | |
| PLT < 100 × 109/L or WBC < 4 × 109/L | Presence | 124 | 1.84 | 1.06-3.21 | 0.030 | 1.77 | 1.01-3.08 | 0.044 |
| Absence | 36 | ref | ref | ref | ref | ref | ref | |
| Splenomegaly | Presence | 152 | 0.68 | 0.30-1.57 | 0.375 | 0.81 | 0.34-1.89 | 0.627 |
| Absence | 8 | ref | ref | ref | ref | ref | ref | |
| PV > 14 mm or SV > 10 mm | Presence | 32 | 1.02 | 0.61-1.69 | 0.927 | 1.08 | 0.64-1.82 | 0.746 |
| Absence | 128 | ref | ref | ref | ref | ref | ref | |
| Esophageal and gastric varices | Presence | 105 | 1.27 | 0.82-1.96 | 0.281 | 1.43 | 0.91-2.25 | 0.120 |
| Absence | 55 | ref | ref | ref | ref | ref | ref | |
| Grade III/IV/V complications | Presence | 10 | 1.11 | 0.48-2.54 | 0.812 | 2.79 | 1.18-6.58 | 0.019 |
| Absence | 150 | ref | ref | ref | ref | ref | ref | |
- Abbreviations: AFP, alpha-fetoprotein; HR, hazard ratio CI, confidence interval; PLT, platelet; PV, portal vein; RFS, recurrence - free survival; SV, spleen vein; WBC, white blood cell.
3.4 Safety analysis
Complications after treatments are shown in Table 4. There was no significant difference in major complications between the two treatment groups. However, ascites and pleural effusion were more frequent in the partial hepatectomy group (P = 0.032 for ascites; P = 0.017 for pleural effusion). In the two groups, there was no patient who died within 30 days from treatment-related complications. Three patients (2 in the partial hepatectomy group and 1 in the interventional treatment group) developed variceal hemorrhage which was classified as Grade IV complication, and received intensive management in the Intensive Care Unit. Overall, there were 54 complications in the partial hepatectomy group, and 16 in the interventional treatment group (P < 0.001). The differences were mainly in Clavien-Dindo grade I complications (P < 0.001) but not in Grade II/III/IV/V complications (all P > 0.05).
| Treatment group | |||
|---|---|---|---|
| Adverse event |
Partial hepatectomy (n = 80) |
Interventional treatment (n = 80) |
P value |
| Pain, n (%) | 12 (15.0) | 5 (6.2) | 0.122 |
| Arrhythmia, n (%) | 8 (10.0) | 3 (3.7) | 0.210 |
| Ascites, n (%) | 10 (12.5) | 2 (2.5) | 0.032 |
| Postoperative bleeding, n (%) | 2 (2.5) | 2 (2.5) | 1.000 |
| Wound infection, n (%) | 4 (5.0) | 0 (0) | 0.120 |
| Biliary leakage, n (%) | 1 (1.2) | 0 (0) | 1.000 |
| Hepatic insufficiency, n (%) | 1 (1.2) | 0 (0) | 1.000 |
| Lung infection, n (%) | 5 (6.2) | 2 (2.5) | 0.443 |
| Pleural effusion, n (%) | 11 (13.7) | 2 (2.5) | 0.017 |
| Total complication, n (%) | 54 (67.5) | 16 (20.0) | <0.001 |
| Clavien-Dindo classification, n (%) | |||
| Grade I | 44 (55.0) | 13 (16.2) | <0.001 |
| Grade II | 2 (2.5) | 1 (1.2) | 1.000 |
| Grade III | 6 (7.5) | 1 (1.2) | 0.117 |
| Grade IV | 2 (2.5) | 1 (1.2) | 1.000 |
| Grade V | 0 (0) | 0 (0) | 1.000 |
- Abbreviation: AE, adverse events.
4 DISCUSSION
In this study, no significant differences in OS and RFS were found in patients with HBV-related HCC and clinically significant PHT who underwent either partial hepatectomy or interventional treatment (TACE/ablation). The incidence of complications after partial hepatectomy was significantly higher than that after interventional treatment, but the majority of complications were mild (grade I). Even with the advantages of intervention therapy such as reduced expense, faster recovery, ease of repeat treatment, and increased quality of life, the partial hepatectomy can still be an option for patients whose tumor location is not suitable for ablation (such as tumors located on the surface or near important biliary ducts and vessels). To our knowledge, this is the first multi-center RCT study that specifically focused on comparing the outcomes of partial hepatectomy with interventional treatment in this patient population.
The initial design of this study was based on a superiority trial, inspired by our previous study that reported a significantly lower 5-year OS rate in the interventional treatment group (42.6%) compared to the hepatectomy group (63.9%) [12]. The actual 5-year OS rate of 64.9% in the interventional treatment group was higher than initially expected, while the partial hepatectomy treatment group had a similar rate of 69.5% to our previous study. The comparable outcomes suggested that this study failed under the category of a superiority study. We believed that this discrepancy may be attributed to the advancements in interventional treatment techniques and the availability of additional post-treatment options, leading to improved patient prognoses in the interventional treatment cohort compared to previous study.
Despite its robust prognostic value [20, 21], HVPG is not widely used in daily clinical practice. To address this limitation, the EASL-EORTC clinical practice guidelines propose an alternative diagnostic measure for clinically significant PHT by using a platelet count below 100 × 109/L with splenomegaly and esophageal varices [22]. However, there is no standardized clinical criteria for indirect definition of clinically significant PHT. To address this problem, the alternative diagnostic criteria used in our present study have been endorsed by the Expert Consensus on Clinical Diagnosis and Treatment of Portal Hypertension with HCC in China [23].
A meta-analysis revealed that clinically significant PHT was associated with elevated 3- and 5-year mortality rates and increased risks of clinical liver decompensation after partial hepatectomy in patients with HCC [8]. Nonetheless, this meta-analysis has raised more doubts than to clarity and the debate persists on whether PHT ought to be regarded as an absolute contraindication to liver resection [6, 7, 24]. Several recent retrospective studies conducted on HCC patients in China reported that PHT had no significant impact on the complication rate and OS after partial hepatectomy [25-27]. Similar retrospective studies conducted on western HCC patients also produced comparable findings [9, 10, 28, 29]. In our previous propensity score matching study, postoperative complications, RFS and OS of HCC patients with PHT were found to be comparable to those patients without PHT after partial hepatectomy [26].
Based on the premise that partial hepatectomy is not a contraindication for patients with HCC and PHT, the identification of treatment outcomes between partial hepatectomy and other treatment modalities for this specific but not uncommon group of patients becomes important. Interventional treatments including TACE and ablation are options that can be used alone or in combination to achieve tumor eradication with maximum preservation of liver function. In a previously reported retrospective study conducted by us, patients with HBV-related HCC and PHT which met the Milan criteria had significantly better RFS and OS after partial hepatectomy when compared to those patients treated with ablation [12]. Another study found no significant difference in OS between patients treated with partial hepatectomy with radiofrequency ablation in BCLC stage 0/A stage HCC patients with PHT [11]. TACE is commonly used in treatment for intermediate-stage HCC, although little is known about the prognostic impact of PHT on this treatment. In a study involving Western patients with HCC who underwent TACE, 69.1% of patients had PHT [30]. While poorer OS was observed in patients with PHT, it was not found to be a significant prognostic factor on multivariable analysis. Previous SURF trial also showed that, for patients with largest HCC diameter ≤ 3 cm and ≤ 3 HCC nodules, RFS did not differ significantly between the surgery and RFA groups [31]. Although our study population differed from that of the SURF study, our findings similarly support that thermal ablation has an effect comparable to resection for early-stage HCC. Based on a prior conducted study in our center, TACE-RFA was superior to RFA alone in achieving OS for patients with HCC less than 7 cm [32], which led us to use the interventional treatment strategy of TACE/ablation for the control group in this study.
The differences between Chinese patients with HCC and PHT compared with those in Europe and the United States can well be explained by the different etiological backgrounds leading to cirrhosis [1]. Thus, it is crucial to consider the differences in the background etiologies of HCC and to assess their impact on prognosis of patients with HCC and PHT. HCC patients with HCV have been found to have significantly higher postoperative mortality and poorer postoperative survival than patients with HBV [33, 34]. Therefore, the recommendation to consider partial hepatectomy to be a contraindication for patients with HCC and PHT basing on data coming from studies on western populations may not be applicable to Chinese patients who predominantly have HBV-related HCC.
This study has limitations. First, while HVPG is currently regarded as the gold standard for accurate assessment of portal pressure changes, it has the limitations, including invasiveness, complexity and high costs. Alternative standards should be used on daily clinical practices to diagnose PHT. In this study, clinically significant PHT was indirectly diagnosed by the commonly used surrogate criteria. Second, all patients included in this study had HBV infection. Thus, generalizability of the findings of this study to HCC of other etiologies beyond HBV remains to be determined.
5 CONCLUSIONS
This study shows that the partial hepatectomy treatment did not meet prespecified significance for improved OS and RFS versus interventional treatment for patients with HBV-related HCC within the Milan criteria and with clinically significant portal hypertension. However, partial hepatectomy is still a safe procedure and should be considered as a treatment option rather than a contraindication.
AUTHOR CONTRIBUTIONS
Concept and design: Yichuan Yuan, Wei He, Binkui Li, and Yunfei Yuan. Acquisition, analysis, or interpretation of data: Yichuan Yuan, Hong Peng, Wei He, Bin Chen, Yun Zheng, and Jiliang Qiu. Drafting of the manuscript: Yichuan Yuan and Wei He. Statistical analysis: Yichuan Yuan and Wei He. Administrative, technical, or material support: Ruhai Zou, Chenwei Wang, Wan Yee Lau. Supervision: Binkui Li and Yunfei Yuan. All the authors approved this version manuscript to be submitted.
ACKNOWLEDGEMENTS
We sincerely appreciate the patients who participated in our study and their families and health providers. We also thank the investigators and staff who contributed to this study at the medical centers. We solemnly remember professor Wan Yee Lau, one of the authors, who deceased February 7, 2024. This work was supported by the grants from the Sun Yat-Sen University Clinical Research 5010 Programme (2012010) and the National Natural Science Foundation of China (82272887).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This trial was approved by the institutional review board of Sun Yat-sen University Cancer Center (Approval No.5010-2012-02) and registered in clinicaltrials.gov (NCT01642446). Written informed consent for participation was obtained from all the patients.
Open Research
DATA AVAILABILITY STATEMENT
The datasets analyzed during the current study are available from the corresponding author upon reasonable request.




