Efficacy, safety and biomarkers of SG001 for patients with previously treated recurrent or metastatic cervical cancer: an open-label, multicenter, phase Ib trial
Jing Zuo and Wei Duan contributed equally.
Abbreviations
-
- AE
-
- adverse event
-
- CI
-
- confidence interval
-
- CR
-
- complete response
-
- DCR
-
- disease control rate
-
- DoR
-
- duration of response
-
- EAS
-
- efficacy analysis set
-
- ECOG
-
- Eastern Cooperative Oncology Group
-
- FAS
-
- full analysis set
-
- IRC
-
- Independent Review Committee
-
- NE
-
- not evaluable
-
- NGS
-
- next-generation sequencing
-
- non-SCC
-
- nonsquamous cell carcinoma
-
- ORR
-
- objective response rate
-
- OS
-
- overall survival
-
- PD
-
- progressive disease
-
- PD-1
-
- programmed death-1
-
- PD-L1
-
- programmed death-ligand 1
-
- PFS
-
- progression-free survival
-
- PR
-
- partial response
-
- r/mCC
-
- recurrent or metastatic cervical cancer
-
- SCC
-
- squamous cell carcinoma
-
- SD
-
- stable disease
-
- SS
-
- safety set
-
- TMB
-
- tumor mutational burden
-
- TRAE
-
- treatment-related adverse event
-
- TTR
-
- time to response
Cervical cancer (CC) is one of the most common gynecological cancers, ranking fourth in incidence and mortality rates among women worldwide and second in China [1]. Approximately 15%-61% of patients with CC develop recurrent or metastatic (r/m) disease in the first two years after initial therapy completion, with a 5-year survival rate of 17% [2]. Platinum-based chemotherapy is the first-line treatment for r/mCC.
The immune checkpoint inhibitors targeting the programmed death-1 (PD-1)/PD-ligand 1 (PD-L1) pathway have provided promising therapeutic choices [3, 4]. Based on the results from the KEYNOTE-158 [5] and KEYNOTE-826 trials [3], pembrolizumab has been approved by the U.S. Food and Drug Administration (FDA) as first-line (in combination with chemotherapy, with or without bevacizumab) and second-line or subsequent treatments for patients with PD-L1-positive r/mCC. However, the objective response rate (ORR) of PD-L1 inhibitor monotherapies rarely exceeds 30% in patients with PD-L1-positive r/mCC [4, 6, 7].
SG001 is a fully humanized and high-affinity immunoglobulin G4 monoclonal antibody that targets PD-1 to block its interaction with the ligands PD-L1 and PD-L2. Here, we present the results from an expansion cohort (previously treated r/mCC) of an open-label, multicenter, phase Ib trial of SG001 monotherapy in patients with multiple advanced cancers (NCT03852823). Patients with histologically confirmed r/mCC who had progressed or were intolerant after one or more lines of chemotherapy and had at least one measurable lesion per the Response Evaluation Criteria in Advanced Solid Tumors (RECIST, version 1.1) were enrolled. SG001 (240 mg) was administered intravenously every 2 weeks until progressive disease, intolerable toxicity, or withdrawal. The methods for this study are described in detail in the Supplementary Material file.
A total of 91 patients were enrolled (Supplementary Figure S1). Ninety (98.9%) patients had received prior platinum-based therapy and 83 (91.2%) patients had undergone previous radiotherapy. In addition, 73 (80.2%) patients had squamous cell carcinoma (SCC), and 78 (85.7%) patients had distant metastasis. Forty-three (47.3%) patients had PD-L1-positive tumors (combined positive score ≥ 1). The baseline characteristics are summarized in Supplementary Table S1.
The Independent Review Committee (IRC)-assessed ORR was 25.3% (95% CI, 16.7-35.5), 29 (31.9%) of patients had stable disease (SD), and the disease control rate (DCR) was 63.7% (95% CI, 53.0-73.6) (Figure 1A and Supplementary Table S2). The median time to response (TTR) was 1.4 months (95% CI, 1.4-2.7). The median duration of response (DoR) had not been reached, with a 12-month DoR rate of 62.4% (95% CI, 35.7-80.5) (Supplementary Table S2). Thirty-nine patients exhibited a reduction in the target lesion size from baseline (Figure 1B). Similar results of the ORR, DCR, DoR and TTR by the investigators were also observed (Supplementary Table S2).
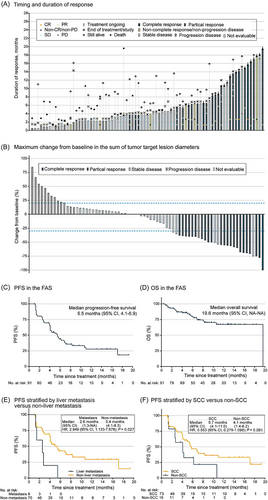
Characteristic of treatment response by IRC. (A) Timing and duration of responses in the EAS. The length of each bar represents the duration of treatment for each patient. (B) Best change from baseline in target lesion size in EAS. The dashed lines at −30% and +20% represent the cut-offs for PR and PD. The Kaplan-Meier curves of (C) PFS and (D) OS in the FAS. Kaplan-Meier curves of PFS stratified by (E) liver metastasis versus non-liver metastasis and (F) SCC versus non-SCC. Abbreviations: CI, confidence interval; CR, complete response; EAS, efficacy analysis set; FAS, full analysis set; HR, hazard rate; IRC, Independent Review Committee; NA, not available; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; SCC, squamous cell carcinoma; SD, stable disease.
The median progression-free survival (PFS) was 5.5 months (95% CI, 4.1-6.9), with a 6-month PFS rate of 43.8% (95% CI, 31.6-55.4) per the IRC (Figure 1C). A total of 66 patients (72.5%) were still alive at the data cut-off date, with a 12-month overall survival (OS) rate of 65.8% (95% CI, 52.3-76.3) (OS curve with censored data was shown in Figure 1D). In the 78 patients with distant metastasis, 8.8% patients (n = 8) with liver metastasis showed a significantly shorter median PFS than those without liver metastasis (IRC: 2.6 months versus 5.4 months, P = 0.027; investigator: 2.1 months versus 4.2 months, P = 0.021) (Figure 1E, Supplementary Figure S2A). Compared to patients with non-SCC, patients with SCC showed a longer median PFS (IRC: 5.7 months versus 4.1 months, P = 0.091; investigator: 5.7 months versus 3.2 months, P = 0.027) (Figure 1F, Supplementary Figure S2B).
In the subset of patients with PD-L1-positive tumors (n = 43), the IRC-assessed ORR was 30.2%, with a median PFS of 7.1 months (Supplementary Figure S3A). Confirmed responses were also observed in those with PD-L1-negative tumors (n = 45), with an ORR of 20.0% and a median PFS of 4.3 months (Supplementary Figure S3A-B). To the best of our knowledge, our study revealed a relatively high ORR of PD-1 inhibitor monotherapy for ≥ second-line treatments in this setting in either the PD-L1-positive population or the PD-L1-negative population compared to the previous studies [6, 7]. Next-generation sequencing (NGS) testing data were obtained from 55 patients. Patients with high tumor mutational burden (TMB ≥ 4.92 mutations/Mb, n = 45) showed a higher ORR and longer median PFS than those with low TMB (n = 10) (IRC-assessed ORR: 33.3% versus 20.0%, P = 0.416; median PFS: 6.8 months versus 4.1 months, P = 0.022) (Supplementary Figure S3C-D). For the definition of TMB-high, the pre-specified threshold is 10 mutations/Mb in the KEYNOTE 158 study; accordingly, only 16% of patients in the CC cohort were regarded as TMB high [8]. In this study, the cut-off value of TMB was determined to be 4.92 mutations/Mb, resulting in more patients (81.8%) being classified as TMB-high. Our cut-off value was similar to that reported in a previous study [9].
Furthermore, the efficacy of combining PD-L1 and TMB was also investigated. No response was observed in patients with PD-L1-negative/TMB-low tumors (n = 6), whereas the IRC-assessed ORRs were 32.1%, 35.3% and 50.0% in patients with PD-L1-positive/TMB-high tumors, PD-L1-negative/TMB-high tumors, and PD-L1-positive/TMB-low tumors, respectively (Supplementary Figure S3E). The median PFS was longer in pooled patients with either PD-L1-positive tumors or TMB-high tumors or both than in those with PD-L1-negative/TMB-low tumors (IRC: 5.7 months versus 4.1 months, P = 0.081, Supplementary Figure S3F; investigator: 6.8 months versus 2.8 months, P = 0.023). Our combination analysis showed that patients with either PD-L1-positive tumors or high TMB or both had a benefit, with ORRs all over 30%, which is consistent with the results of the CLAP study [9], showing that the combination of TMB status and PD-L1 expression has a better prediction value.
A total of 74.7% (n = 68) of patients experienced one or more treatment-related adverse events (TRAEs). TRAEs of grade 3-4 were reported in 18.7% of patients (Supplementary Table S3), which was comparable to other similar studies with a reported rate of 12.2%-21.1% [4, 5, 10]. Grade ≥ 3 TRAEs that occurred in three or more patients were anemia (4.4%) and decreased lymphocyte count (3.3%). Three (3.3%) patients discontinued treatment because of TRAEs (abnormal hepatic function, interstitial lung disease, and toxic epidermal necrolysis) and one patient discontinued treatment due to AE assessed as not related to the SG001. No TRAEs leading to death occurred. Immune-related AEs of any grade associated with SG001 occurred in 36.3% (n = 33) patients, and 7.7% (n = 7) experienced at least one or more immune-related AEs of ≥ grade 3. The grade ≥ 3 immune-related AE that occurred in two or more patients was abnormal hepatic function (2.2%). The immune-related AEs (i.e., hypothyroidism and hyperthyroidism) reported here were consistent with those of other PD-1 inhibitors [8, 9].
This study was limited by a single-arm trial with no historical or concurrent control group. Despite this limitation, our exploration of PD-1 inhibitor monotherapy may still provide some theoretical basis for the role played by PD-1 inhibitors in later combination therapy for synergistic activities and greater clinical benefits [6]. The benefit of SG001 monotherapy in PD-L1-positive r/mCC has been further verified in an ongoing phase II study (NCT04886700). A phase III study of SG001 plus chemotherapy with/without bevacizumab in patients with PD-L1-positive r/mCC is underway (NCT05715840).
In summary, SG001 monotherapy exhibited clinically meaningful efficacy with minimal safety concerns in previously treated r/mCC patients. Of note, not only PD-L1 expression but also TMB could be predictors of the effectiveness of PD-1 inhibitor monotherapy. The encouraging response results and manageable safety profiles of SG001 revealed its significant potential in combination therapy for r/mCC.
AUTHOR CONTRIBUTIONS
Conception and design: Jing Zuo, Lingying Wu, Wei Duan, Mingxuan Zhao and Xiugao Yang. Acquisition, analysis, and interpretation of the data: Jing Zuo, Lingying Wu, Wei Duan, Mingxuan Zhao, Zhendong Chen, Jie Lin, Huaqiu Shi, Ou Jiang, Youzhong Zhang, Meiyu Fang, Li Wang, Wei Wang, Yong Huang, Junyan Yu, Xiaoxue Zhang, Weiqing Pu, Deshun Hao, Fenglin She, Xiugao Yang, Xiao Zhang, Miao Niu, and Yan'e Song. Administrative, technical, and material support: Jing Zuo, Lingying Wu, Wei Duan, Deshun Hao, Fenglin She, Ying Chen, and Qizhi Tang. Supervision: Jing Zuo, Lingying Wu, Wei Duan, Mingxuan Zhao, Zhendong Chen, Jie Lin, Huaqiu Shi, Ou Jiang, Youzhong Zhang, Meiyu Fang, Li Wang, Wei Wang, Yong Huang, Junyan Yu, Xiaoxue Zhang and Weiqing Pu. Writing—review and editing: Jing Zuo, Lingying Wu, Wei Duan and Mingxuan Zhao.
ACKNOWLEDGMENTS
The authors would like to thank the patients who participated in this trial and their families, as well as the investigators, study coordinators, study teams, and nurses who assisted. Writing and editorial assistance in the preparation of this article was provided by Cuixia Gao and Tingyuan Yang of CSPC Zhongqi Pharmaceutical Technology (Shijiazhuang) Co., Ltd.
FUNDING INFORMATION
This study was supported by the CSPC Zhongqi Pharmaceutical Technology (Shijiazhuang) Co., Ltd.
CONFLICT OF INTEREST STATEMENT
Xiaoxue Zhang, Weiqing Pu, Deshun Hao, Fenglin She, Xiugao Yang, Ying Chen, Qizhi Tang, Xiao Zhang, Miao Niu, and Yan'e Song are employees of CSPC Zhongqi Pharmaceutical Technology (Shijiazhuang) Co., Ltd. The others have no conflicts of interest to declare.
ETHICS APPROVAL STATEMENT AND CONSENT TO PARTICIPATE
The study was approved by the independent ethics committee National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (ID. 20/353-2137) and each participating center and was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice. All patients signed informed consent in advance of participation. ClinicalTrials.gov identifier: NCT03852823.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




