Protein tyrosine phosphatases: emerging role in cancer therapy resistance
Min Zhao, Wen Shuai, and Zehao Su contributed equally to this work.
Abstract
Background
Tyrosine phosphorylation of intracellular proteins is a post-translational modification that plays a regulatory role in signal transduction during cellular events. Dephosphorylation of signal transduction proteins caused by protein tyrosine phosphatases (PTPs) contributed their role as a convergent node to mediate cross-talk between signaling pathways. In the context of cancer, PTP-mediated pathways have been identified as signaling hubs that enabled cancer cells to mitigate stress induced by clinical therapy. This is achieved by the promotion of constitutive activation of growth-stimulatory signaling pathways or modulation of the immune-suppressive tumor microenvironment. Preclinical evidences suggested that anticancer drugs will release their greatest therapeutic potency when combined with PTP inhibitors, reversing drug resistance that was responsible for clinical failures during cancer therapy.
Areas covered
This review aimed to elaborate recent insights that supported the involvement of PTP-mediated pathways in the development of resistance to targeted therapy and immune-checkpoint therapy.
Expert opinion
This review proposed the notion of PTP inhibition in anticancer combination therapy as a potential strategy in clinic to achieve long-term tumor regression. Ongoing clinical trials are currently underway to assess the safety and efficacy of combination therapy in advanced-stage tumors.
List of abbreviations
-
- ALK
-
- anaplastic lymphoma kinase
-
- AKT
-
- protein kinase B
-
- AP-1
-
- activator protein-1
-
- BRAF
-
- V-raf murine sarcoma viral oncogene homolog B1
-
- BRCA
-
- breast cancer susceptibility gene
-
- CAR-T
-
- chimeric antigen receptor T-cell immunotherapy
-
- CDK4/6
-
- cyclin-dependent kinase 4/6
-
- CTL
-
- cytotoxic T lymphocyte
-
- CTLA-4
-
- cytotoxic T lymphocyte associate protein-4
-
- CSF1
-
- colony-stimulating factor-1
-
- CSF1R
-
- colony-stimulating factor-1 receptor
-
- CRISPR
-
- clustered regularly interspaced palindromic repeats
-
- DCR
-
- disease control rate
-
- DLT
-
- dose limiting toxicity
-
- DUSP4
-
- dual-specificity phosphatase 4
-
- DUSP6
-
- dual-specificity phosphatase 6
-
- EGFR
-
- epidermal growth factor receptor
-
- EMT6
-
- experimental mammary tumour-6
-
- ERK
-
- extracellular regulated protein kinase
-
- Gab2
-
- Grb2-associated-binding protein 2
-
- GDP
-
- guanosine diphosphate
-
- Grb2
-
- growth factor receptor-bound protein 2
-
- GTP
-
- guanosine triphosphate
-
- HCC
-
- hepatocellular carcinoma
-
- IFN-γ
-
- interferon γ
-
- IFNGR
-
- interferon-γ receptor
-
- IL-6
-
- interleukin-6
-
- IRF
-
- interferon regulatory factor
-
- ITIM
-
- immune receptor tyrosine-based inhibitory motif
-
- JAK
-
- Janus kinase
-
- JNK
-
- c-Jun N-terminal kinase
-
- KRAS
-
- kirsten rat sarcoma virus
-
- MAPK
-
- mitogen-activated protein kinase
-
- MEK
-
- mitogen-activated extracellular signal-regulated kinase
-
- MHC-I
-
- major histocompatibility complex class I
-
- MKP2/3
-
- mitogen-activated protein kinase phosphatases 2/3
-
- mTOR
-
- mammalian target of rapamycin
-
- NFAT
-
- nuclear factor of activated T-cells
-
- NF-κB
-
- nuclear factor kappa-B
-
- NRAS
-
- neuroblastoma RAS viral oncogene homolog
-
- NSCLC
-
- non-small cell lung cancer
-
- ORR
-
- objective response rate
-
- PARP
-
- poly-ADP ribose polymerase
-
- PD-1
-
- programmed cell death protein 1
-
- PDAC
-
- pancreatic ductal adenocarcinoma
-
- PDGFR
-
- platelet-derived growth factor receptor
-
- PD-L1
-
- programmed death-ligand 1
-
- PI3K
-
- phosphoinositide 3-kinase
-
- PTP
-
- protein tyrosine phosphatase
-
- PTPN2
-
- protein tyrosine phosphatase non-receptor 2
-
- PTPN11
-
- protein tyrosine phosphatase non-receptor 11
-
- QD
-
- quaque die
-
- RAS
-
- rat sarcoma virus
-
- RasGAP
-
- RAS GTPase-activating protein
-
- RAF
-
- Raf protein kinase
-
- RIPR
-
- receptor inhibition by phosphatase recruitment
-
- RNA-seq
-
- RNA-sequencing
-
- SCC
-
- squamous cell carcinoma
-
- SHP2
-
- src homology-2 domain-containing phosphatase 2
-
- Slamf6+
-
- signaling lymphocytic activation molecule family member 6-positive
-
- STAT3
-
- signal transducer and activator of transcription 3
-
- TAM
-
- tumor-associated macrophage
-
- TC-PTP
-
- T cell protein tyrosine phosphatase
-
- TCR
-
- T cell receptor
-
- Tim-3+
-
- T cell immunoglobulin domain and mucin domain-3-positive
-
- TNF-α
-
- tumor necrosis factor-α
-
- TRAE
-
- treatment-related adverse event
-
- WT
-
- wild type
1 BACKGROUND
The homeostasis of protein tyrosine phosphorylation is regarded as an important mechanism for the maintenance of intracellular signal transduction. Recruitment of protein tyrosine phosphatases (PTPs) acts as a prerequisite in the process of dephosphorylation of signal transduction proteins, which underlay the broad participation of PTPs in a diverse array of signaling pathways [1-3]. It is now well-known that some PTPs function in a positive manner downstream of growth-stimulatory and immune-suppressive signaling pathways, thereby regulating the duration and amplitude of cell proliferation and immune tolerance [4, 5]. Under the given circumstances, malfunction of PTPs seemed to be oncogenic to support tumorigenesis in different types of human cancer, ranging from hematological malignancy to solid tumors [6]. This malfunction was observed in two distinct forms: genetic mutations of PTPs during tumorigenesis and feedback activation of PTPs following cancer therapy, leading to the emergence of drug resistance [7, 8]. The past decades have witnessed progress in the discovery of pro-tumorigenic PTPs under the genetic context of specific driver mutations. Notably, Src homology-2 domain-containing phosphatase 2 (SHP2) was the firstly identified oncogenic phosphatase in the PTP family [9, 10]. Less insights were concentrated on the feedback regulation of PTPs in resistance to cancer therapy.
Recent studies have identified that PTP-mediated pathways accounted for one of the possible mechanisms of resistance to targeted therapy and immune checkpoint therapy. In the context of non-small cell lung cancer (NSCLC) cells resistant to sotorasib (Kirsten rat sarcoma virus with G12C mutation [KRASG12C] inhibitor), a in vivo clustered regularly interspaced palindromic repeats (CRISPR)-Cas9 screening unveiled the presence of sustained proliferative signaling in a SHP2-dependent manner [11]. Additionally, preclinical loss-of-function screening in melanoma mice treated with anti-programmed cell death protein 1 (PD-1) antibody identified that deletion of protein tyrosine phosphatase non-receptor 2 (PTPN2) rendered susceptibility to immune-checkpoint therapy through transcriptional activation of interferon γ (IFN-γ) response genes [2]. These evidences indicated that perturbation of PTPs served as a central signaling hub to buffer stress following cancer therapy. It occurred through promoting constitutive activation of growth-stimulatory signaling pathways or influencing over the immune-suppressive tumor microenvironment. Therefore, targeting PTP inhibition in anticancer combination therapy was probably a potential strategy to overcome clinical resistance and unleash full potential of anticancer agents. Hence, this review focused on the molecular mechanism of PTPs in resistance to targeted therapy and immune-checkpoint therapy. It aimed to highlight the potential role of PTPs as drug-resistant targets in frontline adjuvant therapy. Then, this review provided an overview of the current state of PTP inhibition-based combination regimens in clinical trials, presenting the existent obstacles as well as possible solutions. This review ultimately proposed a prominent strategy of targeting PTP inhibition in anticancer therapy to overcome the long-standing conundrum of drug resistance.
2 PTPs MEDIATE RESISTANCE TO TARGETED THERAPY
2.1 Feedback activation of SHP2 in resistance to cancer therapy targeting KRAS mutant
Under physiological condition, SHP2 (encoded by protein tyrosine phosphatase non-receptor 11 [PTPN11]) functioned in a scaffolding- or phosphatase-dependent manner to promote the transition of inactive guanosine diphosphate (GDP)-bound KRAS into active guanosine triphosphate (GTP)-bound KRAS [12]. It then triggered downstream cascade activation of the mitogen-activated protein kinase (MAPK) pathway [13]. Genetic mutations in KRAS disrupted the guanine exchange cycle, typically by locking KRAS in the persistently activated GTP-bound state [14], thereby providing growth advantage for MAPK pathway-driven cancers [15]. In a preclinical model of pancreatic ductal adenocarcinoma (PDAC) with mutated KRAS, gene set enrichment analysis revealed that deletion of PTPN11 induced a clear loss of KRAS signaling signature, including downstream mitogen-activated extracellular signal-regulated kinase (MEK), protein kinase B (AKT), and interleukin-6 (IL-6)/Janus kinase (JAK)/signal transducer and activator of transcription 3 (STAT3) signaling [16, 17]. These results indicated that mutant KRAS still depended on SHP2 for signal intensification and downstream cascade activation.
Feedback activation of SHP2 following inhibition of the rat sarcoma virus (RAS)-Raf protein kinase (RAF)-MEK pathway conferred to therapeutic resistance in KRAS mutant-driven cancers (Figure 1A). In preclinical models of pancreatic and lung cancers harboring mutant KRAS, treatment with MEK inhibitors (selumetinib or trametinib) induced a chronic activation of extracellular regulated protein kinases (ERK) over time, leading to a transient delay in tumor growth [16]. Ruess et al. [16] observed a significant increase in phosphorylation level and phosphatase activity of SHP2 in resistant pancreatic cancer cells, suggesting a SHP2-dependent positive-feedback loop for amplification of KRAS activity to compromise MEK inhibition. Scientists also claimed that combination therapy of trametinib and SHP-099 (SHP2 inhibitor) benefited to impeding ERK reactivation in response to MEK inhibition, resulting in sustained growth inhibition in KRAS-mutated PDAC [18]. In clinic, gain-of-function mutation of KRASG12C was a well-known driver mutation that accounted for the pathogenesis in 13% of NSCLC [19]. A phase II clinical trial of sotorasib (a KRASG12C inhibitor) observed objective response in 37.1% of patients with NSCLC, whereas others bypassed inhibition to resume proliferation (NCT03600883) [20, 21]. To identify the candidate genes mediating drug resistance, Xue et al. [11] conducted a genome-wide CRISPR-Cas9 screen in NSCLC cells and discovered sustained proliferative signaling in an epidermal growth factor receptor (EGFR)- and SHP2-dependent manner. These results restated the feedback regulation loop of SHP2 in the adaptive reactivation of KRAS signaling. Co-targeting SHP2 (RMC-4630) and KRASG12C (sotorasib) has been observed to attenuate the onset of drug-induced resistance, and significantly enhanced the antiproliferative effect in lung cancer cells [13] (Figure 1B).
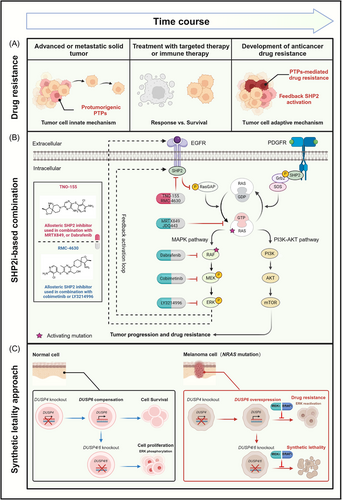
The dependence of KRAS on SHP2 phosphatase for full activation underlay the molecular mechanism of drug-induced resistance [16]. These findings manifested that in clinical settings, mutation-specific inhibitors of KRASG12C would be most effective when combined with SHP2 inhibitors to achieve sustained tumor regression. Currently, clinical trials of such combination regimen have observed objective response in KRASG12C-mutated solid tumors [22, 23].
2.2 Synthetic lethality of dual-specificity phosphatase 4 (DUSP4) and dual-specificity phosphatase 6 (DUSP6) in MAPK pathway resistant cancer
Synthetic lethality pioneered the next generation of cancer therapeutics for tackling targets that were not classically “druggable” [24]. It referred to recessive lethality under a combination of mutations in two or more separate genes, while inactivation of only one of these genes had little effect on cell viability [25]. The recent success of poly-ADP ribose polymerase (PARP) inhibitors in breast cancer susceptibility gene (BRCA)-mutated ovarian cancer was the first clinical trial to apply synthetic lethality to cancer therapy [26]. In the treatment of cancer, synthetically lethal pairs were dependent on the genetic context, namely driver mutations in cancer cells. This approach not only favored a selective cell-killing effect on cancer cells but also spared normal cells due to a lack of fixed genetic alteration, thereby providing a clinical advantage over traditional chemotherapy [27]. Therefore, identification of synthetic lethal pairs benefited the discovery of drug-sensitive targets under the genetic context of driver mutations, thus addressing the issue of treatment resistance to anticancer agents [28].
DUSP4/6, encoding MAPK phosphatases 2/3 (MKP2/3), were negative feedback regulators of the MAPK pathway through dephosphorylation of ERK, p38, and C-Jun N-terminal kinase (JNK) [29, 30]. Interestingly, Ito et al. [31] developed a CRISPR paralog targeting library screen and uncovered a novel synthetic lethal interaction between DUSP4/6 and oncogenic neuroblastoma RAS viral oncogene homolog (NRAS) in melanoma cells. Dual knockout of DUSP4 and DUSP6 selectively impaired growth of NRAS-mutated melanoma cells, in contrast to cell survival when it occurred to wild type (WT) cells or single knockout of DUSP4 or DUSP6. Indeed, 155 clinical specimens from The Cancer Genome Atlas suggested that melanoma tumors harboring oncogenic NRAS mutation showed increased expression of DUSP4/6 than that of WT tumors. These findings indicated that the synthetic lethal effect of DUSP4/6 was dependent on the genetic context of NRAS mutation. Besides, ERK, a shared substrate of DUSP4/6, engaged in the resistance of cancer cells with pre-existing oncogenic mutations in the MAPK pathway [32, 33]. Ito et al. [31] further discovered that double knockout of DUSP4/6 led to enhanced phosphorylation of ERK. Pharmacological inhibition of ERK with SCH772984 reduced the growth perturbation upon double knockout of DUSP4/6, such that resistant cells characterized as ERK reactivation provided genetic context vulnerable to DUSP4/6 knockout (Figure 1C). Notably, A375 melanoma cells resistant to dabrafenib became cross-sensitized to DUSP4/6 inhibition. Then it provided the opportunity to reduce drug resistance through intermittent cyclical drug treatment altering between V-raf murine sarcoma viral oncogene homolog B1 (BRAF) inhibitors and DUSP4/6 inhibition.
In conclusion, the compensatory relationship between functionally redundant genes, DUSP4 and DUSP6, masked their potential to be therapeutic targets in single-gene perturbation screens. The CRISPR paralog targeting library screens uncovered double knockout of DUSP4/6 as an alternative strategy in NRAS-mutated melanoma resistant to therapeutics targeting the MAPK pathway [31]. Small-molecule inhibitors targeting DUSP4/6 are deficient in current clinical settings, and further efforts in drug discovery would substantiate the synthetic lethal effect of dual inhibition of DUSP4/6 and NRAS in drug-resistant melanoma.
3 PTPs MEDIATE RESISTANCE TO IMMUNE-CHECKPOINT THERAPY
3.1 SHP2 inhibition in alleviation of colony-stimulating factor-1 (CSF1)-induced resistance to immune-checkpoint blockade
CSF1 is a key regulator to remodel the tumor microenvironment toward immunosuppression by promoting the differentiation of myeloid cells into M2-like tumor-associated macrophages (TAMs) [34]. Programmed death-ligand 1 (PD-L1) expression by TAMs triggered the activation of immune-checkpoint pathways to suppress the cytotoxicity of tumor-specific T cells [35] (Figure 2A). Humanized monoclonal antibodies directed against immune checkpoints were among the most effective cancer therapy in clinic [36, 37]. However, approximately 80% of patients failed to benefit from immunotherapy due to inadequate response or adaptive resistance [38, 39]. Neubert et al. [40] examined the gene expression in tumor biopsies from metastatic melanoma patients stratified into responders and non-responders following anti-PD-1 therapy. The non-responders exhibited a positive correlation in gene expression profiles between CD8A and CSF1, CSF1 receptor (CSF1R), CD163, indicating the co-enrichment of CD8+ T cells with TAMs. This study showed that two major cytokines secreted by activated CD8+ T cells, IFN-γ and tumor necrosis factor-α (TNF-α), were well-known drivers to induce CSF1 expression in the tumor microenvironment. The CSF1/CSF1R signaling in turn drove the recruitment of pro-tumorigenic, M2-like TAMs to human melanoma, thereby contributing to an immunosuppressive microenvironment and limited response to immune-checkpoint therapy (Figure 2A). These findings suggested that T cell-induced CSF1 expression promoted tumor adaptive resistance to immune-checkpoint therapy [40].
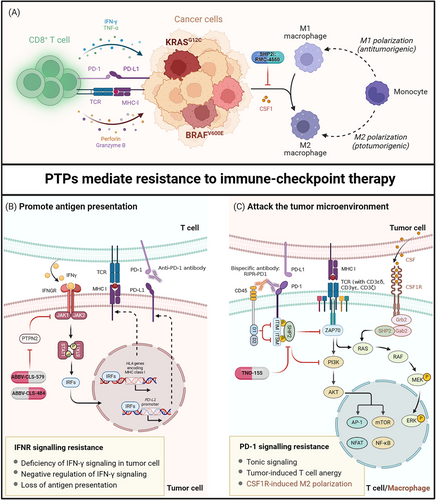
A previous research suggested that SHP2 functioned in a positive manner downstream of CSF1/CSF1R signaling to regulate polarization of TAMs toward an immunosuppressive M2 phenotype [41]. It was likely that SHP2 inhibition represented an alternative strategy to abrogate CSF1-mediated resistance to antitumor immunity. Another study found that pharmacologic inhibition of SHP2 with an allosteric inhibitor, RMC-4550, led to significant depletion of CSF1-differentiated bone marrow-derived macrophages [17]. It was attributed that SHP2 inhibition selectively induced caspase-3/7 activation in M2 macrophages, contributing to a profound shift in polarized macrophage populations toward immune susceptibility. Besides, the tumor xenograft model suggested that RMC-4550 induced significant tumor growth delay in CT26 colon carcinoma, whereas anti-CSF1R treatment had minimal effect. These differences in antitumor immunity resulted from that, in addition to CSF1R signaling, SHP2 was also a downstream molecule of PD-1 signaling to restrain T cell immunity [42]. Thus, SHP2 inhibition harbored the potential to induce CD8+ T cell infiltration and simultaneously impede the resistance dynamics by suppressing CSF1R signaling. Targeting SHP2 inhibition in cancer cells benefited to creating a less immunosuppressive tumor microenvironment susceptible to immunotherapy [43] (Figure 2B). Combination therapy of RMC-4550 and anti-PD-1 antibody resulted in tumor regression in 20% of mice bearing experimental mammary tumour-6 (EMT6) breast carcinoma, and those mice were resistant to subsequent tumor reimplantation, implying long-lasting antitumor immunity [44].
Taken together, the multiple regulatory roles of SHP2 downstream of both CSF1R and PD-1 signaling provided an enormous therapeutic advantage for patients suffering inadequate response or resistance to immune therapy. Clinical trial has initiated the combination regimen of SHP2 inhibitors (TNO-155) and anti-PD-1 antibody (spartalizumab) to evaluate the safety and efficacy in selected malignancies [45]. Meanwhile, rapid development of SHP2 allosteric inhibitors would dramatically accelerate the progression of combination therapy into clinical treatment [46].
3.2 Enforced recruitment of CD45 to block tonic signaling induced immune resistance
Tonic signaling referred to a non-coordinated and sustained activation signaling that accounted for T cell exhaustion in chimeric antigen receptor T-cell (CAR-T) immunotherapy [47, 48]. Likewise, recent study identified some tonically activated signaling in immune-checkpoint therapy. In Jurkat T cells, application of nivolumab (anti-PD-1 antibody) was efficient to block extracellular ligand-activated signaling, yet it failed to inhibit sustained intracellular downstream signaling, leading to persistent inhibition of T cell function [49]. Furthermore, deletion of the extracellular domain of PD-1 was unable to recapitulate the effect of PD-1 knockout on T cell activation [49]. These results reflected the existence of a ligand-independent mechanism of immune suppression, as evidenced by the phosphorylation of PD-1 in resting T cells [49]. These findings were consistent with previous observations of constitutive phosphorylation of PD-1 immune receptor tyrosine-based switch motif in non-stimulated peripheral blood T cells [50]. Thus, the ligand-independent activation of PD-1 signaling, defined as tonic signaling, accounted for tumor intrinsic resistance to immunotherapy.
CD45 was a receptor-like PTP bearing abundant distribution on the cell surface, resembling the subcellular distribution of immune-checkpoint receptors [51]. To remove tonic signaling-induced intracellular PD-1 phosphorylation, Fernandes et al. [49] utilized their overlapped cell distribution to develop a receptor dephosphorylation strategy through enforced recruitment of CD45 to the proximity of PD-1. This approach was termed as receptor inhibition by phosphatase recruitment (RIPR), which referred to a hetero-bispecific antibody RIPR-PD-1 exhibiting binding affinity toward both CD45 and PD-1. RIPR-PD-1 prompted cis-ligation between the intracellular phosphatase domain of CD45 and the phosphorylated motifs of PD-1, thus inhibiting both tonic and ligand-activated signaling (Figure 2B). This approach potentiated T cell activation with enhanced expression of CD69 and CD25, as well as the secretion of cytokine IL-12 in T cells. In addition, Fernandes et al. [49] also engineered a mouse version of RIPR-PD-1 to substantiate that it was more efficient to reduce tumor volume by approximately 45% than anti-PD-1 antibody in MC38 carcinoma. Therefore, the development of RIPR-PD-1 represented a promising strategy to achieve dual blockade of tonic and ligand-induced signaling in T cells, thereby reversing drug resistance arising from inadequate response to immune-checkpoint therapy.
3.3 PTPN2 mediate the IFN-γ pathway in resistance to immune-checkpoint blockade
Defective mismatch repair-induced genetic lesions have been considered as a central contributor to the development of cancer [52, 53]. High mutational burdens in cancer translated into potential neo-antigens to activate cytotoxic T lymphocytes (CTLs) for the immune attack [54], of which IFN-γ played an integral role in antigen presentation [55, 56]. Current clinical trials have substantiated the positive correlation between IFN-γ signaling and immune-checkpoint therapy [57-59]. Transcriptome analysis of 101 tumor biopsies from patients with advanced melanoma, being treated with monotherapy of nivolumab (anti-PD-1) or combined with ipilimumab (anti-cytotoxic T lymphocyte associate protein-4, anti-CTLA-4), identified that the expression of IFN-γ response genes in tumor-infiltrating T cells were the main drivers of clinical response to immune-checkpoint therapy [57]. The JAK-STAT signaling axis was downstream of interferon-γ receptor (IFNGR) to direct the transcriptional activation of IFN-γ response genes [60]. Thus, deficiency of the IFN-γ pathway resulting from loss-of-function mutations in JAK1/2 conferred tumor innate resistance to immune-checkpoint therapy [61].
PTPN2, encoding T cell-PTP (TC-PTP), was identified as a cancer immunotherapy target due to the antagonistic effect on IFN-γ sensing [2]. Manguso et al. [2] conducted a pooled loss-of-function genetic screening in melanoma mice treated with anti-PD-1 antibody, and identified that deletion of PTPN2 rendered susceptibility to immune-checkpoint therapy. Transcriptional analysis of PTPN2-null B16 cells revealed a substantial increase in the expression file of IFN-γ response genes following IFN-γ exposure, which increased the level of antigen-loaded major histocompatibility complex class I (MHC-I) on the surface of tumor cells. Then it attracted accumulation of cytotoxic CD8+ T cells into the tumor microenvironment, leading to significant growth inhibition in melanoma. Knockout Jak1 or Stat1 in PTPN2-null cells demonstrated a significant growth advantage following anti-PD-1 immunotherapy, indicating that PTPN2 was upstream of the JAK-STAT axis to modulate IFN-γ sensing by tumor cells [2] (Figure 2C). LaFleur et al. [62] further dissected that an increase in the number of CTLs could be attributed to the regulation of T cell subpopulation. This study suggested that PTPN2 deletion promoted differentiation of signaling lymphocytic activation molecule family member 6-positive (Slamf6+) progenitor exhausted cells into T cell immunoglobulin domain and mucin domain-3-positive (Tim-3+) terminally exhausted cells that were cytotoxic [62]. To evaluate the impact of PTPN2 on immune-checkpoint therapy, researchers injected MC38 tumor cells into WT or PTPN2-null mice following anti-PD-1 therapy and found that deletion of PTPN2 resulted in complete tumor clearance, in contrast to the progressive tumor growth observed in WT mice [63]. It reiterated that loss of PTPN2 rendered tumors more vulnerable to immune-checkpoint therapy.
In conclusion, deficiency in the IFN-γ pathway accounted for an integral resistance mechanism to immune therapy [57]. Loss of PTPN2 abrogated the negative effect of JAK/STAT1 on the expression of IFN-γ response genes, increasing IFN-γ sensing by tumor cells [2]. Indeed, RNA-sequencing (RNA-seq) analysis of 996 patients with glioma identified that transcriptional level of PTPN2 was increased in advanced glioma and associated with poor immune response in patients [7]. These findings supported PTPN2 inhibition as a therapeutic strategy to mitigate resistance to immune therapy. Combination therapy of PTPN2 inhibitors (ABBV-CLS-484/579) and anti-PD-1 antibodies have progressed into clinical trials to evaluate the safety and efficacy in advanced or metastatic tumors [64].
4 TARGETING PTP INHIBITION TO OVERCOME CANCER THERAPY RESISTANCE
4.1 Targeting PTP inhibition in targeted therapy
Preclinical evidences have substantiated the therapeutic potential of targeting PTP inhibition to combat the emergence of drug resistance in targeted therapy. In clinical trials, it was best characterized by the combination of SHP2 inhibitors in MAPK pathway therapeutics (Table 1). Of note, recruitment of SHP2 was the priority to promote transition of inactive RAS-GDP into active RAS-GTP [12], which made it a crucial convergent node to block abnormal activation of the MAPK pathway, ranging from upstream receptor tyrosine kinases to downstream cascade signaling effectors. Mutations in the EGFR gene were the most common feature in patients suffering from relapsed NSCLC [65]. Genetic screening of clinical samples revealed that SHP2 was downstream of EGFR to provide parallel survival input signaling to promote resistance to the third-generation EFGR inhibitors. Preclinical evidences supported the notion that allosteric inhibition of SHP2 could restore sensitivity to EGFR inhibitors in cancers driven by EGFR mutation [43]. A phase I clinical trial of TNO155 in combination with a third-generation EGFR inhibitor, nazartinib, was the first-in-human trial to characterize the safety and tolerability of the combined treatment in EGFRL858R/T790M mutant NSCLC (NCT03114319) [66]. The same medication therapy was later found in drug combination including RMC-4630 and osimertinib (NCT03989115) [67], ERAS-601 and cetuximab (NCT04670679) [68]. Downstream of receptor tyrosine kinases, hyperactivation of the RAS-RAF-MEK-ERK signaling axis was observed in a high percentage of tumors, most frequently resulting from activating mutation of KRAS and BRAF genes [69]. KRAS mutants depended on SHP2 phosphatase for cancer progression [16], of which KRASG12C mutation was most sensitive to SHP2 inhibition compared to KRASG13D and KRASQ61H [70, 71]. A couple of phase I/II clinical trials of TNO155 in combination with KRASG12C inhibitors, MRTX849 (NCT04330664) or JDQ443 (NCT04699188) [72, 73], were successively initiated to evaluate its molecular effects and clinical efficacy toward advanced solid tumors harboring KARSG12C mutation, so was it regarding RNC-4630 and AMG-510 (NCT04185883) [74]. The dependence of class 3 BRAF mutants on SHP2-mediated upstream signal for tumor growth conferred sensitivity to SHP2 inhibition [17]. Dual inhibition of SHP2 and BRAF was approved to put into clinical trial for advanced and metastatic colorectal cancer with BRAFV600E mutation (NCT04294160). However, long-term treatment also induced chemoresistance of cancer cells against small-molecule inhibitors [75, 76]. Then inhibition of downstream effectors, MEK or ERK, has been proposed as a complementary strategy [77]. Inhibition of SHP2 activity with RMC-4630 possessed therapeutic potential to synergize with MEK (NCT03989115) or ERK inhibitors (NCT04916236) for the treatment of relapsed and refractory solid tumors resistant to KRAS or BRAF inhibitors (Table 1) [67, 78]. In summary, targeting SHP2 inhibition in MAPK pathway-driven cancers was expected to be less susceptible to drug resistance in clinical therapy.
| Intervention | NCT | Phase | Treatment protocol | Indication | Status |
|---|---|---|---|---|---|
| SHP2 (encoded by PTPN11) | |||||
| TNO155 | NCT05541159 | Phase I (pharmacokinetics study) | TNO155 | Renal impairment | Not yet recruiting |
| NCT03114319 | Phase I | TNO155 plus nazartinib (EGFR inhibitor) | Advanced EGFR mutant NSCLC, KRASG12-mutant NSCLC, esophageal squamous cell carcinoma, head and neck squamous cell carcinoma, melanoma | Recruiting | |
| NCT04000529 | Phase I | TNO155 plus spartalizumab (anti-PD-1 antibody) | NSCLC, esophageal squamous cell carcinoma, head and neck squamous cell carcinoma, gastrointestinal stromal tumors, colorectal cancer | Recruiting | |
| TNO155 plus ribociclib (CDK4/6 inhibitor) | |||||
| NCT04330664 | Phase I/II | TNO155 plus MRTX849 (KRASG12C inhibitor) | Advanced or metastatic solid tumor | Recruiting | |
| NCT04699188 | Phase I/II | TNO155 plus JDQ443 (KRASG12C inhibitor) | KRASG12C mutant solid tumor, NSCLC, colorectal cancer, pulmonary cancer | Recruiting | |
| TNO155 plus spartalizumab (anti-PD-1 antibody) | |||||
| NCT04294160 | Phase I | TNO155 plus dabrafenib (BRAFV600E inhibitor) | BRAFV600 colorectal cancer | Recruiting | |
| RMC-4630 | NCT03634982 | Phase I | RMC-4630 | Relapsed/refractory solid tumor | Recruiting |
| NCT04916236 | Phase I | RMC-4630 plus LY3214996 (ERK1/2 inhibitor) | Pancreatic cancer, colorectal cancer, NSCLC, metastatic KRAS mutant tumor | Recruiting | |
| NCT05054725 | Phase II | RMC-4630 plus sotorasib (KRASG12C inhibitor) | NSCLC with KRASG12C mutation | Active, not recruiting | |
| NCT03989115 | Phase I/II | RMC-4630 plus cobimetinib (MEK inhibitor) | Relapsed/refractory solid tumor | Recruiting | |
| RMC-4630 plus osimertinib (EGFR inhibitor) | EGFR positive locally advanced or metastatic NSCLC | ||||
| JAB-3068 | NCT03518554 | Phase I | JAB-3068 | NSCLC, head and neck squamous cell carcinoma, esophageal cancer, other metastatic solid tumors | Recruiting |
| NCT03565003 | Phase I/II | JAB-3068 | Recruiting | ||
| JAB-3312 |
NCT04121286 and NCT04045496 |
Phase I | JAB-3312 | NSCLC, colorectal cancer, pancreatic ductal carcinoma, esophageal squamous cell carcinoma, head and neck squamous cell carcinoma, breast cancer | Recruiting |
| NCT05288205 | Phase I/IIa | JAB-3312 plus glecirasib (KRASG12C inhibitor) | Advanced solid tumor with KRASG12C mutation | Recruiting | |
| ERAS-601 | NCT04670679 | Phase I | ERAS-601 plus cetuximab (anti-EGFR antibody) | Advanced or metastatic solid tumor | Recruiting |
| NCT04866134 | Phase Ib/II | ERAS-601 plus ERAS-007 (ERK inhibitor) | Advanced or metastatic solid tumor | Active, not recruiting | |
| RLY-1971 | NCT04252339 | Phase I | RLY-1971 | Advanced or metastatic solid tumor | Recruiting |
| SH3809 | NCT04843033 | Phase I | SH3809 | Advanced solid tumor | Recruiting |
| BBP-398 | NCT04528836 | Phase I | BBP-398 | Solid tumor | Recruiting |
| NCT05621525 | Phase I | BBP-398 |
Advanced solid tumor, Advanced or metastatic NSCLC |
Recruiting | |
| NCT05375084 | Phase I | BBP-398 plus nivolumab (anti-PD-1 antibody) | NSCLC, solid tumor | Recruiting | |
| NCT05480865 | Phase I | BBP-398 plus sotorasib (KRASG12C inhibitor) | Solid tumor, metastatic NSCLC | Recruiting | |
| NCT06032936 | Phase I | BBP-398 plus Osimertinib (EGFR inhibitor) | Advanced or metastatic NSCLC with EGFR mutations | Recruiting | |
| HBI-2376 | NCT05163028 | Phase I | HBI-2376 | NSCLC, colorectal cancer, Pancreatic cancer | Recruiting |
| HS-10381 | NCT05378178 | Phase I | HS-10381 | Advanced solid tumor | Recruiting |
| ET0038 | NCT05354843 | Phase I | ET0038 | Advanced solid tumor | Recruiting |
| BPI-442096 | NCT05369312 | Phase I | BPI-442096 | NSCLC, pancreatic cancer, colorectal cancer | Not yet recruiting |
| PF-07284892 | NCT04800822 | Phase I | PF-07284892 plus lorlatinib (ALK inhibitor) | Solid tumor | Active, not recruiting |
| PF-07284892 plus binimetinib (MEK1/2 inhibitor) | |||||
| PF-07284892 plus encorafenib (BRAFV600E inhibitor) | |||||
| PF-07284892 plus cetuximab (anti-EGFR antibody) | |||||
| BR790 | NCT05715398 | Phase I/IIa | BR790 plus anlotinib (RTKs inhibitor) | NSCLC | Not yet recruiting |
| NCT05505877 | Phase I/IIa | BR790 plus tislelizumab (anti-PD-1 antibody) | Advanced solid tumor | Recruiting | |
| NCT06032936 | Phase I | BR790 plus osimertinib (EGFRT790M inhibitor) | NSCLC | Recruiting | |
| TC-PTP (encoded by PTPN2) | |||||
| ABBV-CLS-579 | NCT04417465 | Phase I | ABBV-CLS-579 | Locally advanced or metastatic tumor | Recruiting |
| PD-1 inhibitor | |||||
| ABBV-CLS-484 | NCT04777994 | Phase I | ABBV-CLS-484 | Relapsed or refractory NSCLC, head and neck squamous cell carcinoma, and advanced clear cell renal cell carcinoma | Recruiting |
| PD-1 inhibitor | Gastric or gastroesophageal junction adenocarcinoma | ||||
- Abbreviations: ALK, anaplastic lymphoma kinase; CDK4/6, cyclin-dependent kinase 4/6; EGFR, epidermal growth factor receptor; ERK1/2, extracellular regulated protein kinase; MEK, mitogen-activated extracellular signal-regulated kinase; NSCLC, non-small cell lung cancer; PD-1, programmed cell death protein 1; RTKs, receptor tyrosine kinases; PTP, protein tyrosine phosphatase.
Up to date, three clinical trials have released their preliminary safety and efficacy profiles in European Society for Medical Oncology Congress 2023 and World Conference on Lung Cancer 2023. A phase I/IIa clinical trial of glecirasib (KRASG12C inhibitor) in combination with JAB-3312 (SHP2 inhibitor) enrolled 144 patients with KRASG12C mutated solid tumors (NCT05288205) [79]. Those patients were treated with glecirasib (400 mg or 800 mg daily) in combination with varied doses of JAB-3312. Dose escalation study concluded with one dose-limiting toxicity (DLT), grade 3 pneumonitis, with a rate of 39.8% across all dose levels. Preliminary evaluation in 58 KRASG12C NSCLC patients exhibited prominent efficacy as evidenced by a disease control rate (DCR) of 100%. Importantly, the objective response rate (ORR) reached 86.7% in patients administrated with 800 mg glecirasib with 2 mg JAB-3312. Compared to the 37.1% ORR in monotherapy of KRASG12C inhibition (NCT03600883) [80], such drug combination regimen manifested promising clinical advantage for NSCLC patients. Subsequent clinical trials would recruit larger patient cohorts to confirm its efficacy [22]. A phase Ib/II clinical trial of JDQ443 (KRASG12C inhibitor) plus TNO155 (SHP2 inhibitor) recruited 50 patients with advanced, KRASG12C-mutated solid tumors (NCT04699188) [81]. Those patients received a daily dose of 200 mg JDQ443 in combination with varied doses of TNO155. This combination regimen was well tolerated with a 36% rate of grade 3 treatment-related adverse events (TRAEs), including neutropenia, anemia, and increased creatine phosphokinase. Preliminary anti-tumor effect was observed in enrolled patients, especially NSCLC. Among those NSCLC patients previously treated with KRASG12C inhibitor (n = 12), the ORR and DCR were 33.3% and 66.7%. In other NSCLC patients received KRASG12C inhibitor for the first time (n = 12), JDQ443 plus TNO155 yielded an ORR of 33.3% and a DCR of 83.3%. Dose-expansion study is enrolling for future safety and efficacy evaluation [23]. In addition, a phase I/Ib study of RMC4630 (SHP2 inhibitor) and LY3214996 (ERK inhibitor) recruited 11 KRASG12C-mutated solid tumors (NCT04916236) [82]. Those patients were divided into 2 cohorts. Cohort 1 was treated with 140 mg RMC-4630 and 100 mg LY3214966. Cohort 2 was treated with 100 mg RMC-4630 and 200 mg LY3214966. Preliminary evaluation identified two instances of DLTs in cohort 1, grade 3 thrombocytopenia and renal insufficiency. Those DLTs limited the tolerability of the combination regimen in cohort 1. By contrast, no DLTs were observed in cohort 2. The ORR in both cohorts was 0%. Patients enrollment is still underway for the evaluation of next dose level [82]. Collectively, phase I/II clinical trials have shown clinical benefit of targeting SHP2 inhibition in KRASG12C-mutated solid tumors. If such efficacy could be further confirmed in larger patient cohorts, it is anticipated that these combination regimens would develop as the first-line treatment for KRASG12C mutant-driven cancers.
4.2 Targeting PTP inhibition in immune-checkpoint therapy
Immune-checkpoint therapy that blocked extracellular ligand-receptor interaction was unable to persistently suppress intracellular events mediated by phosphatase-dependent tonic signaling. It may account for the inadequate efficacy of immune therapy in clinical treatment [49]. Targeting PTP inhibition in immune-checkpoint therapy represents an efficient strategy to unleash the pre-existing antitumor immunity, transforming a cold tumor into an inflamed one to improve therapeutic response [83]. It was best characterized as synchronous inhibition of extracellular PD-1-PD-L1 interaction and intracellular SHP2 dephosphorylation to overcome tumor-intrinsic resistance to immune-checkpoint blockade [84]. A phase I clinical trial of TNO155 in combination with anti-PD-1 antibody, spartalizumab, was expected to provide clinical benefit to patients with advanced malignancies (NCT04000529) [85]. In addition, deficiency of the IFN-γ pathway was an important resistance mechanism of anti-PD-1 immunotherapy [86]. Preclinical research found that cancer cells resistant to PD-1 blockade became cross-sensitive to PTPN2 depletion through enhanced IFN-γ sensing [2]. Inhibition of PTPN2 with small-molecule inhibitors, ABBV-CLS-579/484, was expected to restore the efficacy of immune-checkpoint therapy. A phase I clinical trial of ABBV-CLS-579 in combination with PD-1 inhibitor, was the first-in-human trial to characterize the safety and tolerability of combined treatment in locally advanced or metastatic tumors (NCT04417465) [87]. The same medication therapy was later found in drug combination including ABBV-CLS-484 and anti-PD-1 antibody for the treatment of relapsed or refractory gastric or gastroesophageal junction adenocarcinoma (NCT04777994) [88] (Table 1). Therefore, the identification of immunosuppressive PTPs as immune biomarker was necessary to distinguish responsive and non-responsive patients, which provided better guidance on design of immune therapy and the prediction of disease prognosis.
4.3 The necessary cautionary tale
Despite that emerging evidences supported the conclusion that PTP inhibitors benefited to overcoming drug resistance in cancer therapy, a minority of preclinical studies have found that deletion of PTPs in specific cell types could contribute to accelerated tumor progression. For instance, loss of SHP2 in Kupffer cells and hepatocytes generated a tumor-promoting microenvironment [89]. It resulted from that SHP2 deficiency could trigger apoptosis of Kupffer cells, which in turn induced compensatory recruitment of bone marrow-derived monocytes into the liver. Those monocytes further differentiated into non-Kupffer cells with TAM function, leading to immunosuppressive microenvironment prompt for tumor progression [89]. Another study also found that hepatocyte-specific deletion of SHP2 conferred to hepatic inflammation and necrosis, leading to regenerative hyperplasia and development of hepatocellular carcinoma (HCC) in aged mice [90]. Consistently, decreased SHP2 expression was observed in a subfraction of human HCC specimens [90]. These results raised caution on the application of SHP2 inhibitors in liver cancer therapy. In contrast to the pro-tumorigenic role of PTPN2 in immune inhibition, overexpression of PTPN2 in murine epidermis acted as a tumor suppressor to attenuate skin tumor formation [91]. Likewise, examination of human squamous cell carcinoma (SCC) unveiled that PTPN2 expression was significantly decreased in advanced stage of SCC [91]. Thus, multiple PTPs harbored double-edged role in regulating carcinogenesis. The design of PTP inhibition-based combination regimens should put cancer type in the first place. In addition, preliminary clinical trials of PTP inhibition-based combination therapy have revealed several grade 3 TRAEs, including pneumonitis, neutropenia, anemia, and increased creatine phosphokinase [22, 23]. Patients with certain underlying diseases who barely tolerate such adverse effects cannot receive those drug combination therapy. As yet, there are none reports indicating that PTP inhibition-based combination regimens are toxic in clinical therapy.
5 CONCLUSIONS
As a key regulator in tyrosine dephosphorylation of signal transduction proteins, PTP-mediated pathways represent an attractive mechanism for supporting the development of drug resistance. That is, the perturbation of PTPs evolved as an adaptive mechanism of cancer cells to offset stress following anticancer therapy, leading to compromised efficacy of targeted therapy and immune-checkpoint therapy [2, 11]. Preclinical evidences have substantiated that deletion or inhibition of PTPs was sufficient to block sustained activation of growth-stimulatory signaling or create a less immune-suppressive microenvironment [2, 92]. Thus, targeting PTP inhibition in anticancer combination therapies seemed to be a potential strategy to overcome clinical resistance.
In clinical settings, there are 3 major challenges in developing PTP inhibition-based combination therapy for the treatment of cancer. The first challenge was the lack of highly potent and specific PTP inhibitors. In concern of the highly conserved and electropositive catalytic pocket in the PTP family, the development of orthosteric inhibitors encountered obstacles in optimizing the bioavailability and selectivity [93]. Alternatively, allosteric inhibitors emerged as a novel strategy to induce or stabilize a catalytically incompetent conformation of PTPs. It was best characterized by the development of highly potent and selective inhibitors of SHP2, such as TNO-155, RMC-4630, and JAB-3312 [94]. Furthermore, blockade of protein-protein interaction between PTPs and upstream molecules was a prominent drug design strategy to circumvent the inherent disadvantage of PTP catalytic pocket. This approach was competent to attenuate the recruitment and activation of PTPs, harboring excellent efficacy similar to that of multidrug combinations. A preclinical research has repurposed the methylene blue, a U.S. Food and Drug Administration-approved chemical for treating methemoglobinemia, as a protein-protein interaction inhibitor to block the interaction between PD-1 and SHP2 [95]. Followed by development of potent PTP inhibitors, subsequent challenge was how to discover the best drug combinations. In addition to traditional unbiased chemical screens, genome-wide small interfering RNA and CRISPR-Cas9-mediated synthetic lethal drug-sensitization screens benefited to uncovering novel therapeutic combinations targeting PTP inhibition under particular genetic events [96-98]. Nevertheless, genetic screening was incompetent to guide combination therapies targeted at epigenetic alternations of PTPs that rewired signal transduction as a mechanism of resistance. System biology appeared to be an alternative approach to make up for those disadvantages. It was a systemic and network-based biology method integrating multi-omics data including genetic, transcriptomic, and proteomic information into mathematical modeling for predicting the efficacy of potential drug combinations [99, 100]. Once a rationally designed combination was selected, the next challenge was how to implement a drug combination in clinical trials. Thereinto, dose-finding study was a necessary step in the early phase of the clinical setting. It required multiple dose de-escalation cycles to optimize the toxicity-efficacy balance of drug combinations. In cases of narrow therapeutic index of each drug, phase I stage often initiated with dose-finding study to achieve maximal therapeutic effect of combination therapy in clinical trials, while sparing less toxicity [101]. In other cases that full doses of anticancer drugs caused tolerable adverse events, multiple cycles of dose escalation or de-escalation studies were required to find the best stoichiometry ratio between PTP inhibitors and anticancer drugs [3]. Likewise, glecirasib was identified as a highly selective covalent inhibitor of KRASG12C. As reported in the 2022 American Society of Clinical Oncology meeting, glecirasib exhibited a well-tolerated safety profile with the recommended phase II dose as 800 mg quaque die (QD) (NCT05009329) [102]. Then, phase I/II clinical trial of glecirasib in combination with JAB-3312 (SHP2 inhibitor) adopted the dose-escalation study to identify the optimal combination dosages (NCT05288205) [22]. Afterward, upon recruiting patients in later clinical trial, defining the disease setting was critical to evaluate the efficacy of PTP inhibition-based drug combination on clinical resistance. In the case that innate resistance to cobimetinib (MEK inhibitor) might be reversed by combination with RMC-4630 (SHP2 inhibitor), the efficacy of combination therapy was best conducted when MEK inhibitor was firstly administrated, instead of in a setting when resistance has already occurred (NCT03989115) [18]. In another case which adaptive resistance to dabrafenib (BRAFV600E inhibitor) might be overcome by deletion of DUSP4 and DUSP6, intermittent cyclical drug treatment was performed when a part of tumor tissue remained sensitive to dabrafenib, and DUSP4/6 inhibition was used to target the residual resistant tissue [31]. These cautions highlighted the importance of identifying biomarkers to monitor the clinical process to initiate a combination before the resistance occurred. In-depth research dedicated to overcoming these challenges would accelerate the application of PTP inhibition in combating drug resistance, leading to long-term remission in cancer therapy.
In conclusion, the identification of PTP-mediated pathways in resistance to cancer therapy indicated the future trends of PTP inhibition as the frontline adjuvant therapy. Targeting PTP inhibition in anticancer combination regimens to circumvent drug resistance has achieved certain success in preclinical studies [44, 71]. Currently undergoing clinical trials would further substantiate the efficacy of PTP inhibition-based combination therapies, providing a prominent strategy in our arsenal against drug resistance.
DECLARATIONS
AUTHOR CONTRIBUTIONS
Min Zhao, Wen Shuai, and Zehao Su drafted the manuscript under the guidance of Qiu Sun and Guan Wang. Ping Xu and Aoxue Wang revised this manuscript. All authors approved the final version for submission.
ACKNOWLEDGEMENTS
This manuscript acknowledges financial support from the National Natural Science Foundation of China (82273770 and 22177083), Natural Science Foundation of Sichuan Province (2022NSFSC1290), 135 Project for Disciplines of Excellence–Clinical Research Incubation Project, West China Hospital, Sichuan University (ZYJC21016), and West China Nursing Discipline Development Special Fund Project, Sichuan University (Grant HXHL21011).
CONFLICT OF INTEREST STATEMENT
All authors declare no conflict of interest.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




