Combination of photodynamic therapy and stimulator of interferon genes (STING) agonist inhibits colorectal tumor growth and recurrence
Abbreviations
-
- BMDMs
-
- bone marrow-derived macrophages
-
- cGAS
-
- cyclic GMP-AMP synthase
-
- CRC
-
- colorectal cancer
-
- DCs
-
- dendritic cells
-
- H&E
-
- hematoxylin and eosin
-
- IFN
-
- interferon
-
- IgG
-
- immunoglobulin G
-
- IL
-
- interleukin
-
- MHC
-
- major histocompatibility complex
-
- NIR
-
- near-Infrared
-
- PDT
-
- photodynamic therapy
-
- ROS
-
- reactive oxygen species
-
- STING
-
- stimulator of interferon genes
-
- dLNs
-
- tumor-draining lymph nodes
-
- TME
-
- tumor microenvironment
-
- VP
-
- Verteporfin
Great progress has been made in the clinical use of photodynamic therapy (PDT) for the treatment of patients with superficial tumors [1]. However, cancer recurrence and metastasis have limited the application of PDT in the treatment of solid tumors and advanced cancers. In this context, combining PDT with other complementary immunotherapy regimens may overcome these limitations of PDT [2]. Therefore, we aimed to elucidate the inhibitory efficiency of PDT in combination with an agonist stimulator of interferon genes (STING) in colorectal cancer (CRC) models and to explore the underlying regulatory effects on the host immune system. STING agonists play an important role in the induction of innate anti-tumor immunity on tumor growth and recurrence potential. All methods used in this work are described in the Supplementary Material.
First, we showed that the photosensitizer verteporfin® was internalized into CRC MC38 and CT26 cells 3 h after incubation and effectively induced cell death by generating reactive oxygen species (ROS) after irradiation (Figure 1A-B and Supplementary Figure S1). PDT-induced dying tumor cells could be phagocytosed by dendritic cells (DCs) (Figure 1C), which in turn stimulated the phenotypic maturation of DCs [3]. Furthermore, ADU-S100 (synthetic cyclic dinucleotides that activate the cyclic GMP-AMP synthase [cGAS]-STING pathway) amplified this immunological effect of PDT-treated cells on DC activation, as shown by the increased expression of CD86 and major histocompatibility complex (MHC)-II (Figure 1D and Supplementary Figure S2). We explored the effect of PDT-treated cells and ADU-S100 on the polarization state of macrophages. Although PDT-treated cells did not alter the polarization state of bone marrow-derived macrophages (BMDMs) in vitro, treatment with ADU-S100 could re-polarize anti-inflammatory M2-type BMDMs [4] toward the pro-inflammatory M1 phenotype; shown by the increased mRNA expression of M1 markers, surface polarization protein level and production of inflammatory mediators, as well as the decreased expression of key M2 markers (Supplementary Figure S3).
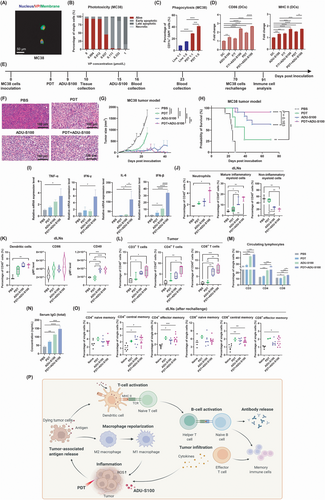
The accumulation of photosensitizers at tumor sites is a prerequisite for PDT. We confirmed that verteporfin was efficiently accumulated in tumors but was also found in metabolic organs (Supplementary Figure S4). Importantly, since the near-infrared (NIR)-laser is a local treatment, no side effect is expected in other organs. Next, we investigated the anti-tumor effects of PDT in combination with ADU-S100 via immunocompetent syngeneic CRC mouse models (Figure 1E). The results showed that the combination of PDT with ADU-S100 exerted a highly suppressive effect on tumor growth and cell proliferation compared to the mild and moderate effects of PDT and ADU-S100 monotherapy, respectively (Figure 1F-G and Supplementary Figure S5A-C). Kaplan-Meier survival curve analysis revealed that the combination of PDT with ADU-S100 almost completely eradicated the MC38 tumors of tumor-bearing mice (survival probability, 91%) compared with PDT (survival probability, 22%) or ADU-S100 (survival probability, 64%) monotherapy (Figure 1H). The same trend was confirmed in the CT26 model (Supplementary Figure S5D-G).
PDT and ADU-S100 have been reported to exert anti-tumor effects by converting the tumor microenvironment (TME) and the tumor-draining lymph nodes (dLNs) from an immunosuppressive to a pro-inflammatory state [2, 5]. As shown in Figure 1I, 3 h after the first ADU-S100 treatment, tumors treated with combinational therapy showed a higher expression of genes encoding pro-inflammatory cytokines [9] than tumors subjected to monotherapies. These cytokines play a key role during initial inflammation and the transition to T-cell-mediated immune responses.
Next, we analyzed the immune cell populations in the tumor, spleen and dLNs ten days after tumor inoculation. The results showed that either treatment alone or PDT and ADU-S100 combined induced the formation of a pro-inflammatory dLNs state, but the combinational therapy triggered the most significant changes, including infiltration of dLNs by neutrophils, increasing levels of mature inflammatory myeloid cells and decreasing proportions of non-inflammatory myeloid cells (Figure 1J). Rapid recruitment and an increase in neutrophil numbers are manifestations of acute inflammation and can initiate anti-tumor adaptive immune responses under inflammatory conditions [6]. Moreover, combinational therapy increased DC numbers and induced relatively enhanced DCs activation in dLNs compared with control or PDT alone (Figure 1K). The tumors treated with the combination exhibited significantly more infiltration of cytotoxic T lymphocytes (CTLs, CD8+ T cells) than helper (CD4+) T cells into the TME compared to tumors treated with monotherapies or control (Figure 1L). Of note, changes in the proportions of CD4+ lymphocytes and CTLs in the spleen and dLNs were not significantly different (Supplementary Figure S6). These data proved that the combinational therapy amplified the effects of monotherapies on the immune-enhancing transformation of the TME and dLNs.
Local treatments have the potential to trigger systemic immune responses and, in some cases, the potential to exert an abscopal effect [7]. Hence, we examined the levels of circulating lymphocytes and total serum immunoglobulin G (IgG) in mice that received different treatments. On day 16, few changes and moderate increases in circulating CD3+ and CD4+ lymphocytes and CTLs were observed in PDT-treated mice and ADU-S100-treated mice. Importantly, the combination treatment significantly increased the levels of CD3+ and CD4+ T cells in the blood compared to PDT, and the increase in CTLs in the combined treatment group was higher compared to the monotherapy groups (Figure 1M and Supplementary Figure S7A-B). Of note, elevated expression of costimulatory CD40 on CD19+ B cells, as well as increased IgG levels in blood serum, were observed on day 23 after combinational therapy (Figure 1N and Supplementary Figure S7C-D). This was probably mediated by the activation of CD4+ and CD8+ cells. These activated cells produce interferon (IFN)-γ, which in turn can activate CD19+ B cells to undergo isotype switching to produce more IgG. These data collectively suggest that the PDT and ADU-S100 combined treatment could establish systemic immune responses in vivo, including an enhanced proliferation of lymphocytes and antibody production in peripheral blood, which is essential for complete tumor eradication [8, 9].
Next, we examined the potential of established peripherally initiated systemic immunity to produce long-term immune memory and regulate distant tumors. Indeed, the mice that were cured by monotherapy and combined treatment all resisted tumor growth after rechallenging; this was in contrast to naïve controls that exhibited rapid tumor growth. Through immune memory phenotype analysis of the blood, dLNs and spleen of these mice, a trend of increased infiltration of functional memory CD4+ and CD8+ T cells was observed in ADU-S100 and combinational therapy treatment groups compared to control (Figure 1O and Supplementary Figure S8). Importantly, the mice cured by combinational therapy exhibited the most significant changes, especially in the dLNs (Figure 1N). This finding suggests that combined treatment resulted in the most prominent development of systemic memory immunity after tumor cell rechallenge following tumor eradication.
Moreover, both primary and distant tumor growth were inhibited or ablated by PDT, ADU-S100 or the combination treatment in the MC38 bilateral tumor model (Supplementary Figure S9). Although the combination treatment did not exert a higher tumor control effect on the distant untreated tumors than ADU-S100 treatment alone [10], it increased the survival rate of the mice bearing two MC38 tumors to 40% compared to that in the PDT (0%) and ADU-S100 (20%) groups (Supplementary Figure S9C). A similar effect of delaying untreated tumor growth was also observed in the CT26 bilateral model (Supplementary Figure S10). Together with previous results, the rationale underlying our current working model is shown in Figure 1P.
In conclusion, we provided first-hand evidence that combinational PDT and STING agonist treatment has an amplified inhibitory regulation on tumor growth and recurrence by the induction of anti-tumor immunity in established CRC models. Further studies are needed to identify the function of important factors in the TME, such as other immune cells and endothelial cells, during treatment.
DECLARATIONS
AUTHOR CONTRIBUTIONS
Original data collection and analysis, or interpretation - Yang Hao, Zili Gu, Sen Ma; writing - original draft preparation - Yang Hao; writing - review and editing - Zili Gu, Timo Schomann, Yuanyuan He, Xiaoxu Dong, Alireza Haghparast; figure - Yang Hao, Zhenfeng Yu; supervision - Peter ten Dijke and Luis J Cruz; Final additions - Peter ten Dijke and Luis J Cruz. All authors contributed to the article and approved the submitted version.
ACKNOWLEDGMENTS
We are grateful to Ferry Ossendorp (Department of Immunology, Leiden University Medical Center) for valuable discussion, Marcel Camps (Department of Immunology, Leiden University Medical Center) and the Ear-Nose-Throat (ENT) department for technical assistance and appreciate the support of all group members.
COMPETING INTERESTS
The authors declare that they have no competing interests. The authors declare that they have no conflict of interest.
FUNDING
Yang Hao received finacial support from the China Scholarship and Jilin Province Chinese Postdoctoral International Exchange Program (YJ20220406). Zili Gu, Zhenfeng Yu and Sen Ma received financial support from the China Scholarship Council, and Peter ten Dijke received funding from Cancer Genomics Centre Netherlands. Timo Schomann and Alireza Haghparast received funding from the European Commission, grants H2020-MSCA-RISE CANCER (777682) and H2020-WIDESPREAD-05-2017-Twinning SIMICA (852985).
AVAILABILITY OF DATA AND MATERIALS
The data presented in this study are available on request from the corresponding authors.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was performed in line with the principles of the Dutch Animal Ethical Commission with the license of project AVD116008045 and approved by the Animal Experimental Committee from the Leiden University Medical Center (LUMC).
CONSENT FOR PUBLICATION
Not applicable.




