Weakened humoral immune responses of inactivated SARS-CoV-2 vaccines in patients with solid tumors
Zhiwei Chen, Peng Zhu, Zuojin Liu, Bin Zhu, Guobing Yin, and Jia Ming contributed equally to this work.
Abbreviations
-
- AEs
-
- adverse events
-
- anti-RBD-IgG
-
- spike receptor-binding domain IgG antibody
-
- atyMBCs
-
- atypical memory B cells
-
- COVID-19
-
- coronavirus disease 2019
-
- IQR
-
- interquartile range
-
- MBCs
-
- memory B cells
-
- NAbs
-
- neutralizing antibodies
-
- RBD
-
- receptor binding domain
-
- SARS-CoV-2
-
- severe acute respiratory syndrome coronavirus 2
Dear Editor
Coronavirus disease 19 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), remains a pandemic. Cancer patients have a higher risk of poor outcomes for SARS-CoV-2 infection than the general population [1]. Vaccines were shown to effectively prevent SARS-CoV-2 infection, severe disease progression, and mortality. Inactivated SARS-CoV-2 vaccines (BBIBP-CorV and CoronaVac) have been approved and widely used in China, with the former shown to be more effective than the latter [2]. Hence, there is an urgent need to investigate the safety and humoral immune responses of inactivated vaccines in cancer patients.
Here, we performed a prospective observational study to evaluate receptor-binding domain IgG antibody (anti-RBD-IgG), neutralizing antibodies (NAbs) and memory B cells (MBCs) responses, and monitor adverse events (AEs) in patients with solid tumors and healthy controls from 21-105 days after full-course of inactivated SARS-CoV-2 vaccines. The detailed methods are described in the Supplementary File.
From July to December 2021, 419 participants were enrolled in the study. Among them, 270 had solid tumors and 149 were healthy controls. The median age of the cancer patients was 49 years (interquartile range (IQR): 43-59), of whom 57.4% were females (Supplementary Table S1). The median time after 2nd-dose vaccination was 59.0 days (IQR: 36.5-83.0). Furthermore, 38.9% (105/270) of patients underwent anti-cancer therapy 3 months before the first vaccination dose. Of the 105 patients with ongoing treatment, 23 patients were treated with chemotherapy, 10 received immunotherapy, and 72 received other therapies.
Safety profile evaluation of inactivated SARS-CoV-2 vaccines in cancer patients showed that the overall AEs for 7 days were similar between cancer patients and healthy controls (13.3% vs.11.4%; Supplementary Table S2). The common local AEs (>3%) in cancer patients were pain at the injection site (9.3%), and the common systemic AEs were muscle pain (4.1%) and fatigue (4.1%). The most common AEs (>3%) in healthy controls was local pain at the injection site (6.0%). Most AEs were mild between the two groups, and no serious AEs were observed (Supplementary Table S2). Hence, inactivated SARS-CoV-2 vaccines were relatively safe for cancer patients.
We also investigated the antibody responses in cancer patients and healthy controls. The titers (2.12 IU/mL [IQR: 0.68–5.40] vs. 3.76 IU/mL [IQR: 1.36-8.48], P < 0.001) of anti-RBD-IgG were significantly lower in cancer patients than that in healthy controls (Figure 1A). Additionally, titers of NAbs were also lower in cancer patients (P < 0.001) than that in healthy controls; which was similar with that in previous studies of other COVID-19 vaccines [3-5]. However, Thomas et al. reported that the BNT162b2 vaccine had similar efficacy between the two groups [6]. The vaccine type, racial category, and cancer type may have contributed to this discrepancy. Similar results for antibody responses were observed in the age and sex subgroups analyses (Supplementary Figures S1 and S2). Younger (age <60 years) patients seemed to have higher anti-RBD-IgG and NAbs titers. Further analysis was performed on different vaccine types, and a similar trend was observed in BBIBP-CorV, CoronaVac, and mixed group vaccines (Supplementary Figure S3).
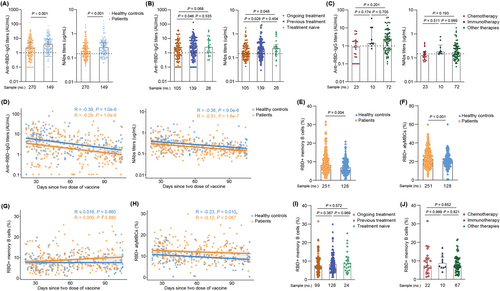
Considering that anti-cancer treatment may compromise antibody responses, we further analyzed antibody response in several treatment subgroups. Anti-RBD-IgG titers in the ongoing treatment group were significantly lower than those in the previous treatment group (1.64 IU/mL [IQR: 0.56–4.48] vs. 2.34 IU/mL [IQR: 0.80-6.82], P = 0.046). Similar results were observed for the titers of the NAbs (Figure 1B). Next, we stratified the ongoing treatment group into three subgroups. The chemotherapy subgroup had the poorest trend of anti-RBD-IgG response than the immunotherapy subgroup (0.87 IU/mL [IQR: 0.24–1.80] vs. 1.40 IU/mL [IQR: 1.26-8.43], P = 0.174); however, this trend was not statistically significant (Figure 1C). The NAbs response showed the same trend as the anti-RBD-IgG response.
To better assess the durability of circulating antibody titers, a single-term linear model was conducted based on data obtained from patients who underwent a full-course vaccination. Although the anti-RBD-IgG and NAbs titers were higher in healthy controls than in cancer patients, the antibody titers showed a continuous decrease in both groups (Figure 1D), which was concordant with a previous study [7]. Altogether, antibody responses to inactivated vaccines were inferior in cancer patients, especially in those undergoing chemotherapy.
We also evaluated RBD-specific MBCs responses in cancer patients. Surprisingly, the frequencies (percentage of total B cells) of RBD+ MBCs were higher in cancer patients than that in healthy controls (7.9% vs. 6.8%, P = 0.004) (Figure 1E). Further analysis of the four subsets of the RBD+ MBCs responses showed that the percentage of resting MBCs (rMBCs) and atypical MBCs (atyMBCs) was significantly reduced and increased (P < 0.001; Supplementary Figure S4B and Figure 1F) in cancer patients, respectively; which was also observed in patients with common variable immunodeficiency [8]. rMBCs respond to antigen rechallenge by proliferating and differentiating into antibody-producing B-cells. AtyMBCs are short-lived activated cells, with a low binding capacity to Spike protein [9]. Hence, although the overall frequency of RBD+ MBCs was higher in cancer patients, immune memory was impaired. Analysis of the durability of circulating MBCs from 21-105 days of post-full-course vaccination showed that the frequency of RBD+ MBCs was similar between the two groups at the early time point (21-40 days), which then increased over time in cancer patients (Figure 1G); this finding was similar to a previous study [7]. The percentages of all four subsets of MBCs in both groups were relatively stable during the interval time (Figure 1H and Supplementary Figure S4D-F).
In addition, the overall frequency of RBD+ MBCs and four subsets were similar among the ongoing treatment, previous treatment and treatment-naive groups. (Figure 1I and Supplementary Figure S5A-D). Supplementary Figure S5E shows the frequency of rMBCs and Supplementary Figure S5E shows the frequency of intMBCs in ongoing treated cancer patients. Further subgroup analysis of patients undergoing treatment indicated that the chemotherapy subgroup showed the lowest trend of RBD+ MBCs compared to that of the immunotherapy subgroup, but this difference was not statistically significant (Figure 1J). Notably, although there was no statistical significance, the percentage of active MBCs was lower in patients undergoing chemotherapy but higher in patients receiving immunotherapy (Supplementary Figure S5G), which indicated that the immune reactivation post-inactivated SARS-CoV-2 vaccination may be impaired in patients undergoing chemotherapy. Altogether, memory B cells responses to inactivated vaccines were impaired in cancer patients, particularly in those receiving chemotherapy. The gating strategy and representative results are shown in Supplementary Figure S6.
This study had several limitations. First, the dynamic changes in antibody titers in each individual were lacking in this cross-sectional analysis, which could have been affected by different factors (i.e., scheduled chemotherapy administration and planned surgery). Second, an assessment of the third “booster” dose and immunity against variants of concerns was not performed, which hindered further analysis, such as the immunity against Delta or Omicrons variants after inactivated vaccination. Third, only patients with solid tumors were included in our study, while patients with hematologic malignancies were excluded, which might have impacted the results, given the higher immunosuppressive status of the latter [10].
In summary, our data revealed that inactivated SARS-CoV-2 vaccines were well-tolerated. However, antibody responses and durable humoral immune responses were weakened, particularly in patients who received chemotherapy, indicating that cancer patients should be given priority for vaccination with SARS-CoV-2 vaccines and booster doses.
DECLARATIONS
AUTHOR CONTRIBUTIONS
Concept and design: HR, DCC, LNH. Funding acquisition: HR, MLP, MC. Participant recruitment: ZWC, PZ, ZJL, BZ, GBY, JM, RS, QBP, TL, XWJ, BW, SBL, HXC, JJW, YLH, ZJL, YH. Experiment execution: RS, QBP, MC, MLP. Acquisition, analysis or interpretation of data: ZWC, PZ, ZJL, BZ, GBY, JM, RS, QBP, LNH, DCC, HR. Drafting and critical revision of the manuscript: ZWC, LNH, DCC, HR. All authors contributed to the article and approved the submitted version.
ACKNOWLEDGMENT
We thank the Health Care Center and department of clinical laboratory of the Second Affiliated Hospital, Chongqing Medical University for their support. This work is supported by the National Science and Technology Major Project of China (2017ZX10202203-007, 2018ZX10302206-003). We also acknowledge the support of the National Natural Science Foundation of China (81772198), Natural Science Foundation of Chongqing, China (cstc2020jcyj-msxmX0389).
COMPETING INTERESTS
The authors declare that they have no competing interests.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Ethics Committee of the Second Affiliated Hospital of Chongqing Medical University and conformed with the ethical guidelines of the Declaration of Helsinki (Ratification No. 133/2022). Written informed consent was obtained from all the participants. This study has been registered at ClinicalTrials.gov (NCT05043246).
CONSENT FOR PUBLICATION
Not applicable.




