Genomic profiling and the impact of MUC19 mutation in hepatoid adenocarcinoma of the stomach
Mengxuan Zhu, Erbao Chen, Shan Yu and Chen Xu contributed equally to this work
Abbreviations
-
- HAC
-
- hepatoid adenocarcinoma
-
- AFP
-
- α-fetoprotein
-
- HAS
-
- hepatoid adenocarcinoma of the stomach
-
- WES
-
- whole-exome sequencing
-
- AFPGC
-
- AFP-producing gastric cancer
-
- CNV
-
- copy number variation
-
- MUC19
-
- mucin 19
-
- KEGG
-
- Kyoto encyclopedia of genes and genomes
-
- TCGA
-
- the cancer genome atlas
-
- STAD
-
- stomach adenocarcinoma
-
- CRC
-
- colorectal cancer
-
- HCC
-
- hepatocellular carcinoma
-
- TMB
-
- tumor mutation burden
-
- NAB
-
- neoantigen burden
-
- OS
-
- overall survival
-
- GC
-
- gastric cancer
-
- DFS
-
- disease-free survival
-
- SEM
-
- standard error of mean
Hepatoid adenocarcinoma (HAC) is a rare pathological subtype of extrahepatic tumor, featured by hepatoid differentiation and α-fetoprotein (AFP)-production [1, 2]. Hepatoid adenocarcinoma of the stomach (HAS), accounting for 0.3% to 1.0% of all gastric cancers (GCs), has attracted increasing attention due to its high degree of malignancy [3]. Compared with classic GC, HAS showed a higher rate of vascular invasion, lymph node metastasis, and liver metastasis, with only 9.0% survival rate at 5 years [4]. Currently, there is no effective treatment for HAS, and little is known about its pathogenesis. Herein, we investigated the molecular features of HAS and identified potential therapeutic targets for HAS.
In this study, we conducted whole-exome sequencing (WES) on 40 paired tumor and normal samples, including 25 HAS (defined as the presence of histologically contained hepatoid differentiation areas), 6 HAC of other organs, and 9 AFP-producing gastric cancer (defined as the serum AFP level >20 ng/mL, without hepatoid differentiation areas in tumor tissues). All patients underwent radical surgery at Shanghai Zhongshan Hospital (Shanghai, China) between July 2013 and September 2017. Their clinicopathological characteristics are presented in Supplementary Table S1. The materials and methods are available in the Supplementary Material.
The gene mutation and copy number variation (CNV) landscapes of the 40 enrolled patients were characterized by gender, age, AFP level, and stage (Figure 1A). In HAS patients, the top 5 most frequently mutated genes were TP53 (44.0%), TTN (44.0%), MUC19 (40.0%), CCNE1 (28.0%) and CDC27 (28.0%) (Supplementary Table S2). KEGG pathway analysis of genetic alterations showed multiple molecular pathways, such as the Wnt signaling pathway, were remarkably enriched in HAS compared with normal gastric tissues (Supplementary Figure S1A). Further, compared with TCGA datasets, MUC19, CCNE1, CDC27, GOLGA6L2, RGS3, VSTM2B, CTBP2, PLEKHF1, and SLC25A5 mutations were almost undetectable in STAD, CRC and HCC (Supplementary Figure S1B-D, Supplementary Table S2), while some genes displayed higher frequency mutation in STAD than HAS (Supplementary Table S3).
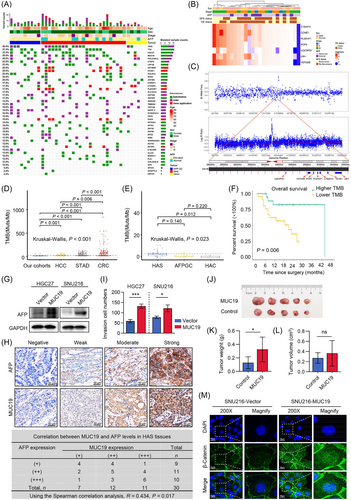
For CNV analysis, C19orf12, CCNE1, PLEKHF1, POP4, UQCRFS1, URI1 and VSTM2B were the most frequently amplificated genes in HAS tumor tissues (Figure 1B). Interestingly, we noticed several HAS samples had increased CNVs at the chr19q12 locus and presented a representative case in Figure 1C. Six patients with the amplification of 4 genes (VSTM2B, CCNE1, PLEKHF1, POP4), located at chr19q12, were identified and survival analysis of all patients revealed that these six patients had worse overall survival (OS) than those without these CNVs (Supplementary Figure S2A, P = 0.068). Besides, in 25 patients with HAS, five patients with 4 co-mutated amplication genes (VSTM2B, CCNE1, PLEKHF1, POP4) had a significantly worse OS than those without these CNVs (Supplementary Figure S2B, 11.1 months vs. 19.3 months, P = 0.045). Similarly, Lu et al. [5] reported AFPGC patients with amplification of CCNE1 at 19q12 has worse prognosis.
Tumor mutation burden (TMB) and neoantigen burden (NAB) have been reported as immune-related signatures which could predict response to immunotherapy [6-8]. In our cohort, the TMB of tumors was significantly and positively correlated with NAB (Supplementary Figure S3A). Compared with the TCGA datasets, the TMB of our cohort was significantly lower than patients with HCC, STAD, or CRC (Figure 1D). TMB of HAS was higher than HAC of other organs (Figure 1E, 4 Muts/Mb vs. 1.2 Muts/Mb, respectively, P = 0.012), while neither TMB nor NAB of HAS was significantly different from those of AFPGC (Supplementary Figure S3B). Survival analysis revealed that HAS patients with low TMB or NAB levels had a poor OS (Figure 1F, Supplementary Figure S3C). Additionally, we found patients with HAC of other organs had a significantly worse OS and disease-free survival (DFS) than HAS patients (Supplementary Figure S3D, E). Considering gallbladder cancer is much more malignant than gastric cancer, we further analyzed the survival differences between patients with hepatoid adenocarcinoma of the gallbladder and HAS. The results indicated that patients with hepatoid adenocarcinoma of the gallbladder had a worse prognosis than HAS patients (Supplementary Figure S3F, P = 0.011).
We also found that HAS and AFPGC patients had similar serums AFP levels (Supplementary Figure S4A). Survival analysis of HAS and AFPGC patients showed patients with lower AFP (≤ 20 ng/mL) had a significantly better OS (Supplementary Figure S4B, P = 0.044). Moreover, both TMB and NAB tended to be negatively correlated with AFP, but the P values were not significant (Supplementary Figure S4C, D). Meanwhile, the TMB and NAB differences were not statistically significant between patients with low and high AFP values (Supplementary Figure S4E, F).
Given that the mucin (MUC) family genes are considered biomarkers and promising therapeutic targets in several cancers [9], we narrowed our focus to these genes. Within the members of MUC family detected in our HAS cohort, MUC19 stood out because of the highest mutation frequency (Supplementary Figure S5A-B). As shown in Supplementary Figure S5C-D, ten MUC19 mutation sites were found in HAS patients and six mutation sites were successfully validated by Sanger sequencing.
Considering the MUC family genes have been reported to promote cancer development, we stably overexpressed MUC19 in HGC27 and SNU216 cells using the dCAS9-SAM system to explore MUC19 functions (Supplementary Figure S6A-C). Then, the relationship between MUC19 and AFP was detected. As shown in Figure 1G, MUC19 overexpression upregulated AFP levels in GC cells. The MUC19 and AFP levels were also examined by immunohistochemistry staining (IHC) in another 20 HAS tissues, which were collected as the validation cohort. Spearman correlation analysis further confirmed the positive relationship between MUC19 and AFP expression (Figure 1H). Next, cell proliferation and invasion capabilities were assessed [10]. Overexpression of MUC19 slightly increased cell proliferation in HGC27 cells, while it had no significant effect in SNU216 cells (Supplementary Figure S6D, E). Moreover, the elevated MUC19 significantly promoted cell invasion in both HGC27 and SNU216 cells (Supplementary Figure S6F, Figure 1I). Futhermore, we established xenograft models by subcutaneously injecting HGC27 cells into nude mice. Consistent with previous results, overexpression of MUC19 facilited tumor growth in vivo (Figure 1J-L).
To gain mechanistic insight into how MUC19 promotes HAS development, we detected Wnt target genes and β-catenin expression in GC cells based on previous KEGG analysis results. The data indicated that MUC19 increased nuclear localization of β-catenin and activated the Wnt/β-catenin pathway (Supplementary Figure S7A-C, Figure 1M). The β-catenin staining in HAS samples showed similar results (Supplementary Figure S7D). Furthermore, Wnt antagonist XAV939 treatment partially reversed the MUC19-induced cell invasion, whereas AFP levels were not influenced (Supplementary Figure S7E-F). Therefore, we concluded that MUC19 contributed to the aggressive malignancy phenotypes of GC cells by activating the Wnt/β-catenin signaling pathway. Considering previous studies have reported that AFP activated the Wnt/β-catenin pathway, we speculated MUC19 might upregulate AFP expression, and thus activating the Wnt/β-catenin signaling. This estimation will be further investigated in our future work.
To summarize, we depicted the genomic mutation and CNV profiles of HAS patients. The TMB, NAB and AFP levels were identified as significant prognostic factors for OS of HAS patients. Most importantly, our study revealed that MUC19 upregulated AFP expression in GC cells and played a critical role in HAS development; thus, it could be a novel diagnostic marker and therapeutic target for HAS.
DECLARATIONS
ACKNOWLEDGMENTS
This study was supported by the Natural Science Foundation of Shanghai (No. 19ZR1409500) and National Natural Science Foundation of China (No. 82172925).
CONFLICT OF INTEREST
No conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
Conceptualization and design: TSL and QL; Data analysis and interpretation: MXZ, EBC, CX and SY; Writing the original draft: MXZ and EBC; Collecting samples: YYY, XC, WL, PFZ, YW and YHC; Performing WES: BFL, SRZ, YTQ, LJH, WWS. All the authors read, reviewed, and approved the final manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the ethics committee of Zhongshan Hospital (No. B2020-094R) and obtained patients’ consent to participate.
CONSENT FOR PUBLICATION
All authors have consent for publication.
Open Research
DATA AVAILABILITY STATEMENT
The data will be available from the corresponding author upon reasonable request.




