Epi-immunotherapy for cancers: rationales of epi-drugs in combination with immunotherapy and advances in clinical trials
Yang Xu, Ping Li and Yang Liu contributed equally.
Abstract
Over the last two decades, several epi-drugs, immune checkpoint inhibitors (ICIs) and adoptive cell therapies have received clinical approval for use in certain types of cancer. However, monotherapy with epi-drugs or ICIs has shown limited efficacy in most cancer patients. Epigenetic agents have been shown to regulate the crosstalk between the tumor and host immunity to alleviate immune evasion, suggesting that epi-drugs can potentially synergize with immunotherapy. In this review, we discuss recent insights into the rationales of incorporating epigenetic therapy into immunotherapy, called epi-immunotherapy, and focus on an update of current clinical trials in both hematological and solid malignancies. Furthermore, we outline the future challenges and strategies in the field of cancer epi-immunotherapy.
Abbreviations
-
- AE
-
- adverse event
-
- AITL
-
- angioimmunoblastic T-cell lymphoma
-
- ALL
-
- acute lymphoblastic leukemia
-
- AML
-
- acute myeloid leukemia
-
- AZA
-
- azacytidine
-
- BC
-
- breast cancer
-
- BCOR
-
- BCL6 corepressor
-
- BET
-
- bromodomain and extraterminal domain
-
- CAR-T
-
- chimeric antigen receptor T
-
- CDA
-
- cytidine deaminase
-
- cHL
-
- classic Hodgkin lymphoma
-
- CIK
-
- cytokine induced killer
-
- CLL1
-
- C-type lectin-like molecule-1
-
- CR
-
- complete response
-
- CRC
-
- colorectal cancer
-
- CRi
-
- CR with insufficient recovery
-
- CSC
-
- cancer stem cell
-
- CTAs
-
- cancer testis antigens
-
- CTCL
-
- cutaneous T-cell lymphoma
-
- CTL
-
- cytotoxic T lymphocyte
-
- CTLA4
-
- cytotoxic T-lymphocyte-associated protein 4
-
- CXCL9
-
- CXC-chemokine ligand 9
-
- DAC
-
- decitabine
-
- DLBCL
-
- diffuse large B cell lymphoma
-
- DLI
-
- donor lymphocyte infusion
-
- DMNTi
-
- DNA mythelytransferase inhibitor
-
- DNMT3A
-
- DNA methyltransferase 3A
-
- ENKTL
-
- extranodal NK/T cell lymphoma
-
- ENT
-
- entinostat
-
- epi-drugs
-
- epigenetic drugs
-
- ER
-
- estrogen receptor
-
- ERVs
-
- endogenous retroviruses
-
- EZH2
-
- enhancer of zeste 2
-
- FL
-
- follicular lymphoma
-
- FLT3
-
- fms-like tyrosine kinase 3
-
- GATA3
-
- GATA-binding protein 3
-
- GVHD
-
- graft versus host disease
-
- HDACi
-
- histone deacetylase inhibitor
-
- HI
-
- h ematological improvement
-
- HLA
-
- human leukocyte antigen
-
- HMA
-
- h ypomethylating agent
-
- HNSCC
-
- head and neck squamous cell carcinoma
-
- HR-MDS
-
- higher-risk MDS
-
- ICI
-
- immune checkpoint inhibitor
-
- IDH2
-
- isocitrate dehydrogenase 2
-
- IFN
-
- interferon
-
- IHC
-
- immunohistochemistry
-
- IRF
-
- interferon regulatory factor
-
- KIR
-
- Killer cell immunoglobulin-like receptor
-
- KMT2D
-
- lysine methyltransferase 2D
-
- LSD1
-
- lysine-specific demethylase 1
-
- MAGE
-
- melanoma-associated antigen
-
- MAGE-A3
-
- MAGE family member A3
-
- mCR
-
- marrow CR
-
- MDS
-
- myelodysplastic syndrome
-
- MDSC
-
- m yeloid-derived suppressor cell
-
- MHC
-
- major histocompatibility complex
-
- miRNA
-
- microRNA
-
- MPN
-
- myeloproliferative neoplasm
-
- MSI
-
- microsatellite instability
-
- MSS
-
- microsatellite stability
-
- ncRNA
-
- non-coding RNA
-
- NKTCL
-
- natural killer/T cell lymphoma
-
- NLRC5
-
- nucleotide oligomerization domain-like receptor subfamily C5
-
- NSCLC
-
- non-small cell lung cancer
-
- NY-ESO-1
-
- New York esophageal squamous cell carcinoma 1
-
- OC
-
- ovarian cancer
-
- ORR
-
- overall response rate
-
- PD
-
- progressive disease
-
- PD-1
-
- programmed cell death protein-1
-
- PD-L1
-
- PD-1 ligand 1
-
- PFS
-
- progression-free survival
-
- piRNA
-
- piwi-interacting RNA
-
- PMBCL
-
- primary mediastinal large B-cell lymphoma
-
- PMN-MDSC
-
- polymorphonuclear MDSC
-
- PR
-
- partial response
-
- PRAME
-
- preferentially expressed antigen in melanoma
-
- PTCL
-
- peripheral T-cell lymphoma
-
- R/R
-
- relapsed or refractory
-
- SCT
-
- stem cell transplantation
-
- SD
-
- stable disease
-
- SGC
-
- salivary gland cancer
-
- siRNA
-
- small interfering RNA
-
- TAP1
-
- transporter 1
-
- T-BET
-
- T-box protein in T cells
-
- TCL
-
- T-cell lymphoma
-
- Tcm
-
- central memory T cell
-
- TCR
-
- T-cell receptor
-
- TET2
-
- ten-eleven translocation 2
-
- Th
-
- T helper cell
-
- THU
-
- tetrahydrouridine
-
- TIL
-
- tumor infiltrating lymphocyte
-
- TIM3
-
- T cell immunoglobulin mucin-3
-
- TMB
-
- tumor mutation load
-
- TME
-
- tumor microenvironment
-
- TNBC
-
- triple-negative breast cancer
-
- TNFRSF4
-
- TNF receptor superfamily member 4
-
- Treg
-
- regulatory T cell
-
- WT-1
-
- Wilms tumor 1
1 BACKGROUND
Immunotherapy, the science of enhancing the immune system to combat cancer, has taken center stage in cancer therapeutics thanks to recent clinical successes in treating various tumors with immune checkpoint inhibitors (ICIs) and adoptive cell therapy [1, 2]. However, it is only effective in a few hematological malignancies and solid cancers, while many patients fail to achieve sustained complete response (CR) and suffer from disease relapse or experience immune-related adverse events (AEs), highlighting the needs for novel treatment strategies [3, 4]. Epigenetics refers to heritable alteration in gene expression without direct changes or mutations in the DNA sequence. Aberrant epigenetic mechanisms imposed by DNA methylation, histone modifications, chromosome remodeling and non-coding RNA play a crucial role in driving cancer initiation and progression [5]. Epigenetic drugs (epi-drugs), such as DNA methyltransferase inhibitors (DMNTis) and histone deacetylase inhibitors (HDACis), have been proven to be effective in many different types of cancer [6]. There has been growing interest in epigenetic regulation of cancer immunity [7] because epigenetic dysregulations not only are restricted to cancer cells, including cancer stem cells (CSCs) but also contribute to the dysfunction of immune cells in the tumor microenvironment (TME).
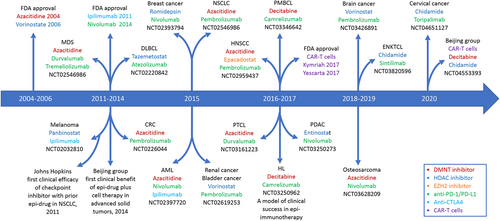
Recently, emerging strategies to enhance the anticancer potency of immunotherapies have been pursued. One of these involves incorporating epigenetic therapy into immunotherapy, called epi-immunotherapy [8]. Accumulating preclinical studies have shown that epigenetic modulation can sensitize tumors to ICIs or cell therapy, and various epi-immunotherapies for the patients with different tumor types are currently being evaluated in numerous clinical trials (Figure 1). In light of recent advances in the field of cancer epigenetics and immunology, we provide an updated, clinically oriented review of the evolving landscape of the combination of epigenetic modifiers with immunotherapy in both hematological cancers (Table 1) and solid tumors (Table 2). Although most results are reported from phase I and phase II clinical trials, unless otherwise specified, we primarily focus on the therapeutic efficacy of this strategy while omitting the toxicity or AEs because most epi-immunotherapies did not present new safety concerns in these trials.
| Condition | Epi-immmunotherapy | NCT Identifier | Phase | Status |
|---|---|---|---|---|
| AML | Aza + Pembro | NCT02845297 | II | Active, not recruiting |
| Aza + Pembro | NCT03769532 | II | Recruiting | |
| Aza + Nivo | NCT03825367 | I/II | Recruiting | |
| Aza + Nivo ± Ipili | NCT02397720 | II | Recruiting | |
| Aza + Nivo | NCT04128020 | I | Withdrawn | |
| Aza + Nivo + Relatli | NCT04913922 | II | Recruiting | |
| Aza + Nivo | NCT03092674 | II/III | Active, not recruiting | |
| Aza + Ave | NCT02953561 | I/II | Terminated | |
| Aza + Ave | NCT03390296 | I/II | Active, not recruiting | |
| Aza + Liri | NCT02399917 | II | Terminated | |
| Aza + Lenalidomide | NCT04490707 | III | Recruiting | |
| Dec + Pembro | NCT02996474 | I/II | Completed | |
| Dec + Nivo | NCT03358719 | I | Completed | |
| Dec + Cam | NCT04353479 | II | Not recruiting | |
| Guadec + Atezo | NCT02892318 | I | Completed | |
| MDS | Aza+ Pembro | NCT03094637 | II | Active, not recruiting |
| Aza+ Atezo | NCT02508870 | I | Completed | |
| Aza + Liri | NCT02599649 | I/II | Terminated, | |
| Aza + Durva + Treme | NCT02117219 | I | Completed | |
| Aza + Ipili | NCT02530463 | II | Recruiting | |
| Aza + Dec + MBG453 | NCT04878432 | II | Recruiting | |
| Aza + MBG453 | NCT04266301 | III | Active, not recruiting | |
| Dec + Sparta | NCT05201066 | I | Not yet recruiting | |
| Ent + Pembro | NCT02936752 | Ib | Active,not recruiting | |
| AML, MDS | Aza+ Durva | NCT02775903 | II | Active, not recruiting |
| Aza + NKR-2 | NCT03612739 | I | Withdrawn | |
| Dec + Pembro | NCT03969446 | I | Recruiting | |
| Dec + Sparta ± MBG453 | NCT03066648 | Ib | Active, not recruiting | |
| Dec + Ave | NCT03395873 | I | Terminated | |
| Dec + Ipili | NCT02890329 | I | Recruiting | |
| Guadec + Atezo | NCT02935361 | I/II | Active, not recruiting | |
| PTCL/CTCL | Aza+ Durva | NCT03161223 | I | Recruiting |
| Romi + Pembro | NCT03278782 | II | Recruiting | |
| Romi + Durva ± Aza | NCT03161223 | I | Recruiting | |
| Chida+ Sintili | NCT04512534 | II | Recruiting | |
| Chida + Sintili + Aza | NCT04052659 | II | Not, yet recruiting | |
| Dec+ Pembro + Pralatrexate | NCT03240211 | Ib | Not recruiting | |
| Chida+ Sintili | NCT04296786 | II | Recruiting | |
| NKTCL | Chida+ Sintili | NCT03820596 | I/II | Recruiting |
| DLBCL | Aza+ Ave + utomilumab | NCT02951156 | Ib/III | Terminated |
| CXD101 + Pembro | NCT03873025 | I/II | Withdrawn | |
| Tazemetostat + Atezolizumab | NCT02220842 | Ib | Completed | |
| PMBCL | Dec + Cam + chemo | NCT03346642 | I/II | Unknown |
| HL | Dec+ Cam | NCT03250962 | II | Recruiting |
| Dec + Cam | NCT04510610 | II/III | Recruiting | |
| Dec + Chida + Cam | NCT04233294 | I/II | Recruiting | |
| Dec + Chida + Cam | NCT04514081 | II | Recruiting | |
| Ent + Pembro | NCT03179930 | II | Recruiting | |
| DLBCL, HL | Vor + Pembro | NCT03150329 | I | Recruiting |
| B-cell lymphoma | Dec-primed Tandem 19/20 CAR-T | NCT04697940 | I/II | Recruiting |
| Dec-primed Tandem 19/20 CAR-T + Dec and/or Chida | NCT04553393 | I/II | Recruiting | |
| Dec + PD-1/CD28 CD19 CAR-T | NCT04850560 | I/II | Recruiting | |
| Post-CAR-T relapsed lymphoma | Chida + Cam | NCT04337606 | I/II | Recruiting |
- Abbreviations: AML, acute myeloid leukemia; MDS, myelodysplasia syndrome; CMML, chronic myelomonocytic leukemia; DLBCL, diffuse large B cell lymphoma; PTCL, peripheral T cell lymphoma; CTCL, cutaneous T cell lymphoma; NKTCL, NK/T cell lymphoma; HL, Hodgkin lymophoma; PMBCL, primary mediastinal B cell lymphoma; DMNTi: Aza, Azacytizine; Dec, Decitabine; Guadec, Guadecitabine; HDACi: Vor, Vorinostat; Ent, Entinostat; Chida, Chidamide; Romi, Romidepsin; CXD101; anti-PD-1: Pembro, Pembrolizumab; Nivo, Nivoluzumab; Cam, Camrelizumab;Sintili, Sintilimab; Sparta, Spartalizumab; anti-PD-L1: Durva, Durvalumab; Atezo, Atezolizumab; Ave, Avelumab; anti-CTLA4: Ipili, Ipilimumab;Treme, Tremelimumab (anti-CTLA4); CAR-T, chimeric antigen receptor T cell; others: Liri, Lirilumab (anti-KIR); MBG453 (anti-TIM3), Relatli, Relatlimab (anti-LAG3); utomilumab (anti-4-1BB), NKR-2 (CAR-T), chemo, chemotherapy.
| Condition | Epi-immunotherapy | NCT Identifier | Phase | Status |
|---|---|---|---|---|
| NSCLC | Aza+ Pembro | NCT02546986 | II | Active, not recruiting |
| Aza + Ent + Nivo | NCT01928576 | II | Recruiting | |
| Aza + Durva | NCT02250326 | II | Active, not recruiting | |
| Dec + Pembro | NCT03233724 | I/II | Recruiting | |
| Dec + Nivo | NCT02664181 | II | Active, not recruiting | |
| Guadec + Moc + Pembro | NCT03220477 | I | Active, not recruiting | |
| Ent + Pembro | NCT02437136 | Ib/II | Unknown | |
| Vor + Pembro | NCT02638090 | II | Recruiting | |
| ACY241 + Nivo | NCT02635061 | Ib | Active, not recruiting | |
| Chida + Pembro | NCT05141357 | II | Recruiting | |
| Moc + Nivo | NCT02954991 | II | Active, not recruiting | |
| Moc+ Durva | NCT02805660 | I/II | Terminated | |
| CRC | Aza+ Pembro | NCT02260440 | II | Completed |
| Aza +Romi + Pembro | NCT02512172 | I | Active, not recruiting | |
| Guadec + Nivo | NCT03576963 | I/II | Withdrawn | |
| Chida + Sintili | NCT04724239 | II | Not recruiting | |
| Melanoma | Aza + Pembro | NCT02816021 | II | Recruiting |
| Guadec + Ipili | NCT02608437 | Ib | Unknown | |
| Ent + Pembro | NCT03765229 | II | Recruiting | |
| Ent + Pembro | NCT02437136 | Ib/II | Unknown | |
| Ent + Pembro | NCT02697630 | II | Active, not recruiting | |
| Pano + Ipili | NCT02032810 | I | Active, not recruiting | |
| Moc + Nivo + Ipili | NCT03565406 | Ib | Terminated | |
| Tino + Nivo | NCT03903458 | Ib | Recruiting | |
| NSCLC, CRC | Aza + Pembro+ Epa | NCT02959437 | I/II | Terminated |
| NSCLC, melanoma | Guadec + Nivo + Ipili | NCT04250246 | II | Not recruiting |
| Pano + Sparta | NCT03982134 | I | Withdrawn | |
| NSCLC, RC, melanoma, | Chida + Nivo | NCT02718066 | I/II | Active, not recruiting |
| NSCLC, CRC, melanoma, HNSCC | Aza + NCB059872 + Pembro + Epa | NCT02959437 | I/II | Terminated |
| HNSCC | Dec + Durva | NCT03019003 | I/II | Recruiting |
| Moc + Durva | NCT02993991 | I | Withdrawn | |
| HNSCC, SGC | Vor + Pembro | NCT02538510 | II | Active, not recruiting |
| PDAC | Aza + Pembro | NCT03264404 | II | Recruiting |
| PDAC, CGC | Ent+ Nivo | NCT03250273 | II | Active, not recruiting |
| PDAC, CGC, liver cancer | Guadec + Durva | NCT03257761 | I | Recruiting |
| Breast cancer | Dec + Pembro | NCT02957968 | II | Recruiting |
| Ent + Atezo | NCT03280563 | I/II | Recruiting | |
| Vor+ Pembro | NCT02395627 | II | Terminated | |
| Vor + Pembro | NCT04190056 | II | Recruiting | |
| Romi + Nivo + Cisplatin | NCT02393794 | I/II | Suspended | |
| Ent + Atezo | NCT02708680 | II | Unknown | |
| Ovarian cancer | Ent + Ave | NCT02915523 | Ib/II | Unknown |
| Guadec + Pembro | NCT02901899 | II | Active, not recruiting | |
| Aza + Pembro | NCT02900560 | II | Completed | |
| Breast, ovarian cancers | Aza + Durva | NCT02811497 | II | Completed |
| RO6870810 + Atezo | NCT03292172 | Ib | Terminated | |
| Cervical cancer | Chida + Tori | NCT04651127 | I/II | Recruiting |
| VA + Ave | NCT03357757 | II | Recruiting | |
| RC | Guadec + Durva | NCT03308396 | Ib/II | Active, not recruiting |
| Ent + Nivo + Ipili | NCT03552380 | II | Active, not recruiting | |
| Ent + Atezo + Beva | NCT03024437 | I | Active, not recruiting | |
| UC | Guadec + Atezo | NCT03179943 | II | Active, not recruiting |
| Taze + Pembro | NCT03854474 | I/II | Recruiting | |
| Ent + Pembro | NCT03978624 | II | Recruiting | |
| Chida + Tisle | NCT04562311 | I | Recruiting | |
| Aza + Pembro + Epa + INCB059872 | NCT02959437 | I/II | Terminated | |
| RC, UC | Vor + Pembro | NCT02619253 | Ib | Active, not recruiting |
| Glioblastoma | Vor+ Pembro | NCT03426891 | I | Active, not recruiting |
| Osteosarcoma | Aza + Nivo | NCT03628209 | I | Recruiting |
| Virus-associated cancers | VA + Ave | NCT03357757 | II | Recruiting |
| Solid tumors, lymphoma | Dec + Pembro + radiation | NCT03445858 | I | Recruiting |
| Advanced solid cancers | Epa+ Pembro | NCT02909452 | I | Completed |
| Ent + Nivo+ Ipili | NCT02453620 | I | Active, not recruiting | |
| Guadec + Pembro | NCT02998567 | I | Active, not recruiting | |
| Lira + Ipili | NCT03525795 | I | Completed | |
| Ent + Pembro | NCT02909452 | I | Unknown | |
| Ent + Nivo + Ipili | NCT02453620 | I | Active, not recruiting | |
| Taze + Durva | NCT04705818 | I | Recruiting |
- Abbreviations: NSCLC, non-small cell lung cancer; CRC, colorectal cancer; PADC, pancreatic ductal adenocarcinoma; CGC, cholangiocarcinoma; HNSCC, head and neck squamous cell carcer; SGC, salivary gland cancer; RC, renal cancer; UC, urothelial carcinoma; DMNTi: Aza, Azacytizine; Dec, Decitabine; Guadec, Guadecitabine; HDACi: Vor, Vorinostat; Ent, Entinostat; Chida, Chidamide; Romi, Romidepsin; Pano,Panobinostat; Moc,Mocetinostat; Tino, Tinostamustine,; VA, Valproic acid; ACY241; EZHi: Lira,Lirametostat; Taze, Tazemetostat; LSD1i: NCB059872; BETi: RO6870810; anti-PD-1: Pembro, Pembrolizumab; Nivo, Nivoluzumab; Cam, Camrelizumab; Sintili, Sintilimab; Sparta, Spartalizumab; Tisle, Tislelizumab; Tori,Toripalimab; anti-PD-L1: Durva, Durvalumab; Atezo, Atezolizumab; Ave, Avelumab; anti-CTLA4: Ipili, Ipilimumab;Treme, Tremelimumab; others: Liri, Lirilumab (anti-KIR), Epa, Epacadostat (anti-IDO1); Beva, Bevacizumab (anti-VEGF).
2 THE RATIONALES OF EPI-DRUGS IN COMBINATION WITH IMMUNOTHERAPY FOR CANCERS
The cancer genome is featured by global DNA hypomethylation, which results in the silence of certain tumor suppressor genes as well as endogenous retroviral elements. The nucleosome is formed through the wrapping of genomic DNA around histone octamers connected with linker histones and is further packed into high-order chromatin that resides within the nucleus. Chromatin remodeling complexes regulate the chromatin configuration in an ATP-dependent manner to activate or repress gene transcription. Multiple histone residues are subjected to covalent modifications, through which the accessibility of DNA to transcription factors is modulated. The non-coding RNAs (ncRNAs) represent another layer of complexity of epigenetic regulation. Short ncRNAs of <30 nucleotides in length, including microRNAs (miRNAs) and small interfering RNA (siRNAs), can bind to the 3’ untranslated region and degrade target mRNA or interfere with its translation. Long ncRNAs have a wide variety of gene regulation at multiple levels, including nucleosome positioning and chromosome looping. It has been clear that ncRNAs also function in immune regulation, which has been recently reviewed elsewhere [9, 10].
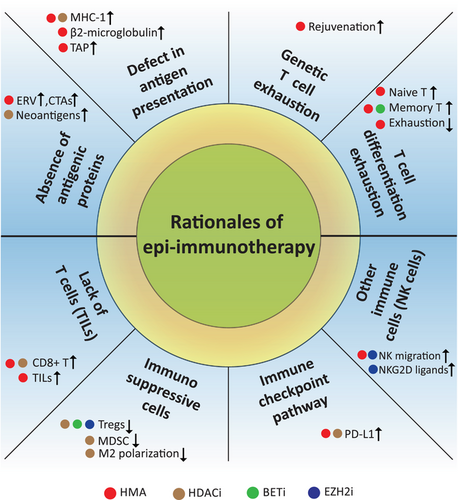
The epigenetic marks on DNA or histones, such as methylation, acetylation, phosphorylation and ubiquitination, are central nodes among a variety of epigenetic modifiers. These modifiers are commonly classified as epigenetic writers, readers and erasers, which function to add, recognize, and remove specific chromatin modifications, respectively. Targeting the epigenetic modifiers with enzymatic activities was initially explored, leading to the development of first-generation epi-drugs, i.e., DNMT or HDAC inhibitors [6]. Chromatin remodelers and ncRNAs do not directly modify DNA or histone, and their therapeutic implications for cancer are under active investigation. A number of excellent reviews have provided details on epigenetic modifiers and their regulatory roles in cancer or immunity [11-14], and we refer to these for more in-depth information on individual epigenetic mechanisms. As discussed below, a brief illustration of the rationales for epi-immunotherapy is shown in Figure 2.
2.1 Direct antitumor effects of epi-drugs
Epi-drugs are chemical agents that alter DNA and chromatin structure and promote the disruption of transcriptional and post-transcriptional modifications [6]. To date, several epi-drugs, including DNMTis and HDACis, have received the US Food and Drug Administration (FDA) approval for a few cancers; for example, azacitidine (AZA) and decitabine (DAC) are the most common DNMTis, also known as hypomethylating agents (HMAs) [13, 15]. More recently, Tazemetostat, an H3K27 methyltransferase enhancer of zeste 2 (EZH2) inhibitor, has been indicated as front-line therapy for epithelioid sarcoma in the US [16]. Nevertheless, clinical response to epi-drugs has been mostly confined to hematological malignancies [17]. DNMTi and HDACi can induce cell cycle arrest, senescence and apoptosis in tumor cells through the re-expression of certain tumor suppressor genes silenced by DNA methylation and histone deacetylation [18-24]. On the other hand, the epigenetic alterations promote CSC self-renewal, proliferation and metastasis and confer treatment resistance [25-28]. Many preclinical studies have shown that DMNTis can inhibit the expression of stemness genes and upregulate differentiation-related genes, thereby significantly reducing the self-renewal and tumorigenesis of CSCs [29-33]. Similarly, HDACis are also capable of controlling the CSC population [34]. Several essential genes involved in the CSC maintenance, such as β-catenin, Stat3 and Notch1, are targeted by HDACi, which alone or in combination can eradicate CSCs to suppress tumor growth [35-39].
However, the best clinical responses to the combined treatment with DNMTi plus programmed cell death protein-1 (PD-1) blockade were found in those patients who received low-dose DNMTi regimens in which the drug dose would not result in cytotoxicity [7]. For example, in a patient tumor-derived xenograft model of colorectal cancer (CRC), low-dose DAC can re-modulate the TME to sensitize the PD-1 blockade [40]. Moreover, Chiappinelli et al. [41] demonstrated that DNMTi stimulated immune signaling through the viral defense mechanisms, and low-dose AZA directly enhanced the anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA4) efficacy in a melanoma model. These striking synergistic effects for cancer intervention can be extended to chimeric antigen receptor T (CAR-T) cell therapy [42, 43]. More importantly, in an open-label phase II study, relapsed or refractory (R/R) classic Hodgkin lymphoma (cHL) patients without previous anti-PD-1 exposure were included, and the CR rate was significantly higher in the patients treated with low-dose DAC plus PD-1 inhibitor camrelizumab than those treated with camrelizumab alone [44]. On the other hand, the efficacy of HDACis alone in clinical trials has been largely restricted to hematological malignancies, and the clinical outcomes in a variety of solid tumors are still disappointing [24, 45]. Collectively, these findings support that modulation of antitumor immune responses, rather than direct antitumor activity of epi-drugs, provide rationales for epi-immunotherapy.
2.2 Enhanced immunogenicity of cancer cells by epi-drugs
The absence of tumor antigens and defects in the antigen-presenting machinery, which result in the lack of recognition by T cells, greatly contribute to primary and adaptive resistance to immunotherapy [46]. Epi-drugs are known to elicit viral mimicry to activate the interferon (IFN) pathway, thereby augmenting immune responses [47]. Accounting for 5%-10% of genomic DNA sequences, human endogenous retroviruses (ERVs) are remnants of germline integrations of exogenous infectious retroviruses during evolution [48, 49]. The cancer-testis antigens (CTAs) are not expressed in healthy tissues other than germ cells but are often abnormally expressed in tumors [50]. ERVs and CTAs, initially suppressed by cytosine methylation in cancer cells, are closely associated with antitumor cytotoxic immune response [51]. Both ERVs and CTAs are reactivated through demethylation following exposure to DMNTi, leading to a state of viral mimicry, through which the neoantigen expression increases immunogenicity and triggers an innate immune response against tumor [41, 52]. During viral mimicry response, double-strand RNA is produced and activates immunogenic pattern recognition receptors, type I and III IFNs are secreted, thus further enhancing antigen processing and presentation through transporter 1 (TAP1) and human leukocyte antigen (HLA)-class I, respectively [48, 51]. DMNTis can upregulate immunogenic CTAs such as New York esophageal squamous cell carcinoma 1 (NY-ESO-1) and melanoma-associated antigen (MAGE) family member A1 (MAGE-A1) [53, 54]. Defect in antigen presentation machinery also contributes to the reduced immunogenicity. Major histocompatibility complex (MHC) class I genes are often silenced due to promotor DNA methylation in cancer, and DMNTi reverses MHC-I gene methylation and increases MHC-I expression in response to IFN [55]. Likewise, β2-microglobulin and TAP1 are also increased when DNA methylation is inhibited, which is responsible for increased antigen processing and presentation [55]. DNMTi can reactivate nucleotide oligomerization domain-like receptor subfamily C5 (NLRC5), an IFN-inducible gene, to increase MHC-I gene expression [56]. HDACi also increases antigen presentation and restores HLA class-I expression in solid tumors [57]. Recently, Truong et al. [58] demonstrated that entinostat (ENT), a selective HDAC1/3 inhibitor, enhanced immunogenicity through neoantigen editing and induced robust specific antitumor response, which was mediated by increased effector T cell infiltration in TME. Inhibition of the histone demethylase lysine-specific demethylase 1 (LSD1) also induces a viral mimicry response following ERVs activation [59]. Additionally, ERV-independent activation is seen in several IFN-responsive genes, such as CXC-chemokine ligand 9 (CXCL9) and CXCL10, which are directly regulated by DNA methylation and histone modifications [60, 61].
The tumor PD-L1 expression appears to associate with the efficacy of PD-1 blockade [62-64]. There is increasing evidence that epi-drugs lead to PD-L1 upregulation in preclinical cancer models [65-67]. This modulation is largely dependent on the reactivation of ERVs and the IFN pathway [68].
2.3 The effects of epi-drugs on T cells
It is well known that epigenetic changes can alter the function and differentiation of T cells [7]. CD4+ T helper (Th) cells predominantly secrete cytokines to stimulate cellular immunity against tumor cells [69]. Low-dose ENT, a class I HDACi, decreases Foxp3 expression in regulatory T cells (Tregs), leading to tumor suppression [70]. CG-745, another HDACi, can also inhibit Treg proliferation and modulate the TME that potentiates anti-PD-1 activity against tumors [71]. Importantly, the combination of HDACis and anti-CTLA4 further improves CD4+ T-cell infiltration and effector functions [72]. In addition, inhibition of EZH2 enhances the pro-inflammatory functions of tumor-infiltrating Tregs and rewires the TME with increased effector T cells [73]. Another study found that CPI-1205, a small-molecule EZH2 inhibitor, can alter Tregs’ phenotype and functions to augment anticancer responses induced by CTLA4 blockade [74]. The bromodomain and extraterminal domain (BET) bromodomain inhibitor JQ1 could synergize with PD-1 inhibitor to promote a robust anticancer response in lung cancer, which was associated with reduced tumor-infiltrating Tregs but increased T-helper type 1 (Th1) cells [75, 76].
It has been established that both intrinsic and extrinsic mechanisms, i.e., terminal differentiation, exhaustion and activation-induced cell death, contribute to T cell dysfunction, which can be rescued by epigenetic reprogramming therapy [76]. T cell exhaustion is dependent on DMNT3a-mediated de novo DNA methylation, block of which by DAC can enhance T cell rejuvenation and sensitize anti-PD-1 therapy in cancer [77]. Recently, we showed that low-dose DAC significantly improves CAR-T cell phenotype and function, which is characterized by increased non-exhausted T cells and naive, early memory T cell differentiation [42, 43]. Loo Yau et al. [78] also demonstrated that low-dose DAC increases CD8+ T cell infiltration and their antitumor activities. Mechanistically, DAC can selectively increase both the number and abundance of a granzyme Bhigh, perforinhigh effector subpopulation. JQ1 had been shown to prevent the transition to effector memory T cells and enhance antitumor response in murine models of CAR-T therapy [79]. The effects of HDACi on T cells are complex and paradoxical, varying by isoform-selective HDAC inhibition. Laino et al. [80] found that low-dose pan-HDACis, but not selective HDACis, impair T cell viability. In patients who received HDAC6-selective inhibitors ACY-1215 and ACY-241, peripheral blood T cells showed increased Th2 transcription factor GATA-binding protein 3 (GATA3) and decreased Th1 transcription factor T-box protein in T cells (T-BET), shifting from exhaustion to central memory phenotype, and enhanced cytotoxicity. HDAC8, often overexpressed in cancers, suppresses the production of T cell-trafficking chemokines; downregulation of HDAC8 promotes global and enhancer acetylation of H3K27 to reactivate chemokine gene transcriptions [81]. In a liver cancer model, selective HDAC8 inhibition synergizes with anti-PD-L1 to eradicate cancer through increased CD8+ T cell infiltration in the TME [81].
2.4 Targeting the TME
In addition to cancer cells, the TME consists of extracellular matrix, vasculature, and stromal cells surrounding the tumor, as well as cytokines, chemokines, and exosomes. These components form a complicated immunosuppressive microenvironment [82, 83]. The epigenetic dysregulation is pivotal in the generation and maintenance of an immunosuppressive TME, resulting in immune evasion of cancer [84, 85]. As mentioned above, epi-drugs can promote maturation of functional Tregs, converting an immunosuppressive TME to an immunocompetent TME. Moreover, epigenetic changes by histone modifications also affect the differentiation and activation of the myeloid cells [86]. Myeloid-derived suppressor cells (MDSCs) include polymorphonuclear MDSCs (PMN-MDSCs) and monocytic MDSCs (M-MDSCs) [87]. Valproic acid, a common HDACi, when combined with an anti-PD-L1 antibody, induces polarization of precursor cells toward M-MDSCs in the bone marrow through transcriptional activation of the interferon regulatory factor (IRF)1/IRF8 pathway [88]. The class I/IV HDACi mocetinostat had been demonstrated to inhibit intratumoral Treg and MDSC populations and increase intra-tumoral CD8+ populations [89]. ENT impairs the immunosuppressive activity only in PMN-MDSCs but not M-MDSCs or macrophages; ricolinostat, an HDAC6 inhibitor, suppresses M-MDSC activity other than PMN-MDSC activity [90]. Interestingly, combined treatment abrogates the activities of MDSC populations and markedly inhibits tumor growth [90].
Macrophages have become promising immune effectors for cancer treatment [91]. The HDAC6 inhibitor, nexturastat A, reduces pro-tumorigenic M2 macrophages [92, 93], while class IIa HDACi improves phagocytic and immunostimulatory functions in macrophages, steering them toward an antitumor phenotype with enhanced capacity to activate cytotoxic T lymphocytes (CTLs) [94, 95]. Some HDACis can suppress M2 macrophage polarization and decrease MDSCs [71]. Although few studies have investigated the effect of HMA on the TME, recent data show that guadecitabine can down-regulate inhibitory accessory cells in the TME and reduce the leukemia-mediated expansion of MDSCs [96]. In a pancreatic ductal adenocarcinoma model, DAC treatment led to increased tumor infiltrating lymphocytes (TILs) and Chi3I3 (Ym1) upregulation, indicating an increase of M2 macrophages in the TME [97].
Epigenetic therapies also play an essential role in modulating natural killer (NK) cell and dendritic cell functions and, therefore, augment antitumor immunity [98, 99]. For example, EZH2 inhibitors can upregulate natural killer cell receptor protein 2D (NKG2D) ligands on cancer cells to enhance NK antitumor responses and induce CXCL10 re-expression, which is necessary and sufficient for NK cell migration [100-102]. Low-dose AZA significantly increases the expression of multiple killer cell immunoglobulin-like receptors in NK cells, thereby boosting NK cell-mediated recognition of leukemia cells [103].
3 ADVANCES IN CLINICAL TRIALS OF EPI-IMMUNOTHERAPY FOR SOLID AND HEMATOLOGICAL CANCERS
3.1 Hematological malignancies
3.1.1 Hodgkin's lymphoma
Currently, there are various FDA-approved ICIs, including antibodies against PD-1 (pembrolizumab and nivolumab), PD-L1 (atezolizumab), and CTLA4 (ipilimumab). In cHL, nivolumab or pembrolizumab alone elicits overall response rates (ORR) of 70%-85% [104-106]. However, the CR rate was only 25%-30%, suggesting that a great proportion of cHLs remain resistant to anti-PD-1 treatment [107, 108]. Nie et al. [44] performed a proof-of-concept study to investigate whether DAC could improve the efficacy of camrelizumab, a PD-1 antibody approved in China, in patients with R/R cHL. In this prospective, open-label, phase II trial (NCT02961101), among anti-PD-1-naïve patients, camrelizumab alone was compared with low-dose DAC (10 mg/day, days 1 to 5) plus camrelizumab. At a median follow-up of 14.9 months, CR rates were 32% and 71% in camrelizumab alone and DAC-camrelizumab combination groups, respectively [44]. When the median follow-up extended to 34.5 months, a greater improvement in CR rate was achieved in patients receiving DAC plus camrelizumab (79% vs. 32%). Progression-free survival (PFS) was longer for those receiving DAC plus camrelizumab compared with camrelizumab alone (35.0 vs. 15.5 months). The benefit of adding DAC to camrelizumab was observed especially in patients who had relatively high tumor burdens or received ≥3 prior lines of therapies. Interestingly, the increase in circulating peripheral central memory T cells was associated with improved clinical response and PFS, suggesting a potential biomarker for epi-immunotherapy in cHL [109]. Lately, Wang et al. [110] updated the clinical results of DAC combined with camrelizumab in cHL patients receiving prior anti-PD-1 (NCT02961101 and NCT03250962). Of 50 patients with progressed or relapsed cHL after anti-PD-1 treatment, the combined treatment resulted in an objective response rate of 52%, CR rate of 36% and longer PFS compared with prior anti-PD-1 monotherapy. The response appears durable once CR is achieved at 24 months. The exploratory studies showed that, when the tumor progresses, the ratio of peripheral CCR7+CD45RA– central memory T cells (Tcm) over total CD8+ or CD4+ cells decrease from the baseline level; after treatment with DAC plus camrelizumab, the Tcm ratio increases and persists in patients who obtained partial response (PR) or better, but not in those with stable disease (SD) or progressive disease (PD) [110].
In preclinical studies using various tumor models, ICIs also had been demonstrated to have synergistic effects when combined with HDACis [111, 112]. In a phase II trial (NCT03179930), R/R cHL patients were given ENT, an oral class I-specific HDACi, plus pembrolizumab [113]. Of 13 evaluable patients, ORR was 92%, including 3 patients who progressed on prior anti-PD-1 therapy, suggesting that the combination of ICI and HDACi have promising clinical activity. The study also showed a reasonable safety profile. Chidamide, a novel, orally active benzamide class of selective inhibitors against HDAC 1, 2, 3 and 10, was evaluated in a clinical study in cHL patients who were resistant to or relapsed after DAC plus camrelizumab treatment [114]. Of 14 evaluable patients, 13 (93%) achieved objective response, including 6 CRs. The toxicities were acceptable without any immune-related AEs. Collectively, compared with ICI alone, the combination of DMNTi or HDACi with ICI resulted in higher CR rate in anti-PD-1-naïve patients with R/R cHL. Epigenetic agents appear to partially reverse the resistance to ICIs since a proportion of patients with prior anti-PD-1 exposure still respond well when an epi-drug is added to anti-PD-1.
3.1.2 B-cell lymphoma
Unlike cHL, diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL) generally do not respond well to PD-1 blocker, although EBV-positive status associates with reasonable efficacy of pembrolizumab [115, 116]. Primary mediastinal large B-cell lymphoma (PMBCL), a DLBCL subtype, is characterized by chromosome 9p24 aberrations and upregulated PD-L1 [117]. A phase Ib and phase II trials (KEYNOTE-013/KEYNOTE-170) have shown that pembrolizumab produced durable responses and acceptable toxicity in R/R PMBCL [118]. To improve the anti-lymphoma activity, several ongoing clinical studies are assessing the combination of PD-1 blockade with DAC or vorinostat in DLBCL, FL and HL (NCT03346642 and NCT03150329). A high frequency of somatic mutations in epigenetic modifier genes, including CREBBP and EZH2, are identified in B cell lymphoma [115]. The mutant EZH2 reprograms germinal center B cells to alter their interactions with follicular Th cells and follicular dendritic cells, promoting B cell transformation in FL [119, 120]. CREBBP mutant-associated program can be reversed by HDAC3 inhibitor, which induces transcription of BCL6 targets to restore immune surveillance [121]. HDAC3 inhibitor can also enable TILs to eradicate DLBCL cells in an MHC-dependent manner and has synergistic effects with PD-L1 antibody in vivo [121].
Recently, multiple new epi-drugs are being evaluated for cytotoxicity against B-cell lymphoma and their mechanisms of action in preclinical and clinical studies. Tazemetostat, an oral first-in-class EZH2 inhibitor, has single-agent activity against DLBCL and FL [122-124]. Interestingly, a phase Ib study (NCT02220842) was performed to assess the efficacy of tazemetostat in combination with atezolizumab on R/R DLBCL [125]. A total of 43 patients were enrolled. However, at the data cut-off, 19 (44%) had discontinued study treatment because of death (n = 17) and withdrawal (n = 2). Best ORR was 16%, including 2 (5%) CRs and 5 (12%) PRs. Among the 5 patients with EZH2 mutations, 3 achieved a response.
3.1.3 T-cell lymphoma
PD-1 is considered a potential therapeutic target of T-cell lymphoma (TCL) because PD-1 is frequently overexpressed in angioimmunoblastic T-cell lymphoma (AITL), natural killer-/T-cell lymphoma (NKTCL), and peripheral T-cell lymphoma (PTCL) [126]. It has been shown that nivolumab results in an ORR of 40% in R/R TCL, and pembrolizumab leads to an ORR of 100% in EBV-associated NK cell lymphoma and TCL [127, 128]. Additionally, several HDACis, including belinostat, romidepsin and chidamide, have been indicated for certain TCL subtypes, but the efficacy of single agents rarely exceeded 30% [129]. It was also well known that azacitidine is effective in follicular Th cell-derived PTCL by targeting recurrent ten-eleven translocation 2 (TET2), DNA methyltransferase 3A (DNMT3A) and isocitrate dehydrogenase 2 (IDH2) mutations [130]. Thus, the incorporation of epi-drugs into PD-1 checkpoint inhibition is worth further clinical assessment in TCL.
Recently, a phase I/II trial of pembrolizumab combined with romidepsin and pralatrexate for R/R AITL and PTCL with follicular Th cells was conducted (NCT03278782) [131]. Among 14 patients who received pembrolizumab in combination with romidepsin, the ORR was 50%, including 5 CRs and 2 PRs, which was durable with 18-month follow-up and with acceptable safety. High level of PD-L1 is predictive of good response [131]. Pembrolizumab is also being tested along with DAC and pralatrexate in a phase I clinical trial (NCT03240211) [132]. In this study, 13 patients with R/R PTCL and cutaneous T-cell lymphoma (CTCL) were enrolled, and the patients who received triplet combination treatment achieved objective responses with a duration of response (DOR) of 18 months. The preliminary data from another phase I/IIa trial (NCT03161223) showed that the epi-immunotherapy was tolerable in PTCL patients, and 3/5 evaluable patients achieved CR when treated with durvalumab, oral azacitidine, and romidepsin [132].
Extranodal NK/T cell lymphoma (ENKTL) is an aggressive Epstein-Barr virus-related lymphoma with a high incidence in Asia [115]. Abnormal PD-1/PD-L1 expression was demonstrated in both neoplastic and immune cells in the TME, which offers opportunities for applying ICIs in this lymphoma subtype [115, 133]. On the other hand, the tumor suppressor epigenetic regulators, lysine methyltransferase 2D (KMT2D) and BCL6 corepressor (BCOR), were frequently mutated in ENKTL [134]. Kwong et al. [135] first demonstrated the effectiveness of pembrolizumab in a small series of patients with R/R ENKTL. In a phase II ORIENT-4 study, sintilimab, another anti-PD-1 antibody, also showed efficacy, and ORR and disease control rate (DCR) were 67.9% (19/28) and 85.7% (24/28), respectively [136]. Recently, a single-arm, open-label, multicenter clinical trial (NCT03820596) was designed to evaluate the safety and efficacy of sintilimab combined with chidamide for R/R ENKTL, showing an impressive response in 36 evaluable patients [137]. In this study, the combination therapy yielded an ORR of 58.3% with CR in 16 (44.4%) patients. Notably, tumor PD-L1 expression could predict clinical response [137]. Furthermore, a single-arm phase II trial is conducted to test the combination of sintilimab, chidamide and azacitidine in patients with R/R PTCLs, and the results are pending [138].
3.1.4 Acute myeloid leukemia and myelodysplastic syndrome
Acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) are highly heterogenous myeloid malignancies with complex molecular genetic abnormalities, and the prognosis remains poor for elderly or refractory patients. Yang et al. [139] first reported that PD-1, PD-L1/L2 and CTLA4 are overexpressed in 8%-34% of bone marrow CD34+ cells from patients with MDS and AML. Currently, there is great clinical interest in immunotherapies for AML, with over 30 clinical studies evaluating ICIs for AML and MDS, which included PD-1, CTLA4, and T cell immunoglobulin mucin-3 (TIM3) blockade [140-142]. However, ICIs, especially anti-PD-1 antibodies, have limited activity in these diseases [143, 144]. Given their potential for induction of checkpoint molecules, HMAs have been combined with PD-1/PD-L1 inhibitors in several studies. In an open-label, phase II study, 70 patients with R/R AML were treated with nivolumab and AZA [145]. The ORR was 33%, including 15 (22%) CR or CR with insufficient recovery of counts (CRi), 1 PR, and 7 hematological improvement (HI). Importantly, the ORR of 58% in HMA-naive R/R AML patients compared favorably with the historical controls treated with AZA alone [145]. Combinations of HMAs with another anti-PD-1 antibody, pembrolizumab, produced similar benefits to those observed with AZA and nivolumab in R/R AML patients [146]. Pembrolizumab-AZA combination therapy also had been investigated in a clinical trial for newly diagnosed AML (NCT02845297), in which 22 unfit, elderly patients were enrolled [147]. Seventeen patients were evaluable with CR/Cri 47% (8/17) and PR 12% (2/17). This front-line treatment resulted in a median OS of 13.1 months [147]. For high-risk MDS (HR-MDS), a phase II trial (NCT03094637) evaluated AZA plus pembrolizumab in patients with HR-MDS after failure of HMA therapy. The combination treatment of pembrolizumab and AZA was well tolerated in 17 therapy-naive patients and showed an ORR of 80%, including 3 CR, 7 marrow CRs and 1 HI [148]. This clinical study showed that the combination therapy might have anticancer activity in certain HMA-failure patients, but no significant improvement in OS was demonstrated. Interestingly, a recent study of triple combination showed encouraging CR/CRi and OS [149]. In this study, 31 R/R AML patients were given nivolumab, AZA and ipilimumab, and the ORR was 60%, including 9 (36%) CR/CRi, 2 (8%) HI lasting over 6 months, and 4 (16%) SD.
The efficacy of PD-1 or PD-L1 blockade appears to vary between AML and MDS [150]. In a single-arm phase I study, avelumab in combination with DAC was evaluated in newly-diagnosed AML patients ineligible for intensive therapy [151]. Only 1 of 5 patient (20%) achieved CR, 3 had SD, and 1 experienced PD. In the first large randomized trial (NCT02845297), 129 AML patients of 65 years or older were randomized to receive AZA plus durvalumab or AZA alone [152]. No significant difference was found in the ORR (31.3% vs. 35.4%) or CR rate (17.2% vs. 21.5%) between 2 arms, and the median OS was 13.0 and 14.4 months, respectively. Similar results were reported in the patients with HR-MDS [152]. In a phase Ib study, the efficacy of atezolizumab, with or without AZA, was evaluated in HMA-failure and HMA-naive MDS patients (NCT02508870) [153]. Despite little effects in HMA-failure MDS patients, atezolizumab, with or without AZA, indeed produces an ORR of 62% (CR, 14%; mCR, 19%; mCR + HI, 10%; HI, 19%) in HMA-naïve patients with median OS not reached.
However, clinical studies in AML and MDS have raised safety concerns about epi-immunotherapy. For example, in HMA-naïve patients receiving AZA and atezolizumab, frequent febrile neutropenia (29%) accounted for 3-month mortality of 29%, which caused early termination of the study (NCT02508870) [146]. In addition, SWOG 1612, a randomized phase III trial in AML and HR-MDS, also showed excessive early deaths in the AZA/nivolumab arm compared with the control arm [154]. Based on our previous studies in cHL [44, 109, 110], we suggest that low-dose HMA should be tested in combination with PD-1 or PD-L1 inhibitors in AML and MDS patients.
Lirilumab is a fully-humanized IgG4 monoclonal antibody that is designed to block the killer cell immunoglobulin-like receptor (KIR)/HLA-C interaction, thereby enhancing NK cell-mediated cytotoxicity against AML in KIR-mismatched haploidentical stem cell transplantation (SCT) [155, 156]. Previously, lirilumab in combination with AZA was evaluated in relapsed AML in a phase Ib/II study (NCT02399917) [157]. Twenty-five patients were included and achieved an ORR of 20%, including a CR/CRi of 8% (2/25) and HI 12% (3/25). The median DOR and overall OS were 2.0 months and 4.0 months, respectively. In another pilot trial (NCT02599649), 8 HR-MDS patients received a median of 4 cycles of treatment with AZA plus lirilumab [158]. Overall, 2 patients achieved CR, 4 had marrow CR, and 2 had SD.
Despite significant advances, epi-immunotherapy has shown variable efficacies. Many patients do not experience a CR, and some are non-responders, highlighting the need to improve our understanding of the clinical features and molecular events associated with response. In AML and HR-MDS, a good response is associated with HMA-naïve status, low leukemia burden, ASXL1 mutation, and high number of pre-treatment CD3+ and CD8+ cells evaluated by mass cytometry (CyTOF) and immunohistochemistry (IHC) [145]. Herbrich et al. [159] analyzed the baseline immune landscape using single-cell CyTOF profiling of serial samples collected from R/R AML patients receiving AZA and avelumab (NCT02953561). The CD4/CD8 ratio and residual T cell profiles were found to predict the response following HMA/PD-L1 inhibition. Importantly, immune landscape studies revealed that AML cells also express other immune checkpoints, in particular, PD-L2, TNF receptor superfamily member 4 (TNFRSF4) and TIM3 could be potential targets for novel epi-immunotherapy [159]. In a recent phase II trial (NCT03066648), sabatolimab (MBG453), an anti-TIM3 antibody, plus DAC or AZA led to an ORR of 58%-70% for HR-MDS, 27%-41% for newly-diagnosed AML, and 24% for R/R AML [160, 161]. The study also showed that combining HMAs with TIM3 inhibition was safe with a relatively durable response in MDS and AML [160, 161]. Based on these data, the STIMULUS MDS-US trial will further assess the feasibility and clinical efficacy of sabatolimab in combination with an oral HMA in patients with advanced MDS [162].
3.2 Solid tumors
3.2.1 Breast cancer
A phase II clinical trial was designed to combine CC-486, an oral HMA, and durvalumab to treat selected immunologically cold tumors, including breast cancer (BC), resulting in marginal clinical response [163]. When combined with anti-PD-1, anti-CTLA4, or both, ENT can significantly prolong tumor-free survival in a HER2/neu transgenic BC model [164]. In ENCORE 602 trial (NCT02708680) [165], 81 patients with refractory triple-negative breast cancer (TNBC) were randomized to receive atezolizumab plus ENT or atezolizumab alone. Unfortunately, no significant differences in ORR, PFS and median OS were observed between the two arms, while more AEs were seen in the combination arm. Similarly, Terranova-Barberio et al. [166] presented the data from a randomized phase II trial (NCT02395627) using triple combination of vorinostat, pembrolizumab, and tamoxifen. In 34 heavily pretreated patients with estrogen receptor (ER)-positive BC, this combination treatment showed limited efficacy. However, comprehensive correlative analysis revealed that an exhausted CD8+ T-cell (PD-1+/CTLA4+) immune signature and an HDACi-dependent decrease in Tregs (CD4+ Foxp3+/CTLA4+) might predict response to epi-immunotherapy. Recently, another HDACi, romidepsin, was combined with cisplatin and nivolumab in a phase I/II study (NCT02393794) [167], in which 51 patients with metastatic TNBC were enrolled. Among 34 evaluable patients, the ORR was 44%, median PFS was 4.4 months, and 1-year PFS rate was 23%; the median OS was 10.3 months, and 1-year OS rate was 43%. The triple combination had safe profiles and impressive efficacy in refractory metastatic TNBC, including PD-L1-negative diseases and those with liver metastasis [167].
3.2.2 Non-small cell lung cancer
Juergens et al. [168] from Johns Hopkins University first reported durable clinical responses to immune checkpoint therapy in advanced NSCLC patients who received prior epi-drug therapy, and later they suggested that AZA-induced PD-L1 upregulation may account for the beneficial effect observed in the combination of epi-drugs and anti-PD-1 [169]. Recently, a Bayesian network meta-analysis revealed that PD-1/PD-L1 inhibitors were more efficacious than control treatment in patients with solid tumors, including non-small cell lung cancer (NSCLC) [170]. However, it is unclear whether the combined use of PD-1/PD-L1 inhibitor and HMA would benefit NSCLC patients [171]. In a phase II trial (NCT02546986), 100 patients with a previous line of platinum-based therapy were assigned to receive pembrolizumab combined with either oral AZA or placebo, and PFS was not improved (median, 2.9 and 4.0 months) [172]. We recently reported an unexpectedly good outcome for 3 advanced NSCLC patients carrying unfavorable ICI biomarkers, such as low tumor mutation load, low microsatellite instability and HLA loss of heterozygosity [173]. Surprisingly, all 3 patients responded well to low-dose DAC in combination with camrelizumab, with mild AEs, suggesting that low-dose DAC sensitized PD-1/PD-L1 inhibitors in NSCLC. High expression of the enzyme cytidine deaminase (CDA) that catabolizes DAC within minutes was reported in NSCLC [174]. The combination of HMA with tetrahydrouridine (THU), a CDA inhibitor, was found to cause > 2-fold DNMT1 depletion and > 5-fold increase in TILs, as well as destruction of NSCLC in vivo. Based on these data, the PRECISE trial (NCT02664181) has been conducted to compare the efficacy of nivolumab alone or in combination with THU and DAC in patients with R/R NSCLC [175]. To investigate the effect of guadecitabine, a second-generation DNA methylation inhibitor, on solid cancers, a phase II dose-escalation trial (NCT02998567) was initiated [176]. Overall, 34 patients were enrolled, of whom 10 of 15 patients with NSCLC (13 patients were resistant/refractory to PD-1/PD-L1 targeting agents) were evaluable, with a DCR of 80% and 5 patients having DCR > 6 months.
Recent clinical trials suggest that the combination of anti-PD-1/PD-L1 and HDACi is a promising option for treating NSCLC patients. A phase Ib study (NCT02635061) assessed the clinical efficacy of ACY-241, a selective HDAC6 inhibitor, plus nivolumab on metastatic NSCLC [177]. Eighteen patients received the treatment. Eight of 13 evaluable patients showed clinical benefit, including 1 CR, 4 PRs and 3 SDs. Immune cell profiling revealed a trend of increased infiltrating cytotoxic T and NK cells following the combined treatment [177]. Two clinical trials (NCT01928576 and NCT02437136) are evaluating the combination of pembrolizumab with HDACis such as vorinostat and ENT on anti-PD-1/PD-L1-naïve or refractory NSCLCs [178]. In a phase I/Ib trial, 33 patients were given pembrolizumab plus vorinostat [179]. Among 30 evaluable patients, 20 had SD or PR. In the subsequent open-label, phase II randomized trial (NCT02638090), patients with metastatic NSCLC were randomized to receive pembrolizumab alone (arm A) or pembrolizumab plus vorinostat (arm B) [180]. Among 47 of 49 patients evaluable for response, the ORR of patients with low pre-treatment TIL count (score = 1) in arm B (66.7%) was obviously higher than that in arm A (33.3%), suggesting that the combination strategy may favor NSCLC patients with a low-TIL count.
3.2.3 Metastatic melanoma
ICIs, including nivolumab, pembrolizumab and ipilimumab, have significantly improved the clinical outcomes of metastatic melanoma and are now in routine use [181-183]. However, more than half of patients experience either primary or acquired resistance [184]. To date, some preclinical studies have shown the therapeutic value of CTLA4 inhibitor combined with HMA [185]. In a phase Ib NIBIT-M4 study (NCT02608437), guadecitabine combined with ipilimumab resulted in immune-related DCR of 42% and ORR of 26% [186]. The interim results of a phase II study (NCT02816021) reported that, in anti-PD-1-naive patients with metastatic melanoma, oral AZA plus pembrolizumab led to a PR 55%, while the anti-PD-1 pretreated patients did not show any response [187].
Epi-immunotherapies using HDACi also have been investigated in metastatic melanoma patients. The ENCORE-601 (NCT02437136), an open-label study, enrolled melanoma patients in which 70% had prior pembrolizumab treatment [188, 189]. With ENT plus pembrolizumab, 10 of 53 patients achieved CR or PR (ORR = 19%). Efficacy results in patients receiving prior PD-1 therapy were consistent with the overall population. Preliminary biomarker analysis suggests that the addition of ENT restores inflammation in the TME necessary for successful re-treatment with anti-PD-1/PD-L1 [188, 189]. An early clinical study (NCT02032810) determined the safety and efficacy of panobinostat, a pan inhibitor of class I, II, and IV HDAC, combined with ipilimumab in advanced melanoma [190]. However, the results with this combination treatment were disappointing. Mocetinostat, an investigational class I and IV HDACi, has demonstrated anticancer activity in patients with hematological malignancies and solid tumor [191]. Preclinical studies showed that mocetinostat promotes the accumulation of central memory CD8 and CD4 T cells and inhibits Treg cell and MDSC functions [192]. In the phase Ib trial (NCT03565406), the efficacy of mocetinostat in combination with ipilimumab was evaluated in 10 patients with unresectable melanoma [193]. At a median follow-up of 16 months, the ORR was 70%, including 2 CRs and 5 PRs; 7 patients developed grade 3-4 immune-related AEs.
3.2.4 Renal and bladder carcinoma
Preclinical and clinical studies have provided a rationale for PD-1, PD-L1 or CTLA4 blockade for the treatment of renal cell carcinoma [194]. Recently, several studies had evaluated the safety and efficacy of epi-immunotherapies using HMA, HDACi, and EZH2 inhibitors. In a phase Ib/II study (NCT03308396), 42 patients with advanced renal cancer were treated with durvalumab and guadecitabine [195]. At a median follow-up of 20.1 months, 66% of patients achieved clinical benefit defined as either PR of SD that lasted ≥6 months. Mechanistically, decreased Tregs and MDSCs might be associated with favorable outcomes, and increased Th17 subpopulations of T cells were associated with immune-related AEs [195]. The results from another phase Ib study showed that vorinostat and pembrolizumab combination was tolerable and active in a subset of ICI-resistant urothelial and renal carcinoma patients [196]. A phase I/II study with pembrolizumab in combination with tazemetostat is ongoing (NCT03854474).
3.2.5 Epithelial ovarian cancer and cervical cancer
To date, most ICI-containing clinical trials for advanced recurrent ovarian cancer (OC) have focused on anti-PD-1/PD-L1 therapy, but the results were disappointing [197]. The combination of ICI with HMA is being explored. In a phase II trial (NCT02901899), guadecitabine plus pembrolizumab brought clinical benefit in 27% of patients with R/R OC. Epic arrays were used to measure global tumor methylation, and showed 0.05% CpG sites being differentially methylated after the treatment [198]. The combination of oral AZA and durvalumab produced a median PFS of 1.9 months and a median OS of 5 months in 28 anti-PD-1-naive patients with platinum-resistant OC in the open-label, phase II multicohort study (NCT02811497) [163]. The biomarker studies did not detect any significant tumor DNA hypomethylation. A phase II randomized study (NCT02915523) recruited 126 advanced OC patients who received avelumab plus either ENT or placebo but found no significant difference in ORR (6% vs. 5%) or OS (NE vs. 11.3 months) [199]. Toripalimab, a humanized PD-1 antibody, in combination with chidamide is being explored in a phase Ib/II, single-arm, multi-center study (NCT04651127), in patients with R/R metastatic cervical cancer.
3.2.6 Colorectal cancer
A recent real-world analysis showed that the earlier use of ICIs resulted in better tumor response in a subset of CRC patients [200]. Since either DMNTi or HDACi combined with ICIs markedly improved treatment outcomes in CRC-bearing mice and DAC-based TME reprogramming could enhance the effect of the PD-1 blockade on CRC with microsatellite stability (MSS) [40], epi-immunotherapies also have been evaluated in a few clinical settings for CRC. Unfortunately, PD-1 blockade (pembrolizumab) combined with AZA appears to have modest activity against metastatic CRC with MSS [201].
3.2.7 Head and neck squamous cell carcinoma
In a phase II trial (NCT02538510), the combination of pembrolizumab and vorinostat was evaluated for recurrent or metastatic head and neck squamous cell carcinoma (HNSCC) and salivary gland cancer (SGC) [202]. Among 25 patients with HNSCC, 8 (32%) achieved PR, and 5 (20%) had SD; the median OS was 12.6 months, and the median PFS was 4.5 months. Four (16%) of 25 patients with SGC had PR and 14 (56%) had SD; the median OS was 14 months and median PFS was 6.9 months [202]. In addition, a phase Ib study (NCT03019003) is investigating the safety and efficacy of oral DAC (ASTX727) combined with durvalumab in recurrent or metastatic HNSCC patients who were resistant to ICI monotherapy.
4 ADVANCES OF EPI-CELL THERAPY FOR SOLID AND HEMATOLOGICAL CANCERS
It has become clear that epigenetic modulation can enhance CAR-T cell persistence and function as well as trafficking within an immunosuppressive TME [203, 204]. In an attempt to decipher the mechanisms underlying a delayed exceptional response to CD19 CAR-T therapy, Fraietta et al. [205] suggested that TET2-disrupted CAR-T cells can acquire a central memory phenotype through altered T cell differentiation as a result of epigenetic modulation. Recently, Weber et al. [206] demonstrated that transient inhibition of CAR signaling, but not immune checkpoint blockade, can restore the functionality of exhausted human CAR-T cells and argued against the concept of the “epigenetically fixed state” in CAR-T cell exhaustion. Collectively, these studies highlight epigenetic reprogramming as a potential strategy to improve CAR-T efficacy.
Although clinical trials of CAR-T therapies targeting CD33, CD123, NKG2D, C-type lectin-like molecule-1 (CLL1), CD70 and fms-like tyrosine kinase 3 (FLT3) for AML are growing, the data on myeloid malignancies remain very limited [143, 150, 207]. We demonstrated that antitumor functions of CD123-specific CAR-T cells are significantly enhanced by low-dose DAC [42]. NKR-2 are CAR-T cells targeting NKG2D ligands [208]. In a phase I clinical trial (NCT02203825), the safety and feasibility of NKR2 in patients with AML, MDS, and multiple myeloma were evaluated [208]. Moreover, EPITHINK, an open-label phase I study (NCT03612739), was designed to investigate the combination therapy of NKR2 and AZA for newly-diagnosed AML/MDS patients ineligible for intensive chemotherapy or transplantation. However, this study was withdrawn by the investigator.
Zebley et al. [209] performed DNA methylation profiling using serial clinical samples from patients with acute lymphoblastic leukemia (ALL) and found that post-infusion CD8 and CD19 CAR-T cells undergo DNA methylation reprogramming that drives cell differentiation toward exhaustion. It was also demonstrated that low-dose DAC-treated CD19 CAR-T cells have stronger anti-lymphoma, proliferation and cytokine-releasing capacities, which is associated with less exhaustion and maintenance of memory phenotype and effector function [43]. Consistent with this result, Li et al. [210] suggested that DAC-primed CD19 CAR-T cells are able to kill more lymphoma cells, and DAC followed by CAR-T cell infusion led to CR in 2 patients with refractory lymphoma. Despite high response rates in R/R B-ALL patients, CD19 CAR-T cells showed limited efficacy in R/R ALL patients with p53-mutation/deletion [211]. Interestingly, Qu et al. [212] reported that ALL patients with p53 alternations achieved and maintained CR after treatment with CAR-T cells and DAC, indicating that DAC application may improve the outcome of CAR-T cell-treated patients.
In addition to CAR-T therapy, the efficacy of DAC combined with other cell therapies also has been evaluated. The studies in patients with solid tumors showed that low-dose DAC combined with cytokine-induced killer (CIK) cells achieved significant clinical benefits [213, 214]. Some clinical studies suggested that AZA and donor lymphocyte infusions (DLIs) can induce remission in post-SCT relapsed myeloid cancers [215, 216]. Sommer et al. [217] investigated DAC in combination with DLIs in 26 patients with relapsed AML, MDS, or MPN after allogeneic SCT. Eighteen patients received DAC-primed DLIs, while 8 were on DAC only. The rates of acute and chronic graft versus host disease were 17% and 6%, respectively. CR/CRi was observed in 15%, PR in 4%, and SD in 58% of patients. Median OS was 4.7 months. PD-L1 expression appears irrelevant to the response, implying that the efficacy of DAC plus DIL does not restrict to patients with low leukemic burden [217]. Qian et al. [218] reported that 2 patients with relapsed AML post allogeneic SCT achieved durable CRs after combined treatment with anti-PD-1, AZA and low-dose DLI, and no severe immune-related AEs or graft versus host disease (GVHD) developed. The anti-PD-1/HMA/DLI combination possibly enhances the graft-vs-leukemia effect of the host's and infused donor's lymphocytes [218]. By serial analysis of samples following transgenic T-cell receptor (TCR) T cell infusion, Nowicki et al. [219] suggested that rapid loss of surface TCR expression is caused by epigenetic silencing through DNA methylation. Thus, whether HMAs could potentiate the efficacy of TCR-T therapy warrants further study.
5 ADVANCES OF EPI-VACCINES FOR SOLID AND HEMATOLOGICAL CANCERS
Unlike vaccines against infectious diseases, therapeutic cancer vaccines have not shown significant responses and clinical benefits in patients, and currently only 2 US FDA-approved vaccines with modest efficacy are indicated for prostate or bladder cancer [220]. Despite feasibility and tolerability, the combination of ICIs with vaccines has not yet been translated into survival improvement in early-phase clinical trials [221, 222]. NY-ESO-1 is a vaccine target for multiple cancers, but its limited expression is a barrier to cancer vaccine efficacy, although it is expressed at higher levels on cancer cells than on normal cells [223]. Preclinical studies demonstrated that DAC could enhance both NY-ESO-1 expression in cancer cells and NY-ESO-1-specific CTL-mediated responses in vitro [224]. The subsequent phase I trial (NCT00887796) showed increased NY-ESO-1 antibodies and T-cell responses, which contribute to disease stabilization or clinical PR in 6 of 10 evaluable patients with relapsed OC [224]. In a randomized phase I/IIb trial (NCT03206047), AEs and best dose of atezolizumab (anti-PD-L1) when given together with guadecitabine and CDX-1401 vaccine (a dendritic cell vaccine against NY-ESO-1) were investigated. A phase I study of guadecitabine in combination with a colon cancer vaccine (GVAX) failed to show any significant immunologic responses in 18 patients with advanced CRC [225].
In a phase I trial enrolling 9 patients with MDS, an HLA-independent NY-ESO-1 vaccine (CDX-1401 plus poly-ICLC adjuvant), when administered in combination with standard decitabine schedules, can induce an NY-ESO-1-specific adaptive immune responses, supporting epigenetic stimulation of vaccine response in myeloid cancers [226]. On the basis of these findings, they are now initiating a second phase I study combining NY-ESO-1 vaccine or DAC with nivolumab for MDS patients (NCT03358719). However, another phase I trial was performed to evaluate the combination of AZA and a multi-peptide therapeutic vaccine targeting NY-ESO-1, MAGE-A3, preferentially expressed antigen in melanoma (PRAME), and Wilms tumor 1 (WT-1). Unfortunately, the trial was terminated due to the lack of clinical benefit despite modest immune response observed in 5 MDS patients [227]. Taken together, epigenetic interventions could theoretically improve therapeutic vaccines, but there is still a long way to achieve clinically meaningful responses [228].
6 PERSPECTIVES AND CHALLENGES
Better understanding the epigenetic determinants of immune response would reveal more potential therapeutic targets. Recently, Griffin et al. [229] found that suppression of SET domain bifurcated 1 (SETDB1), a H3K9 lysine methyltransferase, can enhance antitumor cytotoxic T-cell responses through activation of immunostimulatory genes and presentation of MHC-I peptides as neoantigens, providing a novel epigenetic strategy to improve ICIs’ efficacy. The advent of immunotherapy has significantly revolutionized cancer treatment. Despite encouraging clinical activity in multiple cancer types, especially hematological malignancies, expanding the indications of immunotherapy and overcoming treatment resistance are the major challenges. It is well known that combining epigenetic and immune therapy can overcome tumor resistance and has shown effectiveness in several cancer types. Nowadays, growing clinical trials are currently testing combinations of epi-drugs with immunotherapy, cell therapy, and cancer vaccine, most commonly DNMTi and HDACi. However, several new epi-drugs and immune therapy, such as anti-CTLA4 antibodies, are now being evaluated in the field of epi-immunotherapy. Moreover, novel triplet regimens of synergistic combinations of immunotherapy with epi-drugs are also being investigated in a variety of cancers. In addition, recent preclinical and clinical data have demonstrated that the combination of low-dose DAC or HDACi with ICIs produced compelling antitumor activity in patients with cHL and solid tumors. Based on these exciting findings and because of the complexity of interplay between cancer epigenetics and cancer immunology, the dose, schedule, and combination of epi-immunotherapy should be optimized in the future clinical trials. In addition, the assessment of new regimens in preclinical models may enable rational, hypothesis-driven identification of mechanism-based epi-immunotherapies for clinical testing. Finally, relevant biomarker analysis may shed light on understanding multiple genetic and molecular factors in a longitudinal manner, thereby providing comprehensive and dynamic information regarding response to treatment, and help identify best candidates for epi-immunotherapy.
7 CONCLUSIONS
It is now clear that epigenetic processes play a significant role in regulating immune response against cancer. Numerous preclinical studies have shown that different classes of epi-drugs are able to increase tumor immunogenicity, enhance immune cell functions and modulate immunosuppressive TME, providing strong rationales for cancer epi-immunotherapy. The combination of epi-drugs with ICIs or cell therapy has led to improved efficacies in several clinical trials, especially for hematological malignancies. However, this field still faces many challenges, such as poor response seen in solid cancer, treatment resistance and limited use of vaccine or cell therapy. We anticipate that the development of next-generation epi-drugs, incorporation of appropriate biomarker and optimized treatment strategy will provide further insight and opportunities for epi-immunotherapy.
DECLARATIONS
ACKNOWLEDGMENTS
We thank Dr. Zhaoxing Wu for technical assistance in figure preparation. This work was supported by the funds from the National Natural Science Foundation of China (No. 81830006, 82170219, 81830004, and 81800188), the Science Technology Department of Zhejiang Province (No. 2021C03117), and the Natural Science Foundation of Zhejiang Province of China (LY21H080005).
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
Wenbin Qian, Yang Xu, Weidong Han and Aibin Liang proposed the concepts; Aibin Liang and Weidong Han provided valuable suggestions; Wenbin Qian, Yang Xu, Ping Li, Yang Liu and Wen Lei drafted the manuscript; Yang Xu and Dijia Xin prepared the tables and figures; Yang Xu, Ping Li, Yang Liu, Wenbin Qian and Dijia Xin made the revision; all authors read and approved the final manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.




