Fascin lysine 471 acetylation cooperates with serine 39 phosphorylation to inhibit actin-bundling activity and tumor metastasis in esophageal squamous cell carcinoma
Fa-Min Zeng and Yin-Wei Cheng contributed equally to this work.
List of abbreviations
-
- A
-
- alanine
-
- AcK471-Fascin
-
- Fascin-K471 acetylation
-
- CK
-
- cytokeratin
-
- D
-
- aspartic acid
-
- DFS
-
- disease-free survival
-
- ESCC
-
- esophageal squamous cell carcinoma
-
- FascinS39A/K471Q
-
- a mimic of phosphorylation defective and hyperacetylated Fascin
-
- FascinS39A/K471R
-
- a mimic of phosphorylation and acetylation defective Fascin
-
- FascinS39D/K471Q
-
- a mimic of hyperphosphorylation and hyperacetylated Fascin
-
- FascinS39D/K471R
-
- a mimic of hyperphosphorylation and acetylation defective Fascin
-
- FRAP
-
- fluorescence recovery after photobleaching
-
- G/F-actin ratio
-
- ratio of G-actin to F-actin
-
- GFP
-
- Green fluorescent protein
-
- H&E
-
- hematoxylin and eosin
-
- IHC
-
- immunohistochemistry
-
- Lys-471
-
- lysine 471
-
- OS
-
- overall survival
-
- P
-
- pellet
-
- PCAF
-
- P300/CBP-associated factor
-
- PhoS39-Fascin
-
- Fascin-S39 phosphorylation
-
- PKC
-
- protein kinase C
-
- PTMs
-
- posttranslational modifications
-
- S
-
- supernatant
-
- Ser-39
-
- Serine 39
-
- SHEEC
-
- Shantou Human Embryonic Esophageal Carcinoma
-
- Thr-403
-
- Threonine 403
-
- TMAs
-
- tissue microarrays
-
- TNM
-
- tumor-node-metastasis
-
- WT
-
- wild-type
Fascin actin-bundling protein 1 (Fascin, gene name: FSCN1) is the primary actin-bundling protein that clusters filaments into stable and bundled filopodia [1]. In previous studies, elevated levels of Fascin promoted filopodia formation and enhanced the invasive ability of tumor cells, suggesting that Fascin is a target for therapeutic intervention [2, 3]. Therefore, it is necessary to elucidate the detailed molecular mechanism by which Fascin bundles F-actin filaments.
Rapid association/dissociation of Fascin from filaments is regulated by its post-translational modifications, such as the widely studied phosphorylation of conserved Ser-39 mediated by protein kinase C (PKC) [4]. The phosphorylation/dephosphorylation cycles of Ser-39 serve as a molecular switch to turn Fascin activity on or off to determine filopodia formation [5]. We previously reported that Fascin was acetylated by P300/CBP-associated factor (PCAF) on Lys-471 [6]. We further elaborated on the precise regulatory mechanism of Fascin acetylation to inhibit its oncogenic function by reducing actin-bundling activity in esophageal squamous cell carcinoma (ESCC) cells, highlighting the potential therapeutic value of mediating Fascin acetylation in tumor metastasis [6]. The phosphorylation of Ser-39 at the Fascin N-terminus and acetylation of Lys-471 at the Fascin C-terminus were inhibited Fasin F-actin bundling activity. However, whether Lys-471 acetylation cooperates with Ser-39 phosphorylation to regulate Fascin function is still unknown.The purpose of this study was to explore the role of Fascin Ser-39 phosphorylation and Lys-471 acetylation in ESCC cell function and filopodia formation.
In this study, we engineered plasmids encoding double point mutations that mimic all four possible modification states, FascinS39A/K471R (S39A/K471R, a phosphorylation- and acetylation-defective Fascin mutant), FascinS39A/K471Q (S39A/K471Q, a phosphorylation-defective and constitutively acetylated Fascin mutant), FascinS39D/K471R (S39D/K471R, a constitutively phosphorylated and acetylation-defective Fascin mutant), and FascinS39D/K471Q (S39D/K471Q, a constitutively phosphorylated and constitutively acetylated Fascin mutant), and expressed them individually in endogenous FSCN1-silenced cells (Figure 1A). The effect of silencing endogenous Fascin could be rescued by exogenous wild-type (WT) Fascin. Cells expressing FascinS39A/K471R exhibited stronger oncogenic abilities than those expressing the WT form. In contrast, the expression of FascinS39A/K471Q or FascinS39D/K471Q produced the opposite effect, that is, it further suppressed cell migration (Figure 1B) and cell proliferation (Supplementary Figure S1A) in FSCN1-silenced cells. These data showed that cell oncogenic abilities could be enhanced or rescued by FascinS39A/K471R or FascinS39D/K471R, while could not be rescued by FascinS39A/K471Q or FascinS39D/K471Q, suggesting a functional interplay between phosphorylation of Ser-39 and acetylation of Lys-471. The in vivo tumor growth of cells expressing FascinS39A/K471Q and FascinS39D/K471Q was significantly inhibited (Supplementary Figure S1B and Figure 1C), whereas the deacetylated mutants (S39A/K471R and S39D/K471R) displayed dramatically increased tumor growth (Figure 1C). These data are consistent with the incidence of lymph node metastasis in the different groups (Figure 1D), highlighting only dephosphorylated and deacetylated (S39A/K471R) Fascin to be a more prominent player. Any post-translational modification at either site inhibited the ability of Fascin to promote tumor growth.
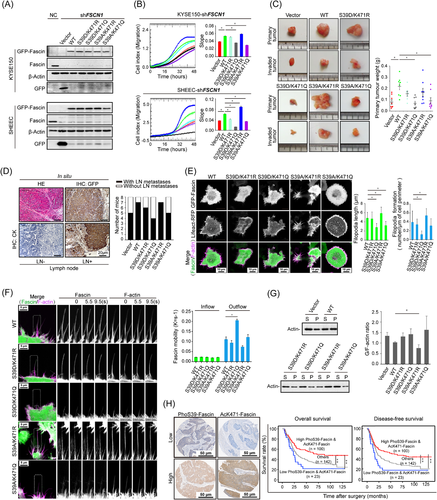
Next, we investigated the functions of these two post-translational modifications in filopodia formation by performing live imaging of cells co-expressing an F-actin reporter gene (Lifeact-RFP) and GFP-tagged WT or double-mutant Fascin. First, the expression of FascinS39D/K471Q significantly reduced filopodial density and length in both KYSE150 (Figure 1E and Supplementary Video S1) and SHEEC (Supplementary Figure S2A) cells. In FSCN1-silenced cells, the double-defective mutant (S39A/K471R), but not the other mutants, rescued filopodia formation, with elongated and overabundant filopodia being evident (Figure 1E and Supplementary Video S1). These data suggested that either Lys-471 acetylation or Ser-39 phosphorylation was sufficient for initiating filopodia formation, while maximal formation of filopodia requires the absence of modifications at both Lys-471 and Ser-39 residues. We further analyzed the dynamics of the filopodia extension-retraction cycle in FSCN1-silenced cells following transfection with the double-mutant-expressing plasmid. Time-lapse imaging showed that WT Fascin, but not FascinS39D/K471Q and FascinS39A/K471Q, could restore filopodia lifetime (Supplementary Figure S2B). Moreover, expression of the deacetylated and dephosphorylated mutant (S39A/K471R) led to long, persistent filopodia with an increased lifetime (Supplementary Figure S2B). Together, these data showed that filopodia assembled in cells with acetylated Fascin were significantly shorter, less stable, and collapse rapidly. In contrast, deacetylated Fascin enhanced filopodia stability and length, which were further enhanced by dephosphorylation of Ser-39 and reduced by phosphorylation of Ser-39.
Our previous results showed that Lys-471 acetylation increases the mobility of Fascin molecules compared to the WT form [6]. Similarly, rapid mobility was observed in KYSE150 (Figure 1F) and SHEEC (Supplementary Figure S2C) cells expressing FascinS39D/K471Q. However, for the mutant Fascin forms with at least one deactivating modification (S39A/K471Q, S39D/K471R, and S39A/K471R), Fascin mobility was reduced, particularly for the mutant with both sites deactivated (S39A/K471R), where mobility was completely abolished (Figure 1F and Supplementary Figure S2C). Previous studies have suggested that monomeric proteins move more rapidly than proteins with short transient interactions, whereas proteins with long transient interactions move the slowest [7]. Thus, our data indicated that at the base of the filopodium, Lys-471 acetylation and Ser-39 phosphorylation cause the majority of Fascin molecules to exist as monomers with fast dynamics due to reduced actin binding. We next explored whether the modification of one amino acid affected the actin bundling of the other. Expectedly, the phosphorylation and dephosphorylation of Ser-39 have previously been shown to decrease and enhance, respectively, the actin-bundling properties of Fascin [4]. Consistent with our earlier findings, expression of only WT and FascinS39A/K471R restored the level of F-actin in KYSE150 and SHEEC cells, as shown by the lower ratio of G/F-actin (Figure 1G and Supplementary Figure S3). In contrast, the other three mutants failed to rescue F-actin levels, but instead increased the G/F-actin ratio compared with that of WT Fascin (Figure 1G and Supplementary Figure S3). These data indicate that both Ser-39 and Lys-471 contribute to the polymerization of F-actin, and phosphorylation of Fascin Ser-39 plays a more important role than the acetylation of Fascin Lys-471 in inhibiting actin-bundling activity.
To assess the clinical significance of the combination of Fascin Ser-39 phosphorylation and Fascin Lys-471 acetylation, we performed immunostaining with phosphorylation- and acetylation-specific antibodies on ESCC samples from the same cohort of patients in our previous work (Figure 1H) [6]. Importantly, Kaplan-Meier survival analysis demonstrated that high levels of both Fascin phosphorylation at the Ser-39 site and acetylation at the Lys-471 site were significantly associated with favorable overall survival (OS; P = 0.006) and disease-free survival (DFS; P = 0.003) compared with the low-level group (Figure 1H).
Here, both in vitro and in vivo biological assays showed that the cellular functions of Fascin are regulated by Lys-471 acetylation in cooperation with Ser-39 phosphorylation, suggesting a functional interplay between these two post-translational modifications. How Ser-39 phosphorylation and Lys-471 acetylation interact requires further exploration. Simultaneously targeting the modifying enzymes that regulate Ser-39 phosphorylation and Lys-471 acetylation could be a good strategy for treating esophageal cancer metastasis. Recently, we identified a novel site, Fascin Thr-403, that could regulate ESCC behavior and actin-bundling activity of Fascin through phosphorylation by AKT serine/threonine kinase 2 [8], demonstrating coordinated regulation of actin-bundling activity among Fascin's series of posttranslational modifications in order to rapidly respond to both internal and external cues. Moreover, Fascin likely regulates nuclear actin and nucleolar morphology by binding to the linker of the nucleoskeleton and cytoskeleton complex [9], and also mediates the remodeling of mitochondrial actin filaments to control mitochondrial oxidative phosphorylation [10]. Further research is needed to investigate the role of Fascin's posttranslational modifications in the nucleus and mitochondria.
DECLARATIONS
ACKNOWLEDGMENTS
We thank Dr. Brian Stramer (King's College London, England, UK) for providing the Lifeact-RFP construct, and Dr. Stanley Lin (Department of Cell Biology and Genetics of Shantou University Medical College) for assistance in revising the manuscript.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
Fa-Min Zeng, Yin-Wei Cheng, and Jian-Zhong He designed the study. Fa-Min Zeng, Yin-Wei Cheng, Jian-Zhong He, and Xiu-E Xu conducted the experiments and analyzed the data. Fa-Min Zeng, Yin-Wei Cheng, Jian-Zhong He, Lian-Di Liao, and Xiu-E Xu acquired and analyzed the data. Fa-Min Zeng and Yin-Wei Cheng wrote the manuscript. En-Min Li and Li-Yan Xu obtained the funding to support the project and supervised the development of this work. All authors read and approved the final manuscript.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This work was approved by the ethical committee of the Central Hospital of Shantou City and the ethical committee of the Medical College of Shantou University, and only resected samples from surgical patients providing written informed consent were included in this study. All animal experiments were conducted with the approval of the Institutional Animal Care and Use Committee of Shantou University.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work was supported by the grants from the National Natural Science Foundation of China (81872372), the Guangdong Basic and Applied Basic Research Foundation (2020A1515011532), the Natural Science Foundation of China-Guangdong Joint Fund (U0932001), the National Cohort of Esophageal Cancer of China (2016YFC0901400), the China Postdoctoral Science Foundation (2018M643134), and 2020 Li Ka Shing Foundation Cross-Disciplinary Research Grant (2020LKSFG07B).




