Colorectal cancer risk following appendectomy: a pooled analysis of three large prospective cohort studies
Disclaimer: Where authors are identified as personnel of the International Agency for Research on Cancer / World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer / World Health Organization.
Abbreviations
-
- HR
-
- hazard ratio
-
- CI
-
- confidence interval
-
- EPIC
-
- European Investigation into Cancer and Nutrition
Dear Editor,
Appendectomy is usually performed to treat acute appendicitis although it may also be conducted in the absence of appendicitis when patients suffer from chronic abdominal pain. Resection is also known to mitigate the risk of ulcerative colitis [1], a risk factor for colorectal cancer. The impact of appendectomy on colorectal cancer risk, particularly in the long term, is unclear. The procedure was initially hypothesized to increase colorectal cancer risk due to decreased immunocompetency. However, recent studies conducted on clinical databases have found little evidence of an association between prior appendectomy and colorectal cancer risk, other than a short-term increase in risk following the procedure in older individuals [2-5]. These studies had various limitations. Firstly, they relied on matched cohorts of appendectomy and non-appendectomy participants, which may have resulted in biases, for example from precautionary removal of the appendix following other abdominal complaints. Secondly, appendectomy data may have been inaccurate due to incomplete medical histories, particularly the omission of appendectomies performed at younger ages. Thirdly, some studies were able to crudely adjust for known colorectal cancer risk factors such as body size, alcohol intake, and smoking status, but not potential confounders such as colorectal cancer screening and family history. In this study, we aimed to address these limitations by conducting a pooled study of over 590,000 participants from three large European prospective studies, the UK Biobank, the European Investigation into Cancer and Nutrition (EPIC), and the French E3N cohort. The large number of incident colorectal cancer cases and high-quality clinical, lifestyle and other epidemiological data collected in these well-characterized cohorts allowed us to robustly examine the association between appendectomy and colorectal cancer overall and across tumor anatomical subsites.
The UK Biobank cohort contributed 472,321 participants, of whom 55,075 (11.7%) had previously undergone appendectomy (Supplementary Materials). A total of 28,929 participants were included from the EPIC study, and 6,262 (21.6%) had reported prior appendectomy. E3N contributed 90,567 participants, of whom 20,418 (22.5%) had reported an appendectomy (Supplementary Table S1). A total of 591,817 participants were therefore included in the pooled study. The median follow-up durations starting from year of reported appendectomy to the end of follow-up were 47 years (range, 0-76 years), 51 years (range, 0-87 years), and 50 years (range, 0-84 years), respectively, for the three cohorts and did not differ appreciably between colorectal cancer cases and non-cases.
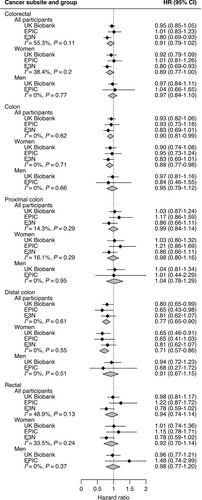
In the UK Biobank, EPIC, and E3N cohorts, 3,171, 576, and 1,080 incident cases of colorectal cancer were recorded during median follow-up times of 7.1 years (range, 0.0-10.0 years), 15.2 years (range, 0.0-18.9 years) and 24.3 years (range, 0.0-25.8 years), respectively. Overall associations between prior appendectomy and colorectal cancer were similar in the three cohort studies, and multivariable adjustment for known colorectal cancer risk factors did not impact the associations (Supplementary Table S2). In the pooled analysis, appendectomy was not associated with lower overall colorectal cancer risk, despite a 9% reduction in risk [hazard ratio (HR) = 0.91, 95% confidence interval (CI) = 0.79-1.02] (Figure 1). Appendectomy was associated with lower colorectal cancer risk in women (pooled HR = 0.89, 95% CI = 0.77-1.00) but not in men (pooled HR = 0.97, 95% CI = 0.84-1.10), and no heterogeneity by sex was detected (I2 = 0, P = 0.35). However, appendectomy was associated with a lower risk of colon cancer overall (pooled HR = 0.90, 95% CI = 0.81-0.99) and distal colon cancer (pooled HR = 0.77, 95% CI = 0.65-0.90), but not with proximal colon cancer (pooled HR = 0.99, 95% CI = 0.84-1.14) or rectal cancer (pooled HR = 0.94, 95% CI = 0.74-1.14). Heterogeneity of association was detected for proximal versus distal colon tumors (I2 = 76.2%, P = 0.04), but not for colon versus rectal tumors (I2 = 0, P = 0.61). These associations have not been published previously.
Associations by age at appendectomy (< 20 or ≥ 20 years) remained similarly unchanged after multivariable adjustments in individual cohorts (Supplementary Table S3). In pooled analyses, prior appendectomy tended to be associated with a lower risk of cancer at most colorectal subsites, although these generally did not meet the threshold for statistical significance (Supplementary Figure S1). Little evidence of association between appendectomy and overall colorectal cancer risk by age at the procedure was found, with no heterogeneity detected (I2 = 0). Likewise, for proximal and distal colon cancer, associations with appendectomy did not differ significantly between the two age groups (I2 = 0, P = 0.81 and I2 = 40.0%, P = 0.20 for the two subsites, respectively).
In a sensitivity analysis using data from the UK Biobank only, the exclusion of participants who had previously reported a diagnosis of ulcerative colitis did not substantially change the appendectomy and colorectal cancer association (Supplementary Table S4). Likewise, the exclusion of participants that had reported previous irritable bowel syndrome, endometriosis, gallstones or gastro-esophageal reflux disease yielded unchanged risk estimates. The exclusion of ulcerative colitis cases from E3N did not appreciably change colorectal cancer-appendectomy associations, nor did the inclusion of all reported appendicitis cases as additional instances of appendectomy (Supplementary Table S5).
To our knowledge, this is currently the first study to observe a reliable inverse association between appendectomy and colon cancer risk. Earlier studies may have included individuals whose appendectomies were related to other intestinal diseases, resulting in a higher short-term risk of colorectal cancer in these groups. In contrast, participants included in the present study were recruited from healthy populations, regardless of prior appendectomy. Appendectomy was thus recorded at all points in the life course prior to the studies’ respective baselines, sometimes early in life. The present study, therefore, leverages a much longer median follow-up from appendectomy (approximately 50 years) than previous studies (< 10 years). This extended follow-up was needed to assess the long-term impact of appendectomy.
Speculations on the mechanisms underlying the observed inverse associations between appendectomy and colon cancer risk could be as follows. First, the appendix is a source of the follicle centers necessary for the development of gut-associated lymphoid tissue, whose absence may limit inflammation and mitigate damaging autoreactivity in the colon [6]. A second possibility is the modification of intestinal microbiota profile and function. The appendix has been described as a “safe house” for commensal bacteria that may be reintroduced into the colon in case of disease [7]. Appendix microbiota exists as biofilms that contact colon epithelial cells and may promote colorectal cancer progression along the predominant adenoma pathway [8]. It also remains to be established why appendectomy was associated with a reduction in overall and distal colon cancer risk but not with the risk of proximal colon or rectal cancer. There is a progressive increase in pH, microbial load, and concentration of microbial metabolites from the proximal colon to the rectum [9], and marked differences exist between distal and proximal tumors in terms of mutation rate and genomic instability [10]. Also, the absence of the appendix and consequent changes in biofilm formation may impact carcinogenesis differentially in the proximal and distal colon. The promotion of carcinogenesis by biofilms has been reported to be common in the proximal but not the distal colon [8].
In conclusion, appendectomy prior to baseline recruitment was robustly and consistently associated with a lower risk of colon cancer, overall and at the distal subsite. Experimental studies are now required to identify which specific physiological changes induced by appendectomy may be beneficial against the development of colon tumorigenesis.
DECLARATIONS
AUTHORS’ CONTRIBUTIONS
N.Murphy and GS, conceptualization of the study; JAR, NM, FA, data analysis; JAR, writing of the manuscript; AF, MC, MCBR, SSMC, MJG, reviewing and editing of the manuscript, and advice on interpretation of results; N.Murphy and GS, study supervision.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The UK Biobank obtained approval from the North-West Multicenter Research Ethics Committee, the National Information Governance Board for Health and Social Care in England and Wales, and the Community Health Index Advisory Group in Scotland. Ethical approval for the EPIC study was obtained from the International Agency for Research on Cancer and the ethical review boards of the participating institutes. The cohort was granted ethical approval by the French National Commission for Computed Data and Individual Freedom (Commission Nationale de l'Informatique et des Libertés). All participants from each of the three cohorts provided written informed consent.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
The French E3N cohort is maintained with the support of the Mutuelle Générale de l'Education Nationale (MGEN), Gustave Roussy and the French League against Cancer (LNCC). E3N-E4N is also supported by the French National Research Agency (ANR) under the Investment for the future Program (PIA) (ANR-10-COHO-0006) and by the French Ministry of Higher Education, Research and Innovation (subsidy #2102 918823). This work has been supported within the context of the ALLERCOLO project funded by Fondation ARC.
CONFLICT OF INTEREST
The authors declare there are no conflicts of interest.
ACKNOWLEDGMENTS
This work was conducted using the UK Biobank Resource under Application Number 25897 and we hereby acknowledge the participants and those involved in building the resource. We also express our gratitude to the Norfolk and Utrecht EPIC centers for the provision of participant data. We thank all women participating in the E3N cohort study run by The French National Institute for Health and Medical Research (Inserm) and acknowledge the continuing support of the MGEN, the Institut Gustave Roussy and the French League Against Cancer (Ligue Contre le Cancer). The work reported in this paper was performed during Agnès Fournier's term as a Visiting Scientist at the International Agency for Research on Cancer.
Open Research
DATA AVAILABILITY STATEMENT
All participant data from this study are available to other researchers. Bonafide researchers can apply to use the UK Biobank dataset by registering and applying at http://ukbiobank.ac.uk/register-apply/. Data from the EPIC and E3N cohorts are available from the authors upon reasonable request, subject to appropriate data sharing agreements. Analysis code and protocols for this study are stored in a centralized online repository and access can be granted by the authors upon request.