United States oncologists’ clinical preferences regarding modes of medicinal cannabis use
ABBREVIATIONS
-
- MC
-
- Medical Cannabis
-
- CI
-
- Confidence Interval;
Almost every state in the United States with a comprehensive medicinal cannabis (MC) law identifies cancer as a qualifying condition for MC use [1, 2]. A growing body of scientific literature indicates that cancer patients use MC for both symptom management (e.g., pain, low appetite, fatigue, mood disturbance) and cancer-directed therapy [3, 4]. While survey research suggests that cannabis is most typically consumed via combustion, cannabis is most often consumed orally among cancer patients [5]. Data from a nationally-representative sample of medical oncologists indicate that 80% of them discuss MC with patients and nearly half recommend cannabis clinically [6]. However, little is known about oncologists’ views on different modes of MC use.
This study, a secondary analysis of the above-mentioned national survey [6], explored oncologists’ preferences regarding different modes (combustion, smoking, vaporization, and ingested) of MC use. We examined provider-level and practice-level predictors of these preferences. We hypothesized that oncologists would favor oral MC use given similar trends among medical providers caring for patients with other serious illnesses [7] and potential stigma in the medical community regarding combustion.
Between November 2016 and January 2017, a nationally-representative sample of board-certified, medical oncologists was surveyed about their views on MC. The corresponding full methodological details are described elsewhere [6]. Briefly, the survey was mailed to a nationally-representative sample [6], MC was defined as non-pharmaceutical cannabis, and the item of interest was presented in a multiple-select response format: “What mode(s) of medical marijuana use do you prefer for your oncology patients?” (possible responses: “smoking,” “vaporizing,” “ingesting orally,” “no preference,” “I don't know,” or “do not support medical marijuana”).
To simplify interpretation, survey participants who “[did] not support medical marijuana use of any sort” were eliminated. Logistic regression was then used to examine continuous independent variables with the categorical dependent variable as oncologists who endorsed any route of use compared to those who selected “don't know/no preference.” The Chi-square test was used to compare remaining independent categorical variables. Specifically, provider-level variables included oncologist's age, sex, yearly income, and years since medical school. We also examined practice-level variables including patient volume, whether oncologists held a medical school appointment, whether they practiced in a state with legal MC, the number of patients for whom they recommended MC or completed MC paperwork, whether they endorsed sufficient knowledge about MC to make clinical recommendations, and whether they believed MC to have antineoplastic effects.
Of the 233 oncologists who responded to the question regarding clinically preferred routes, 15.4% (n = 36) reported that they did not support MC. Of the remaining 197 oncologists, the majority were Caucasian (n = 113, 57.4%) and male (n = 126, 64.0%). The mean age was 52.6 years [standard deviation (SD) = 10.7 years], and the mean duration since medical school graduation was 25.8 years (SD = 11.1 years). Roughly half (54.3%) practiced in a state with MC law. Supplementary Table S1 offers additional demographic characteristics.
In answering the multiple-response format questions, the majority of oncologists favored an oral route of MC use (n = 97; 49.2%), while only 18.3% (n = 36) supported vaporization and 10.2% (n = 20) combustion; 23.8% (n = 47) indicated “no preference” and 21.8% (n = 43) responded “I don't know.” Out of the 197 respondents, over a quarter (31.0%; n = 61) endorsed a single route of use; 18.8% (n = 37) two routes; and 3.0% (n = 6) three routes (Figure 1). Among the 61 participants who support a single route of MC use, the majority (n = 56; 91.8%) favored oral use.
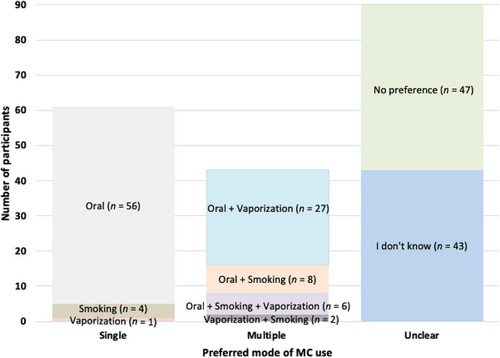
No provider-level variables were related to choosing an oral route for MC. However, regarding practice-level variables, oncologists were more likely to support oral MC use if they reported more frequently “recommend[ing] marijuana use for cancer-related issues” [odds ratio (OR) = 1.03, 95% confidence interval (CI) = 1.01-1.08, P = 0.007), “fill[ing] out paperwork allowing [their patients] to use MC” (OR = 1.08, 95% CI = 1.01-1.15, P = 0.036); or considered themselves to possess “sufficient knowledge about medicinal use of marijuana to make recommendations” (χ2 = 4.53, P = 0.033). Oncologists were also more likely to prescribe oral MC use if they believed MC to have “antineoplastic properties” (OR = 2.70, 95% CI = 1.01-1.15, P = 0.036). These relationships are summarized in Supplementary Table S1.
This study explored the United States oncologists’ clinical preferences regarding modes of MC use for their patients. We found that the oral route was preferred by nearly half (49.2%) of oncologists while nearly as many reported that they had no preference or did not know (45.7%). Far fewer of those surveyed favored vaporization (18.3%) or combustion (10.2%). Provider preference for oral MC use was associated with considering oneself more knowledgeable and clinically experienced regarding MC (e.g., endorsing enough MC knowledge to make recommendations; greater experience in recommending MC), suggesting that, overall, oncologists’ preference for oral MC use may be informed by their clinical familiarity with MC. Finally, oncologists were more likely to select an oral MC use if they believed MC to possess antineoplastic properties.
While the survey was not designed to explore why participants favored one mode of MC use over another, several factors may have contributed to our findings. First, oncologists may have considered oral MC use to be a traditional route of administration for medicinal compounds and, conversely, may have associated combustion or vaporization with recreational drug use. Pediatric oncologists, for instance, similarly favor oral formulations for their patients [8]. Second, oncologists may have associated combustion and vaporization with risks, such as inhalation of carcinogens or risk for fungal infection, respectively. Indeed, oncologic clinical trials on MC since 2000 have relied exclusively on oral administration, despite observational evidence showing that cancer patients turn to a wide variety of other routes of MC use [9]. In turn, the fact that recent oncologic clinical trial evidence has centered on oral MC use may have reinforced this provider preference [10]. Oncologists may have been anecdotally influenced by their patients’ practices as the majority of patients with serious illnesses, including cancer, prefer oral use [4]. Finally, the high percentage of oncologists who did not express a clear preference may be due to the relatively immature clinical trial evidence for MC use or that our survey did not account for the rapid growth of MC use, in the United States or abroad, since the survey's administration.
Limitations of this secondary analysis included that data were cross-sectional and did not account for a rapidly evolving MC landscape. For example, a recent focus on vaporization-related lung injury may dissuade oncologists from approving of this specific route of MC use [11]. Indeed, the answer set that accompanied the route-of-use item in our survey may have not captured other routes of MC use, such as sublingual or topical applications of MC or, potentially, unanticipated ways to use MC (e.g., suppositories). The question also did not allow survey participants to link their mode of user preferences to a particular symptom. Oncologists might prefer that their patients use fast-acting combustion for acute pain but oral administration for cancer-directed therapy. Finally, our survey did not inquire about oncologists’ personal experience with cannabis or their interactions with the MC industry. Nonetheless, our findings were derived from a nationally-representative sample and we acheieved a robust response rate, contributing to the rapidly evolving landscape of MC research.
In a shifting legal and public perception landscape that facilitates increasing use of MC in cancer settings, clinical trials comparing efficacy/risks between modes of MC administration are imperative. However, the current legal climate in the United States has not facilitated such research. Given this reality, our study offers an important glimpse into oncologists’ views on different ways patients use MC and practitioners who consider themselves clinically experienced and knowledgeable regarding MC use, as well as the high percentage of oncologists who consider themselves unable to advise patients on MC use.
DECLARATIONS
AUTHOR CONTRIBUTIONS
TSS: Conception, analysis and interpretation of data; Writing manuscript
MNN: Designing and administering original survey; Organizing data; Conception, analysis and interpretation of data; Writing manuscript
ST: Analysis and interpretation of data; Writing manuscript
PRC: Designing and administering original survey; Organizing data; Conception, analysis and interpretation of data; Writing manuscript
MY: Conception, analysis and interpretation of data; Writing manuscript
DB_J: Designing and administering original survey; Organizing data; Conception, analysis and interpretation of data; Writing manuscript
WP: Conception, analysis and interpretation of data; Writing manuscript
IMB: Designing and administering original survey; Organizing data; Conception, analysis and interpretation of data; Writing manuscript
All authors reviewed and approved the final version of this work and consent for the publication of this article.
ACKNOWLEDGMENTS
This work did not have any associated funding. The authors would like to thank the oncologists who took part in the study. The survey was administered by SK&A Healthcare Databases (http://www.skainfo.com).
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by institutional review boards at the Dana-Farber Cancer Institute and the University of Massachusetts, Boston.
CONFLICT OF INTERESTS
The authors report no conflict of interest.
DATA AVAILABILITY STATEMENT
Data available upon reasonable request to the corresponding author.




