Safety, tolerability, and pharmacokinetics of BAT8001 in patients with HER2-positive breast cancer: An open-label, dose-escalation, phase I study
Abstract
Background
The introductions of anti- human epidermal growth factor receptor-2 (HER2) agents have significantly improved the treatment outcome of patients with HER2-positive breast cancer. BAT8001 is a novel antibody-drug conjugate targeting human epidermal growth factor receptor-2 (HER2)-expressing cells composed of a trastuzumab biosimilar linked to the drug-linker Batansine. This dose-escalation, phase I study was designed to assess the safety, tolerability, pharmacokinetics, and preliminary anti-tumor activity of BAT8001 in patients with HER2-positive locally advanced or metastatic breast cancer.
Methods
This trial was conducted in subjects with histologically confirmed HER2-positive breast cancer (having evaluable lesions and an Eastern Cooperative Oncology Group performance status of 0 or 1) using a 3 + 3 design of escalating BAT8001 doses. Patients received BAT8001 intravenously in a 21-day cycle, with dose escalation in 5 cohorts: 1.2, 2.4, 3.6, 4.8, and 6.0 mg/kg. The primary objective was to evaluate the safety and tolerability of BAT8001. Preliminary activity of BAT8001 was also assessed as a secondary objective.
Results
Between March 2017 to May 2018, 29 HER2-positive breast cancer patients were enrolled. The observed dose-limiting toxicities were grade 4 thrombocytopenia and grade 3 elevated transaminase. The maximum tolerated dose was determined to be 3.6 mg/kg. Grade 3 or greater adverse events (AEs) occurred in 14 (48.3%) of 29 patients, including thrombocytopenia in 12 (41.4%) patients, aspartate aminotransferase increased in 4 (13.8%) patients, γ-glutamyl transferase increased in 2 (6.9%) patients, alanine aminotransferase increased in 2 (6.9%) patients, diarrhea in 2 (6.9%) patients. Objective response was observed in 12 (41.4%; 95% confidence interval [CI] = 23.5%-61.1%) and disease control (including patients achieving objective response and stable disease) was observed in 24 (82.8%; 95% CI = 64.2%-94.2%) patients.
Conclusions
BAT8001 demonstrated favorable safety profiles, with promising anti-tumor activity in patients with HER2-positive locally advanced or metastatic breast cancer. BAT8001 has the potential to provide a new therapeutic option in patients with metastatic HER2-positive breast cancer.
Abbreviations
-
- HER2
-
- human epidermal growth factor receptor-2
-
- ADC
-
- antibody-drug conjugate
-
- IHC
-
- immunohistochemistry
-
- ISH
-
- in situ hybridization
-
- RECIST
-
- response evaluation criteria in solid tumors
-
- PS
-
- performance status
-
- ECOG
-
- Eastern Cooperative Oncology Group
-
- LVEF
-
- left ventricular ejection fraction
-
- CTCAE
-
- Common Terminology Criteria for Adverse Events
-
- DLT
-
- dose-limiting toxicity
-
- MTD
-
- maximum-tolerated dose
-
- AE
-
- adverse event
-
- ECG
-
- electrocardiogram
-
- NCI
-
- National Cancer Institute
-
- TBIL
-
- total bilirubin
-
- PK
-
- pharmacokinetic
-
- PR
-
- partial response
-
- CR
-
- complete response
-
- Cmax
-
- maximum serum concentration
-
- Cmax, ss
-
- maximum serum concentration at steady-state
-
- Cmin, ss
-
- trough plasma concentration at steady-state
-
- Tmax
-
- the time to maximum serum concentration
-
- t1/2
-
- terminal elimination half-life
-
- AUC0-t
-
- area under the concentration-time curve to the last sampling time
-
- AUC0-∞
-
- area under the concentration-time curve to infinity
-
- CL
-
- clearance
-
- Vz
-
- terminal phase distributed volume
-
- Vss
-
- steady-state volume of distribution
-
- DOR
-
- duration of response
-
- PFS
-
- progression-free survival
-
- RP2D
-
- recommended phase 2 dosing
-
- CI
-
- confidence interval
-
- ER+
-
- estrogen-receptor-positive
-
- AST
-
- aspartate aminotransferase
1 INTRODUCTION
The overall percentage of patients with human epidermal growth factor receptor-2 (HER2)-positive breast cancer is approximately 15%–20% [1, 2]. The activation of HER2 promotes cell proliferation, adhesion, invasion, and differentiation, as well as the inhibition of apoptosis through downstream signaling pathways, such as PI3K/AKT/mTOR and RAS/RAF/MAPK [3]. Patients with tumors that have increased HER2 expression (HER2 gene amplification determined by in situ hybridization [ISH] or HER2 protein overexpression assessed by immunohistochemistry [IHC]) usually exhibit an aggressive clinical course and poor survival outcomes [4, 5].
The introductions of anti-HER2 agents, including trastuzumab [6] and other HER2-targeted drugs (e.g. lapatinib [7], neratinib [8], tucatinib [9], pertuzumab [4], ado-trastuzumab emtansine [T-DM1] [10], and trastuzumab deruxtecan [11]) have significantly improved the treatment outcomes of patients with HER2-positive breast cancer. However, continuous administration of these agents is often hampered by toxicities affecting the cardiac [12], gastrointestinal [7, 8] or hematological [13] system. In addition, treatment outcomes are also limited by the development of acquired resistance after initial responses to HER-2 targeted therapies [14, 15]. Thus, continuous development of novel anti-HER2 agent is essential to provide better options for patients who are intolerant or resistant to standard treatment.
The key components of an antibody-drug conjugate (ADC) are cytotoxic agent, linker and monoclonal antibody targeting a tumor-enriched or tumor-specific antigen [16, 17]. T-DM1, an ADC of the HER2 antibody trastuzumab linked to DM1 (a microtubule inhibitor), has been shown to significantly prolong the survival and improve the quality of life of patients with HER2-positive metastatic breast cancer [13, 18]. Based on these results, T-DM1 is recommended as the second-line treatment for HER2-positive metastatic breast cancer patients who have failed at least one trastuzumab-based regimen. In addition, T-DM1 is also approved for the adjuvant treatment in patients with HER2-positive early breast cancer who have residual invasive disease after neoadjuvant taxane and trastuzumab-based treatment in 2019 [19]. Several novel ADCs are currently in clinical development. Harboring different linkers and payloads, some of the ADCs have shown promising results in previous clinical trials [11, 20]. Additionally, trastuzumab deruxtecan is approved for the treatment of previously treated HER2-positive advanced breast cancer based on the efficacy shown in a phase II study.
BAT8001 is a novel ADC that targets HER2-expressing cells and is composed of a trastuzumab biosimilar product (BAT0606) that covalently binds to a drug-linker, batansine (a derivative of maytansine linked to an uncleavable linker by a stable amide bond). BAT8001 contains 6-maleimidocaproic acid (an uncleavable linker), to avoid the release of toxic payload in the blood circulation. Also, the linker and payload of BAT8001 are connected by a stable amide bond to assure its stability. After binding to HER2, the BAT8001-HER2 complex is endocytosed and degraded in lysosomes, resulting in the release of an active metabolite [21, 22]. Preclinical studies suggest that BAT8001 can effectively inhibit the proliferation of HER2-overexpressing cells (SK-BR-3 and BT474) both in vivo and in vitro (data not reported). Additionally, no observable adverse effect level (NOAEL) of BAT8001 in cynomolgus monkey multiple-dose toxicity study was 15 mg/kg, while that of T-DM1 using the same dosing frequency was 10 mg/kg (data not reported). These data indicate that BAT8001 could be used in human trials.
In this first-in-human, phase I, dose-escalation study, we aimed to assess the safety, tolerability, pharmacokinetics, and preliminary anti-tumor activity of BAT8001 in patients with HER2-positive locally advanced or metastatic breast cancer.
2 PATIENTS AND METHODS
2.1 Patients
This open-label, single-center, dose-escalation, phase I study prospectively enrolled patients with advanced or metastatic breast cancer histopathologically or cytologically diagnosed and tested HER2-positive (IHC 3+ and/or ISH +) who sought treatment between March 2017 to May 2018 at the Sun Yat-sen University Cancer Center (Guangzhou, Guangdong, China). The inclusion criteria were as follows: (1) aged 18–70 years old; (2) had at least one measurable tumor according to the response evaluation criteria in solid tumors version 1.1 (RECIST 1.1) [23]; (3) had a performance status (PS) of 0 or 1 on the Eastern Cooperative Oncology Group (ECOG) scale; (4) life expectancy of at least three months; (5) adequate hematological, heart, lung, hepatic and renal function; (6) left ventricular ejection fraction (LVEF) ≥ 50%; (7) cumulative anthracycline dose ≤360 mg/m2 doxorubicin or equivalent. Key exclusion criteria included a medical history of serious lung diseases, cardiac insufficiency (grade ≥3 symptomatic congestive heart failure according to Common Terminology Criteria for Adverse Events [CTCAE] v4.03), myocardial infarction, unstable angina, severe arrhythmia, symptomatic central nervous system, or brain metastases.
This study was registered at ClinicalTrials.gov (NCT04189211) and was performed in accordance with the principles of the Declaration of Helsinki and the Chinese Good Clinical Practice and local legislation. It was approved by the Ethics Committee of Sun Yat-sen University Cancer Center (Approval No.: A2016-052). All patients provided written informed consent to the study.
2.2 Study design and procedures
A “3+3” protocol was used for dose escalation to identify the maximum tolerated dose. BAT8001 was administered by intravenous infusion on day 1, once every 21 days, and treatment cycles were repeated until disease progression or unacceptable toxicity. The initial cohort at dose level of 1.2 mg/kg enrolled 3 patients, with 100% dose escalations to the second cohort, 50% dose escalations to the third cohort, and 33% dose escalations to subsequent dose levels. Doses were escalated up to 6.0 mg/kg. At least 3 patients were evaluated at each dose level. For any cohort, if one patient experienced dose-limiting toxicity (DLT) during the DLT assessment window (21-day period following first dose of BAT8001), that cohort patient number was expanded to up to 6 patients. If 2 or more patients experienced DLT, further enrollment at that dose level and dose escalation were ceased and the previous dose level was considered as the maximum-tolerated dose (MTD).
2.3 Study assessments
Safety evaluations were conducted at each study visit. Safety assessments included documentation of adverse events (AEs), physical examination, vital signs, laboratory test assessments, radiographs, electrocardiogram (ECG) and left ventricular function by echocardiogram, and ECOG PS. Toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) v4.03.
DLT was defined as any of the following treatment-related AE occurring during cycle 1 of each dose level: grade-3 or worse non-hematologic and non-hepatic major organ toxicity (excluding grade 3 diarrhea, nausea, or vomiting in the absence of premedication that responded to therapy); grade ≥4 thrombocytopenia or anemia, or grade ≥4 neutropenia lasting 4 days or associated with fever; LVEF ≤45% and decreased ≥10% from baseline; grade ≥3 cardiac toxicity, new segmental wall-motion abnormalities or cardiac troponin I elevation to 0.2 ng/mL; grade ≥3 elevations in total bilirubin, hepatic transaminases levels; serum aminotransferase >3ULN and total bilirubin (TBIL) >2ULN; patients with baseline grade 2 hepatic transaminase level ≥10×ULN. All safety analyses were descriptive only.
Serum was collected for pharmacokinetic (PK) analysis on day 1–5, 7, 9, 11, 14 and 18 after the first administration of the study drug (cycle 1); day 1 for cycles 2 and 3; and day 1–5, 7, 9, 11, 14, 18 and 21 for cycle 4. Pharmacokinetics of BAT8001, total anti-HER2 antibody, and maytansine derivative (payload) were evaluated using standard noncompartmental methods using WinNonlin version 6.2 (Certara USA Inc; Princeton, NJ, USA).
Tumor response assessments were repeated every two cycles and evaluated as per the Response Evaluation Criteria in Solid Tumors (RECIST v1.1) [23]. Partial response (PR) or complete response (CR) was confirmed on the response 4 weeks after the criteria for response were first met. All patients who received at least one complete cycle (3 weeks) of BAT8001 and had a follow-up assessment were evaluable for tumor response.
2.4 Endpoints
The primary outcomes of this study were to evaluate the safety and the tolerability of intravenous (IV) BAT8001 and to determine the MTD or recommended dose for subsequent clinical trials.
Secondary outcomes included pharmacokinetics and therapeutic efficacy evaluations. The PK parameters included the maximum serum concentration (Cmax) or Cmax at steady-state (Cmax, ss), the trough plasma concentration at steady state (Cmin, ss), the time to Cmax (Tmax), the terminal elimination half-life (t1/2), the area under the concentration-time curve to the last sampling time (AUC0-t), the area under the concentration-time curve to infinity (AUC0-∞), clearance (CL), terminal phase distributed volume (Vz), steady-state volume of distribution (Vss). Anti-tumor efficacy assessment included the proportion of patients achieving an objective response (including CR and PR), duration of response (DOR, measured from the date of first objective response [either complete or partial response] to the first date that progressive disease was documented as per the RECIST v1.1; Any participants who did not progress by the cutoff date were censored at their final tumor assessment date) and progression-free survival (PFS, defined as the time from the date of first dose of study drug to the date of the first objective documentation of disease progression by radiography or death by any cause, whichever occurred sooner).
2.5 Statistical analysis
Total sample size for the dose escalation analysis was not predetermined, and was dependent on practical considerations. For the safety analysis, the evaluable population included patients who received at least one dose of study drug. The pharmacokinetic evaluable population included all patients who received at least one dose of study drug and had at least one posttreatment serum concentration of BAT8001. The efficacy evaluable population included all patients who received at least one dose of study drug and for whom both baseline and posttreatment data for tumor measurement per RECIST were available.
Demographic, safety, and pharmacokinetic data were summarized with descriptive statistics. Point estimates and 95% exact binomial confidence interval (CI) were determined. Kaplan Meier method was used to estimate the median for timetoevent endpoints including duration of response and PFS, with corresponding 95% CIs calculated. For objective responses, the number, proportion, and corresponding two-sided Clopper-Pearson exact 95% CIs were summarized. SAS version 9.3 and higher (SAS Institute, Cary, NC, USA) was used for all statistical analyses.
3 RESULTS
3.1 Patients and treatment
A total of 29 female patients with metastatic breast cancer (median age, 49.0 years; range, 27–65 years) were enrolled. The patients’ demographics and baseline characteristics are summarized in Table 1. All patients had an ECOG PS of 0 (10/29, 34.5%) or 1 (19/29, 65.5%). Most of the patients were heavily pretreated: 19 (65.5%) received 3 or more previous chemotherapy regimens in the metastatic setting. Twenty-two (75.9%) received prior anti-HER2 treatment. Fifteen (51.7%) patients with estrogen-receptor-positive (ER+) breast cancer received prior endocrine therapy before receiving BAT8001 treatment.
| Characteristic | Total (n = 29) | Cohort 1 1.2 mg/kg (n = 3) | Cohort 2 2.4 mg/kg (n = 3) | Cohort 3 3.6 mg/kg (n = 10) | Cohort 4 4.8 mg/kg (n = 7) | Cohort 5 6.0 mg/kg (n = 6) | |
|---|---|---|---|---|---|---|---|
| Age (median [range], years) | 49 (27-65) | 60 (47-65) | 42 (41-65) | 47 (27-61) | 49 (31-64) | 49 (41-57) | |
| ECOG PS (cases [%]) | |||||||
| 0 | 10 (34.5) | 2 (66.7) | 0 (0) | 5 (50.0) | 1 (14.3) | 2 (33.3) | |
| 1 | 19 (65.5) | 1 (33.3) | 3 (100) | 5 (50.0) | 6 (85.7) | 4 (66.7) | |
| ≥2 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| HER2 expression * (detected by IHC; cases [%]) | |||||||
| 2+ | 5 (17.2) | 1 (33.3) | 0 (0) | 1 (10.0) | 3 (42.9) | 0 (0) | |
| 3+ | 24 (82.8) | 2 (66.7) | 3 (100) | 9 (90.0) | 4 (57.1) | 6 (100) | |
| Metastasis (cases [%]) | |||||||
| Lung metastasis | |||||||
| Present | 21 (72.4) | 2 (66.7) | 3 (100) | 9 (90.0) | 2 (28.6) | 5 (83.3) | |
| Absent | 8 (27.5) | 1 (33.3) | 0 (0) | 1 (10.0) | 5 (71.4) | 1 (16.7) | |
| Liver metastasis | |||||||
| Present | 12 (41.3) | 1 (33.3) | 1 (33.3) | 6 (60.0) | 3 (42.9) | 1 (16.7) | |
| Absent | 17 (58.6) | 2 (66.7) | 2 (66.7) | 4 (40.0) | 4 (57.1) | 5 (83.3) | |
| Bone metastasis | |||||||
| Present | 15 (51.7) | 2 (66.7) | 1 (33.3) | 4 (40.0) | 3 (42.9) | 5 (83.3) | |
| Absent | 14 (48.2) | 1 (33.3) | 2 (66.7) | 6 (60.0) | 4 (57.1) | 1 (16.7) | |
| Brain metastasis | |||||||
| Present | 5 (17.2) | 0 (0) | 0 (0) | 3 (30.0) | 0 (0) | 2 (33.3) | |
| Absent | 24 (82.7) | 3 (100) | 3 (100) | 7 (70.0) | 7 (100) | 4 (66.7) | |
| Number of previous chemotherapy regimens (cases [%]) | |||||||
| 1 | 6 (20.7) | 0 (0) | 2 (66.7) | 2 (20.0) | 0 (0) | 2 (33.3) | |
| 2 | 4 (13.8) | 1 (33.3) | 0 (0) | 0 (0) | 1 (14.3) | 2 (33.3) | |
| ≥3 | 19 (65.5) | 2 (66.7) | 1 (33.3) | 8 (80.0) | 6 (85.7) | 2 (33.3) | |
| Prior hormonal therapy (cases [%]) | |||||||
| Yes | 15 (51.7) | 2 (66.7) | 2 (66.7) | 5 (50.0) | 6 (85.7) | 0 (0) | |
| No | 14 (48.3) | 1 (33.3) | 1 (33.3) | 5 (50.0) | 1 (14.3) | 6 (100) | |
| Previous anti-HER2 therapy (cases [%]) | |||||||
| Trastuzumab | |||||||
| Yes | 12 (41.4) | 2 (66.7) | 0 (0) | 3 (30.0) | 3 (42.9) | 5 (83.3) | |
| No | 17 (58.6) | 1 (33.3) | 3 (100) | 7 (70.0) | 4 (57.1) | 1 (16.7%) | |
| Pertuzumab | |||||||
| Yes | 2 (6.9) | 0 (0) | 0 (0) | 1 (10.0) | 1 (14.3) | 0 (0) | |
| No | 27 (93.1) | 3 (100) | 3 (100) | 9 (90.0) | 6 (85.7) | 6 (100) | |
| Previous Radiotherapy (cases [%]) | |||||||
| Yes | 14 (48.3) | 2 (66.7) | 2 (66.7) | 3 (30.0) | 4 (57.1) | 3 (50.0) | |
| No | 15 (51.7) | 1 (33.3) | 1 (33.3) | 7 (70.0) | 3 (42.9) | 3 (50.0) | |
- * All tissue samples tested for IHC 2+ have been verified by ISH to be HER2-positive.
- Abbreviations: ECOG, Eastern Cooperative Oncology Group; PS, performance status; HER2, human epidermal growth factor receptor-2; IHC, immunohistochemical; ISH, in situ hybridization.
3.2 Safety
In the dose-escalation study, 29 patients were evaluable for MTD determination of BAT8001. BAT8001 dose was escalated from 1.2 mg/kg to 6.0 mg/kg (1.2 mg/kg [n = 3], 2.4 mg/kg [n = 3]; 3.6 mg/kg [n = 10]; 4.8 mg/kg, [n = 7]; 6.0 mg/kg [n = 6]). The DLTs observed in cycle 1 were grade-4 thrombocytopenia and grade-3 elevated transaminase. Grade-4 thrombocytopenia occurred in 1 patient who was prescribed with 6.0 mg/kg, 2 patients who was prescribed with 4.8 mg/kg, and 1 patient who was prescribed with 3.6 mg/kg of BAT8001. Grade-3 elevated alanine aminotransferase and aspartate aminotransferase occurred in 1 patient who was prescribed with 6.0 mg/kg. As 2 of 7 patients in the 4.8 mg/kg dose group experienced a DLT, 3.6 mg/kg was determined to be the MTD of BAT8001.All patients had at least one drug-related AE reported in this study. The incidence of AEs is summarized in Table 2. The most frequent drug-related AEs during treatment were increased aspartate aminotransferase (AST; 25/29, 86.2%), thrombocytopenia (24/29, 82.8%), increased alanine aminotransferase (16/29, 55.2%), increased γ-glutamyl transferase (14/29, 48.3%), anemia (13/29, 44.8%), increased alkaline phosphatase (11/29, 37.9%), elevated low-density lipoprotein (11/29, 37.9%), hyperuricemia (10/29, 34.5%), and fever (10/29, 34.5%). Grade 3 or greater AE occurred in 14 (48.3%) patients, including thrombocytopenia in 12 (41.4%) patients, increased AST in 4 (13.8%) patients, increased γ-glutamyl transferase in 2 (6.9%) patients, increased alanine aminotransferase in 2 (6.9%) patients, diarrhea in 2 (6.9%) patients. Transient thrombocytopenia was the only grade 4 BAT8001-related AE (6/29, 20.7%). One patient in the 6.0 mg/kg dose group experienced a severe AE (spinal compression fracture and spinal cord compression) which required treatment interruptions, but were considered not related to the BAT8001 treatment. No patient discontinued the study treatment because of drug-related AEs. No death was reported in this study. Overall, an increased occurrence of grade 3 and grade 4 AEs was observed in higher dose cohorts than in lower doses.
| Cohort 1 1.2 mg/kg (n = 3, cases [%]) | Cohort 2 2.4 mg/kg (n = 3, cases [%]) | Cohort 3 3.6 mg/kg (n = 10, cases [%]) | Cohort 4 4.8 mg/kg (n = 7, cases [%]) | Cohort 5 6.0 mg/kg (n = 6, cases [%]) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BAT8001-related AE | Total (n = 29, cases [%]) | Grade 1-2 | Grade 3 | Grade 4 | Grade 1-2 | Grade 3 | Grade 4 | Grade 1-2 | Grade 3 | Grade 4 | Grade 1-2 | Grade 3 | Grade 4 | Grade 1-2 | Grade 3 | Grade 4 |
| Increased aspartate aminotransferase | 25 (86.2) | 1 (33.3) | 0 (0) | 0 (0) | 3 (100) | 0 (0) | 0 (0) | 7 (70.0) | 2 (20.0) | 0 (0) | 7 (100) | 0 (0) | 0 (0) | 3 (50.0) | 2 (33.3) | 0 (0) |
| Thrombocytopenia | 24 (82.8) | 0 (0) | 0 (0) | 0 (0) | 1 (33.3) | 1 (33.3) | 0 (0) | 6 (60.0) | 1 (10.0) | 2 (20.0) | 3 (42.9) | 2 (28.6) | 2 (28.6) | 2 (33.3) | 2 (33.3) | 2 (33.3) |
| Increased alanine aminotransferase | 16 (55.2) | 0 (0) | 0 (0) | 0 (0) | 3(100) | 0 (0) | 0 (0) | 6 (60.0) | 0 (0) | 0 (0) | 4 (57.1) | 0 (0) | 0 (0) | 1 (16.7) | 2 (33.3) | 0 (0) |
| Increased γ-glutamyl transferase | 14 (48.3) | 0 (0) | 0 (0) | 0 (0) | 2 (66.7) | 0 (0) | 0 (0) | 5 (50.0) | 1 (10.0) | 0 (0) | 2 (28.6) | 0 (0) | 0 (0) | 3 (50.0) | 1 (16.7) | 0 (0) |
| Anemia | 13 (44.8) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 7 (70.0) | 0 (0) | 0 (0) | 2 (28.6) | 0 (0) | 0 (0) | 3 (50.0) | 0 (0) | 0 (0) |
| Increased alkaline phosphatase | 11 (37.9) | 0 (0) | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) | 0 (0) | 4 (40.0) | 0 (0) | 0 (0) | 3 (42.9) | 0 (0) | 0 (0) | 3 (50.0) | 0 (0) | 0 (0) |
| Elevated low-density lipoprotein | 11 (37.9) | 2 (66.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (30.0) | 0 (0) | 0 (0) | 2 (28.6) | 0 (0) | 0 (0) | 4 (66.7) | 0 (0) | 0 (0) |
| Fever | 10 (34.5) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (30.0) | 0 (0) | 0 (0) | 3 (42.9) | 0 (0) | 0 (0) | 3 (50.0) | 0 (0) | 0 (0) |
| Hyperuricemia | 10 (34.5) | 1 (33.3) | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) | 0 (0) | 4 (40.0) | 0 (0) | 0 (0) | 1 (14.3) | 0 (0) | 0 (0) | 3 (50.0) | 0 (0) | 0 (0) |
| Nausea | 7 (24.1) | 2 (66.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (10.0) | 0 (0) | 0 (0) | 1 (14.3) | 0 (0) | 0 (0) | 3 (50.0) | 0 (0) | 0 (0) |
| Anorexia | 6 (20.7) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (30.0) | 0 (0) | 0 (0) | 1 (14.3) | 0 (0) | 0 (0) | 1 (16.7) | 0 (0) | 0 (0) |
| Leucopenia | 5 (17.2) | 1 (33.3) | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (14.3) | 0 (0) | 0 (0) | 2 (33.3) | 0 (0) | 0 (0) |
| Vomiting | 5 (17.2) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (10.0) | 0 (0) | 0 (0) | 1 (14.3) | 0 (0) | 0 (0) | 2 (33.3) | 0 (0) | 0 (0) |
| Hypoalbuminemia | 4 (13.8) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (20.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (16.7) | 0 (0) | 0 (0) |
| Neurotoxicity | 4 (13.8) | 1 (33.3) | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) | 0 (0) | 1 (10.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (16.7) | 0 (0) | 0 (0) |
| Neutropenia | 3 (10.3) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (14.3) | 0 (0) | 0 (0) | 1 (16.7) | 0 (0) | 0 (0) |
| Fatigue | 3 (10.3) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (10.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (16.7) | 0 (0) | 0 (0) |
| Diarrhoea | 3 (10.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (10.0) | 0 (0) | 0 (0) | 1 (14.3) | 0 (0) | 1 (16.7) | 0 (0) | 0 (0) |
| Epistaxis | 3 (10.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (10.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (33.3) | 0 (0) | 0 (0) |
| Rash | 2 (6.9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (20.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Headache | 2 (6.9) | 1 (33.3) | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Increased serum creatinine | 1 (3.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (14.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Peripheral edema | 1 (3.4) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Abdominal pain | 1 (3.4) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Oral ulceration | 1 (3.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (10.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Cough | 1 (3.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (16.7) | 0 (0) | 0 (0) |
| Sore throat | 1 (3.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (10.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Rash itch | 1 (3.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (10.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Alopecia | 1 (3.4) | 0 (0) | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Back pain | 1 (3.4) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Hypertension | 1 (3.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (16.7) | 0 (0) | 0 (0) |
- Abbreviations: AE, adverse event.
3.3 Pharmacokinetics
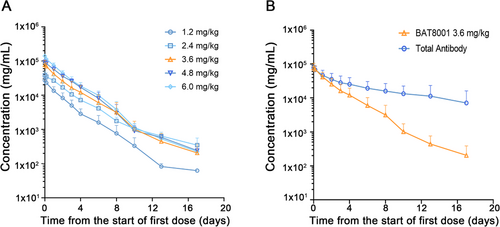
Plasma and serum samples for pharmacokinetic analyses were available for all 29 patients who received BAT8001 doses ranging from 1.2 to 6.0 mg/kg. The serum concentrations of BAT8001 over cycle 1 for the 1.2-, 2.4-, 3.6-, 4.8-, and 6.0-mg/kg treatment groups are shown in Figure 1A. A multi-exponential decline in serum concentrations of BAT8001 was observed, and systemic exposure increased with respect to dose across the 1.2- to 6.0-mg/kg dose range after the cycle 1. The calculated pharmacokinetic parameters for each cohort over cycle 1 are summarized in Table 3. There was no significant accumulation of BAT8001 after IVinjection once every 21 days. Very low concentrations (<1 ng/mL) of free maytansine derivative were detected at all dose levels, and systemic clearance of total anti-HER2 antibody was slower than clearance of BAT8001 (Figure 1B and Supplementary Table S1 and S2). Of these 29 patients, only one had an anti-drug antibody response, with no observed impact on pharmacokinetics of BAT8001.
| Cohort | λZ (1/h) | t1/2 (h) | Tmax (h) | Cmax (ng/mL) | AUC0-t (h×ng/mL) | AUC0-∞ (h×ng/mL) | Vz (mL/kg) | Vss (mL/kg) | CL (mL/h/kg) |
|---|---|---|---|---|---|---|---|---|---|
| Cohort 1 (1.2 mg/kg; n = 3) | 0.0206 ± 0.0038 | 34.40 ± 5.86 | 0.00 ± 0.00 | 27228.77 ± 5960.36 | 1150280.04 ± 426707.90 | 1155573.32 ± 426217.67 | 55.06 ± 14.46 | 55.47 ± 12.13 | 1.17 ± 0.53 |
| Cohort 2 (2.4 mg/kg; n = 3) | 0.0104 ± 0.0004 | 66.74 ± 2.77 | 0.00 ± 0.00 | 38469.27±11371.55 | 2376641.68 ± 824625.87 | 2410407.26 ± 803079.58 | 106.20 ± 46.76 | 89.10 ± 53.68 | 1.10 ± 0.45 |
| Cohort 3 (3.6 mg/kg; n = 10) | 0.0161 ± 0.0105 | 59.38 ± 30.46 | 0.40 ± 1.26 | 80735.83 ± 24625.42 | 3802921.30 ± 1692136.42 | 3826911.91 ± 1715857.82 | 84.92 ± 30.81 | 56.20 ± 12.68 | 1.28 ± 1.01 |
| Cohort 4 (4.8 mg/kg; n = 7) | 0.0091 ± 0.0009 | 76.61 ± 6.52 | 0.57 ± 1.51 | 92880.49 ± 16660.59 | 5052300.32 ± 860926.67 | 5076495.13 ± 862955.42 | 109.70 ± 13.88 | 58.10 ± 12.15 | 0.97 ± 0.18 |
| Cohort 5 (6.0 mg/kg; n = 6) | 0.0108 ± 0.0040 | 70.59 ± 20.49 | 0.00 ± 0.00 | 140088.07 ± 15216.88 | 6284782.56 ± 1018649.74 | 6320177.65 ± 1034866.74 | 99.40 ± 31.47 | 53.69 ± 4.47 | 0.98 ± 0.18 |
- The summary data are presented as mean ± SD.
- Abbreviations: λZ, apparent terminal elimination rate constant; t1/2, terminal elimination half-life; Cmax, maximum serum concentration; Tmax, time of observed maximum serum concentration; AUC0-t, area under the plasma concentration-time curve from zero to the last measurable plasma concentration point; AUC0-∞, area under the concentration versus time curve from time zero extrapolated to infinity; Vz, volume of distribution at end phase; Vss, steady-state volume of distribution; CL, total body clearance.
3.4 Efficacy
All 29 enrolled patients in the 1.2- to 6.0-mg/kg cohorts were considered evaluable for efficacy. Patients were evaluated for tumor response every two cycles according to the RECIST 1.1 criteria. A summary of best overall response is shown in Figure 2. Of the 29 evaluable patients, 12 (41.4%; 95% CI = 23.5%–61.1%) achieved an objective response, 10 of which were confirmed. A total of 24 (82.8%; 95% CI = 64.2%–94.2%) patients achieved disease control. In 7 patients previously treated with trastuzumab and lapatinib, 2 (28.6%; 95% CI = 3.7%–71.0%) patients achieved an objective response and 4 (57.1%; 95% CI = 18.1%–90.1%) patients achieved disease control. For 13 patients previously treated with trastuzumab alone, the objective response was observed in 6 (46.2%; 95% CI = 19.2%–74.9%) patients, including 1 unconfirmed overall response, and disease control was observed in 11 (84.6%; 95% CI = 54.6%–98.1%) patients. For the 3.6 mg/kg dose (MTD) cohort (n = 10), objective response was observed in 4 (40.0%; 95% CI = 12.2%-73.8%) patients, including 1 unconfirmed overall response, and disease control was observed in 8 (80%; 95% CI = 44.4%–97.5%) patients. Notably, 1 patient achieved CR at the 3.6 mg/kg dose (MTD) cohort, and for whom the duration of response was 9 months.
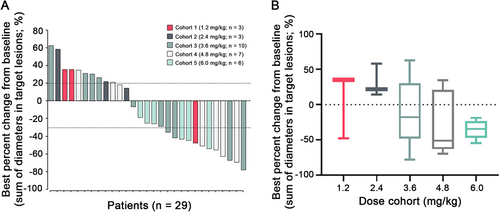
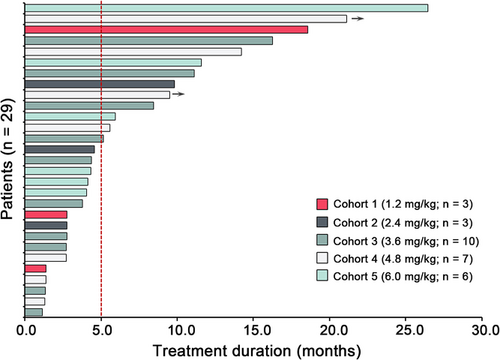
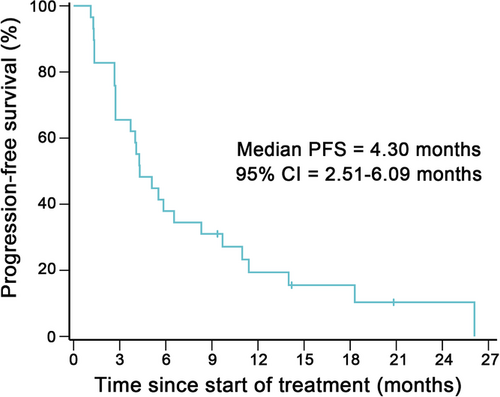
At the study cutoff date, 10 (34.5%) patients had received more than 6 months of treatment and 2 (6.9%) patients were still on treatment (Figure 3). Patients with a partial response were mostly on 3.6mg/kg or higher dose cohorts, except for 1 patient who received 1.2mg/kg of BAT8001 and remained on treatment after 18.6 months since initial dosing (Figure 2 and 3). Of the 12 patients who achieved an objective response, their median duration of response was 4.30 months (95% CI = 0–14.24 months). Of the 29 efficacy-evaluable patients, 26 (89.7%) had a PFS event and their median PFS was 4.30 months (95% CI = 2.51–6.09 months; Figure 4).
4 DISCUSSION
This is the first human study reported for BAT8001, a novel ADC targeting HER2. A total of 29 patients with HER2-positive advanced or metastatic breast cancer were enrolled into the dose-escalation study. BAT8001 doses of 1.2, 2.4, 3.6, 4.8, and 6.0 mg/kg were evaluated. BAT8001 was administered by intravenous infusion every 21 days. The MTD of BAT8001 was determined to be 3.6 mg/kg. The analysis of pharmacokinetic of BAT8001 showed a nonlinear pharmacokinetic profile across the assessed range of doses. Increased Cmax and area under the serum concentration-time curve were observed at higher doses. Systemically free maytansine derivative levels were substantially low at all doses and time points assessed.
Harboring a microtubule inhibitor payload, the toxicity profile of BAT8001 has some similarities to those of T-DM1 [24]. The most common side-effect of BAT8001 is thrombocytopenia. This side-effect is also typical for T-DM1, but less commonly seen in HER2 targeting ADCs with other types of cytotoxic agents [11, 20]. Grade 3/4 BAT8001-related thrombocytopenia events were effectively controlled by use of appropriate medication, such as recombinant human-thrombopoietin, and dose reduction (1 patient in 3.6 mg/kg cohort, 2 patients in 4.8 mg/kg cohort, and 1 patient in 6.0 mg/kg cohort), which did not result in serious bleeding events. Increased hepatic transaminase was reported as a dose-limiting toxicity at the 6.0 mg/kg cohort. This type of AE is often transient and reversible, which has also been reported as a common AE of T-DM1 [24]. Pneumonitis was noted as one of the safety concerns of other HER2-targeting ADCs [25]. However, no such events were recorded in this study. This finding warrants further attention in future studies recruiting a larger cohort of patients.
BAT8001 demonstrated promising efficacy in patients with advanced or metastatic HER2-positive breast cancer. In this heavily pretreated cohort with 76% of patients who relapsed after HER2 inhibitor therapy and 66% of patients who received 3 or more chemotherapeutic regimens for advanced disease, 41.4% and 82.8% of the patients achieved objective response and disease control, respectively. Notably, one patient achieved CR at the MTD cohort. These results were in line with the reported efficacy data of T-DM1 [26]. Considering the limited and heterogenous population in our study cohort, although the efficacy results shown here are impressive, they still need to be validated in future randomized-controlled trials.
The present study has some limitations. First, although we enrolled patients with heavily pretreated metastatic disease, few of these patients had received treatment with pertuzumab and none had received T-DM1 prior to the enrollment. This is because these two drugs were not approved for metastatic breast cancer during the study period. Second, this study only enrolled patients with HER2-positive (defined as IHC 3+ and/or ISH+) tumors. Recently, other HER2 targeting ADCs have shown activity in patients with lower expression of HER2 [27]. But the current study has not provided such data.
In conclusion, results from this phase I, dose escalation study have shown that BAT8001 was well tolerated at a dose of 3.6 mg/kg. BAT8001 demonstrated therapeutic potential in heavily pretreated patients with HER2-positive metastatic breast cancer. Further investigation to confirm these findings is ongoing in a phase III trial to evaluate the efficacy and safety of BAT8001 compared with the combination of lapatinib and capecitabin in patients with locally or metastatic HER2 positive breast cancer that progressed during or after at least one trastuzumab-based regimen (NCT04185649). Additional studies to evaluate the efficacy of BAT8001 in T-DM1 pretreated patients or those with HER2-low solid tumors are under preparation.
ACKNOWLEDGEMENTS
This study was sponsored by Bio-Thera Solutions, Ltd. We thank the patients who participated in this study, as well as their families and caregivers. We thank Jibin Li for guiding the biostatistics analysis, who is employed by Sun Yat-sen University Cancer Center. We thank Weimin Zhang for assistance in medical writing and editorial support, who is employed by Peking University Cancer Hospital and Institute.
AUTHORS' CONTRIBUTIONS
RH, SW, FX, LW, WX, SL, and JW made substantial contributions to the study conception and design. RH, FX, LW, WX, and JW contributed to data acquisition. RH, SW, LW, SL, JY, WT and JW analyzed and interpreted the data. SW served as the primary investigator of the study. All authors read and approved the final manuscript.
FUNDING
This study was supported by the Natural Science Foundation of Guangdong Province (2020A1515010105), Joint Fund of the National Natural Science Foundation of China and Natural Science Foundation of Guangdong Province (U1601224).
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the institutional review board and ethics committee of Sun Yat-sen University Cancer Center. It was carried out in accordance with the principles of the Declaration of Helsinki, Chinese Good Clinical Practice and local legislation. All recruited patients provided written informed consent before enrolment.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The datasets obtained and analyzed during the present study have been deposited in the Research Data Deposit public platform (www.researchdata.org.cn) with the accession code RDDA2020001803 and are available from the corresponding author on reasonable request.
COMPETING INTERESTS
SW received research grants from Pfizer for work outside the submitted work.




