The prognostic significance of non-sentinel lymph node metastasis in cutaneous and acral melanoma patients—A multicenter retrospective study
Abstract
Background
Whether non-sentinel lymph node (SLN)-positive melanoma patients can benefit from completion lymph node dissection (CLND) is still unclear. The current study was performed to identify the prognostic role of non-SLN status in SLN-positive melanoma and to investigate the predictive factors of non-SLN metastasis in acral and cutaneous melanoma patients.
Methods
The records of 328 SLN-positive melanoma patients who underwent radical surgery at four cancer centers from September 2009 to August 2017 were reviewed. Clinicopathological data including age, gender, Clark level, Breslow index, ulceration, the number of positive SLNs, non-SLN status, and adjuvant therapy were included for survival analyses. Patients were followed up until death or June 30, 2019. Multivariable logistic regression modeling was performed to identify factors associated with non-SLN positivity. Log-rank analysis and Cox regression analysis were used to identify the prognostic factors for disease-free survival (DFS) and overall survival (OS).
Results
Among all enrolled patients, 220 (67.1%) had acral melanoma and 108 (32.9%) had cutaneous melanoma. The 5-year DFS and OS rate of the entire cohort was 31.5% and 54.1%, respectively. More than 1 positive SLNs were found in 123 (37.5%) patients. Positive non-SLNs were found in 99 (30.2%) patients. Patients with positive non-SLNs had significantly worse DFS and OS (log-rank P < 0.001). Non-SLN status (P = 0.003), number of positive SLNs (P = 0.016), and adjuvant therapy (P = 0.025) were independent prognostic factors for DFS, while non-SLN status (P = 0.002), the Breslow index (P = 0.027), Clark level (P = 0.006), ulceration (P = 0.004), number of positive SLNs (P = 0.001), and adjuvant therapy (P = 0.007) were independent prognostic factors for OS. The Breslow index (P = 0.020), Clark level (P = 0.012), and number of positive SLNs (P = 0.031) were independently related to positive non-SLNs and could be used to develop more personalized surgical strategy.
Conclusions
Non-SLN-positive melanoma patients had worse DFS and OS even after immediate CLND than those with non-SLN-negative melanoma. The Breslow index, Clark level, and number of positive SLNs were independent predictive factors for non-SLN status.
Abbreviations
-
- BJCH
-
- Beijing Cancer Hospital
-
- CI
-
- confidence interval
-
- CLND
-
- completion of lymph node dissection
-
- DeCOG-SLT
-
- Dermatologic Cooperative Oncology Group-Selective Lymphadenectomy Trial
-
- DFS
-
- disease-free survival
-
- FUSCC
-
- Fudan University Shanghai Cancer Center
-
- HE
-
- hematoxylin and eosin; HR: hazard ratio
-
- MSS
-
- melanoma-specific survival
-
- Non-SLN
-
- non-sentinel lymph node
-
- OS
-
- overall survival
-
- RCT
-
- randomized clinical trial
-
- SMR
-
- Society of Melanoma Research
-
- TJMUCH
-
- Tianjin Medical University Cancer Institute and Hospital
-
- ZJCH
-
- Zhejiang Cancer Hospital
1 INTRODUCTION
Since the past decade, sentinel lymph node biopsy (SLNB) has become the standard management for patients with early-stage melanoma. In the Multicenter Selective Lymphadenectomy Trial-I (MSLT-1) study, the 5-year survival rates of node-positive patients were significantly improved for patients who underwent SLNB and immediate complete lymph node dissection (CLND) over those who underwent CLND until recurrence [1]. In recent years, the value of CLND for patients with sentinel-node metastasis has been denied in several retrospective nonrandomized studies [2-7]. In addition, the German Dermatologic Cooperative Oncology Group-Selective Lymphadenectomy Trial (DeCOG-SLT) [8, 9] and MSLT-II [10] studies have shown that immediate CLND did not increase melanoma-specific survival among patients with melanoma and sentinel-node metastases. Indeed, these trials have provided powerful evidence for the exemption of immediate CLND for SLN-positive patients. However, more than 80% of the enrolled patients in both the DeCOG-SLT and MSLT-II had only one positive SLN, and ∼58% of the patients in the DeCOG-SLT and ∼67% of the patients in the MSLT-II had negative non-SLNs. Considering the relatively early stage of the enrolled patients, exemption of immediate CLND in non-SLN or multiple SLN metastasis patients still warrant further investigation.
The proportion of melanoma subtypes in Asian people is distinct from that in Western populations. While acral melanoma is rare (1-9%) in Caucasians [11-13], it accounts for most melanomas in Asian individuals (58%) [14, 15], especially in the Chinese patient population, and this proportion could reach up to 68% (data from Fudan University Shanghai Cancer Center, FUSCC). Further, conclusions that were drawn from Western populations still require validation in Asian patients for a better understanding of this disease in wider population settings.
This current study was performed to identify the prognostic role of non-SLN status in SLN-positive melanoma patients who underwent immediate CLND and the predictive factors of non-SLN metastasis for Asian acral and cutaneous melanoma patients.
2 PATIENTS AND METHODS
2.1 Patients
Patients with clinically lymph node-negative acral and cutaneous melanoma who underwent wide R0 resection with a negative margin and SLNB at FUSCC (Shanghai, China), Tianjin Medical University Cancer Institute and Hospital (TJMUCH; Tianjin, China), Beijing Cancer Hospital (BJCH; Beijing, China), and Zhejiang Cancer Hospital (ZJCH; Hangzhou, China), from September 1, 2009, to August 31, 2017, were identified. All patients with positive SLN (SLN+) underwent CLND within one month. Some of the patients underwent SLNB and CLND in one operation when the fast-frozen pathology reported SLN+, while the others were recalled for CLND within one month after surgery when the routine paraffin pathology reported SLN+. CLND was performed according to routine surgical procedures in diverse lymph node basins including the ilioinguinal basins, the iliac basins, and the axillary basins. SLN+ melanoma patients who subsequently underwent CLND were enrolled in the current retrospective study. Patients less than 18 years old or with a follow up of less than 1 month were excluded.
2.2 Surgery and pathology
SLNB was routinely performed using technetium-99 sulfur colloid, methylene blue, or both. The pathological methods to detect the SLN and non-SLN metastases were similar to those used in our prior study [16]. Briefly, SLN/non-SLN specimens were dissected every 3 mm or along the longest axis on the largest surface, fixed by 3.7% neutral formaldehyde, conventional dehydrated, and paraffin-embedded followed by hematoxylin and eosin (HE) staining. The SLN status was also estimated by immunohistochemistry (S-100 protein, HMB45, Melan A, and SOX10). The antibodies against S-100 and Melan A were purchased from Dako company (Copenhagen, Danish). The antibody against HMB45 was purchased from MaiXin Biotechnologies (Fuzhou, China). The antibody against SOX10 was purchased from Gene Tech (Shanghai) Company (Shanghai, China). Each section was observed under a light microscope by two pathologists (one attending physician, Min Ren, FUSCC: for diagnosis, and one associate chief physician, Yun-Yi Kong, FUSCC: for confirmation of the diagnosis).
2.3 Data retrieval and follow-up
Clinicopathological variables including age, gender, Clark level, Breslow index, ulceration, number of positive SLNs, non-SLN status, and adjuvant therapy were retrieved. Pathologic nodal (pN) stage and pathological stage were defined according to the 8th edition of the American Joint Committee on Cancer (AJCC) cancer staging manual [17]. Patients were monitored through clinical examination such as routine physical checkups, ultrasound, CT and/or MRI every 3 months for the first 2 years, every 6 months for 3-5 years, and then annually. Patients were followed up through reexamination or telephone follow-up until death or June 30, 2019. The survival of patients was censored at the date of the last follow-up (June 30, 2019). Overall survival (OS) was calculated as the interval between radical surgery and death/last follow-up. Disease-free survival (DFS) was defined as the time interval from radical surgery to local recurrence or distant metastasis. Recurrence or metastasis was confirmed by pathology or imaging follow-up.
2.4 Statistical analysis
Pearson's chi-squared test or Fisher's exact test was used for univariable analysis of the different category groups. Multivariable logistic regression modeling was performed to identify factors associated with non-SLN positivity. Kaplan–Meier estimation and log-rank analysis were used to identify the prognostic factors for DFS and OS. Variables with P < 0.05 in the univariable survival analysis were included in the multivariable Cox regression analysis to identify corresponding independent prognostic factors and for calculating hazard ratios (HRs) and 95% confidence intervals (95% CIs). P-values less than 0.05 were considered statistically significant. All statistical analyses were performed using the Statistical Product and Service Solutions (SPSS, version 22.0; SPSS Company, Chicago, IL) software. This study was approved by the Ethics Committee of FUSCC, TJMUCH, BJCH, and ZJCH. Each participant signed an informed consent document during the preoperative conversation.
3 RESULTS
3.1 Baseline characteristics
A total of 328 (28.0%) SLN+ (FUSCC: n = 150; TJMUCH: n = 81; BJCH: n = 50; ZJCH: n = 47) patients among the 1171 melanoma patients (FUSCC: n = 570; TJMUCH: n = 276; BJCH: n = 173; ZJCH: n = 152) who underwent wide resection and SLNB were included in this study. Their median age was 56 years, with a range from 23 to 91 years. 160 (48.8%) patients were male.
Of the entire cohort, 220 (67.1%) had acral melanomas and 108 (32.9%) had cutaneous melanomas. Of all the acral melanoma patients, 23 (10.5%) had melanomas of the upper extremities, and 197 (89.5%) had melanomas of the lower extremities, including 156 plantar melanomas. Among the cutaneous melanoma patients, only two (1.9%) had melanoma in the head and neck, 62 (57.4%) were in the limbs, and 44 (40.7%) were in the trunk (Figure 1). Most patients (n = 238, 72.6%) had melanoma of Clark level IV and V. More than 1 SLN+ were found in 123 (37.5%) patients, while 99 (30.2%) patients had positive non-SLNs. Most patients (n = 275, 83.8%) had received adjuvant therapy (chemotherapy and/or high dose interferon) after surgery (Table 1). Further, no significant differences were found in Breslow index, ulceration, number of positive SLNs, non-SLN status, N stage, AJCC stage, gender, the Clark level, and adjuvant therapy between the acral and cutaneous groups, while only age was significantly different.
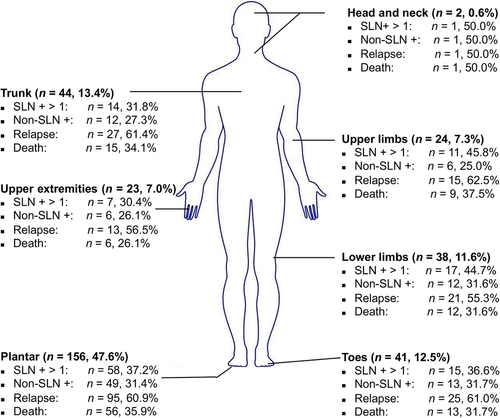
Schematic illustration of the different locations of melanomas and their clinical characteristics.
Abbreviations: SLN +, sentinel lymph node positive; non-SLN +, non-sentinel lymph node positive
| Variable | Cutaneous (n = 108) [cases (%)] | Acral (n = 220) [cases (%)] | Total [cases (%)] | Pearson χ2 | P value |
|---|---|---|---|---|---|
| Age | 13.449 | < 0.001 | |||
| < 60 | 82 (75.9) | 121 (55.0) | 203 (61.9) | ||
| ≥ 60 | 26 (24.1) | 99 (45.0) | 125 (38.1) | ||
| Gender | 2.468 | 0.116 | |||
| Male | 46 (42.6) | 114 (51.8) | 160 (48.8) | ||
| Female | 62 (57.4) | 106 (48.2) | 168 (51.2) | ||
| Breslow index | 3.070 | 0.215 | |||
| ≤ 2 mm | 30 (27.8) | 81 (36.8) | 111 (33.8) | ||
| > 2-4 mm | 40 (37.0) | 65 (29.5) | 105 (32.0) | ||
| > 4 mm | 38 (35.2) | 74 (33.6) | 112 (34.1) | ||
| Clark level | 1.322 | 0.250 | |||
| I-III | 34 (31.5) | 56 (25.5) | 90 (27.4) | ||
| IV-V | 74 (68.5) | 164 (74.5) | 238 (72.6) | ||
| Ulceration | 0.030 | 0.863 | |||
| Absent | 60 (55.6) | 120 (54.5) | 180 (54.9) | ||
| Present | 48 (44.4) | 100 (45.5) | 148 (45.1) | ||
| No. of positive SLN | 0.368 | 0.544 | |||
| 1 positive | 65 (60.2) | 140 (63.6) | 205 (62.5) | ||
| > 1 positive | 43 (39.8) | 80 (36.4) | 123 (37.5) | ||
| Non-SLN status | 0.167 | 0.683 | |||
| Negative | 77 (71.3) | 152 (69.1) | 229 (69.8) | ||
| Positive | 31 (28.7) | 68 (30.9) | 99 (30.2) | ||
| N stage | 0.227 | 0.893 | |||
| 1a | 52 (48.1) | 105 (47.7) | 157 (47.9) | ||
| 2a | 41 (38.0) | 88 (40.0) | 129 (39.3) | ||
| 3a | 15 (13.9) | 27 (12.3) | 42 (12.8) | ||
| AJCC stage* | 0.194 | 0.907 | |||
| IIIA | 30 (27.8) | 66 (30.0) | 96 (29.3) | ||
| IIIB | 23 (21.3) | 44 (20.0) | 67 (20.4) | ||
| IIIC | 55 (50.9) | 110 (50.0) | 165 (50.3) | ||
| Adjuvant therapy | 0.861 | 0.650 | |||
| Yes | 94 (87.0) | 181 (82.3) | 275 (83.8) | ||
| No | 14 (13.0) | 39 (17.7) | 53 (16.2) |
- * AJCC stage refers to the pathological staging system.
- Abbreviations: SLN, sentinel lymph node; non-SLN, non-sentinel lymph node; AJCC, American Joint Committee on Cancer.
3.2 Prognostic factors
During the follow-up period, 197 (60.1%) patients had local recurrence or distant metastasis, and 113 (34.5%) died. The 5-year DFS rate of all patients was 31.5%, and the 5-year OS rate was 54.1%. The Breslow index (P < 0.001 for both DFS and OS), the Clark level (P = 0.002 and P = 0.001, respectively), ulceration (P = 0.012 and P = 0.036, respectively), number of positive SLNs (P < 0.001 and P = 0.003, respectively), non-SLN status (P < 0.001 and P = 0.001, respectively), N stage (P < 0.001 and P = 0.002, respectively), AJCC stage (P = 0.001 and P = 0.006, respectively) and adjuvant therapy (P = 0.031 and P = 0.017, respectively) were significantly associated with DFS and OS. Multivariable survival analysis revealed that non-SLN status (P = 0.003), number of positive SLNs (P = 0.016), and adjuvant therapy (P = 0.025) were independent prognostic factors for DFS (Table 2), while non-SLN status (P = 0.002), the Breslow index (P = 0.027), Clark level (P = 0.006), ulceration (P = 0.004), number of positive SLNs (P = 0.001), and adjuvant therapy (P = 0.007) were independent prognostic factors for OS (Table 3). Patients with more than 1 positive SLN had significantly poorer DFS (HR, 1.430; 95% CI: 1.070-1.912; Table 2) and OS (HR, 7.755; 95% CI: 2.357-27.051; Table 3) than those with only 1 positive SLN (Figures 2A and 3A). For patients with positive non-SLN, the HRs were 1.601 (95% CI, 1.172-2.187; Table 2) for DFS and 5.974 (95% CI, 1.817-16.420; Table 3) for OS (Figures 2B and 3B). Patients with higher N stage tended to have poorer DFS (Figure 2C) and OS (Figure 3C).
| Disease-free survival | |||
|---|---|---|---|
| Multivariate analysis | |||
| Variable | Univariate analysis (P value) | Hazard Ratio (95% CI) | P value |
| Age | 0.720 | Not included | |
| Gender | 0.168 | ||
| Breslow index | < 0.001 | 0.063 | |
| ≤ 2 mm | Reference | ||
| > 2-4 mm | 1.193 (0.803-1.772) | 0.381 | |
| > 4 mm | 1.577 (1.058-2.349) | 0.025 | |
| Clark level | 0.002 | 0.212 | |
| I-III | Reference | ||
| IV-V | 1.264 (0.875-1.827) | ||
| Ulceration | 0.012 | 0.299 | |
| Absent | Reference | ||
| Present | 1.172 (0.868-1.583) | ||
| No. of positive SLNs | < 0.001 | 0.016 | |
| 1 positive | Reference | ||
| > 1 positive | 1.430 (1.070-1.912) | ||
| Non-SLN status | < 0.001 | 0.003 | |
| Negative | Reference | ||
| Positive | 1.601 (1.172-2.187) | ||
| N stage | < 0.001 | Not included | |
| 1a | |||
| 2a | |||
| 3a | |||
| AJCC stage* | 0.001 | Not included | |
| IIIA | |||
| IIIB | |||
| IIIC | |||
| Adjuvant therapy | 0.031 | 0.025 | |
| Yes | Reference | ||
| No | 1.516 (1.096-2.099) | ||
- * AJCC stage refers to the pathological staging system.
- Abbreviations: SLN +, sentinel lymph node-positive; non-SLN, non-sentinel lymph node; AJCC, American Joint Committee on Cancer.
| Overall survival | |||
|---|---|---|---|
| Multivariate analysis | |||
| Variable | Univariate analysis (P value) | Hazard Ratio (95% CI) | P value |
| Age | 0.614 | Not included | |
| Gender | 0.125 | ||
| Breslow index | < 0.001 | 0.027 | |
| ≤ 2 mm | Reference | ||
| > 2-4 mm | 1.126 (0.644-1.9698) | 0.677 | |
| > 4 mm | 1.855 (1.080-3.186) | 0.025 | |
| Clark level | 0.001 | 0.006 | |
| I-III | Reference | ||
| IV-V | 2.056 (1.189-3.555) | ||
| Ulceration | 0.036 | 0.004 | |
| Absent | Reference | ||
| Present | 3.901 (1.262-12.184) | ||
| No. of positive SLN | 0.003 | 0.001 | |
| 1 positive | Reference | ||
| > 1 positive | 7.755 (2.357-27.051) | ||
| Non-SLN status | 0.001 | 0.002 | |
| Negative | Reference | ||
| Positive | 5.974 (1.817-16.420) | ||
| N stage | 0.002 | Not included | |
| 1a | |||
| 2a | |||
| 3a | |||
| AJCC stage* | 0.006 | Not included | |
| IIIA | |||
| IIIB | |||
| IIIC | |||
| Adjuvant therapy | 0.017 | 0.007 | |
| Yes | Reference | ||
| No | 1.924 (1.192-3.106) | ||
- * AJCC stage refers to the pathological staging system.
- Abbreviations: Non-SLN, non-sentinel lymph node; AJCC, American Joint Committee on Cancer.
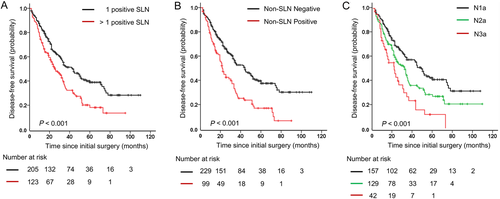
Kaplan-Meier plot curves for the disease-free survival (DFS) of patients in different subgroups. (A) DFS for patients with 1 positive SLN compared with those with more than 1 positive SLN (P < 0.001). (B) DFS for patients with negative non-SLNs compared with patients who had positive non-SLNs (P < 0.001). (C) DFS for patients with different N stages (P < 0.001).
Abbreviations: SLN, sentinel lymph node; non-SLN, non-sentinel lymph node

Kaplan-Meier plot curves for the overall survival (OS) of patients in different subgroups. (A) OS for patients with 1 positive SLN compared with those with more than 1 positive SLN (P = 0.003). (B) OS for patients with negative non-SLNs compared with patients who had positive non-SLNs (P < 0.001). (C) OS for patients with different N stages (P = 0.001).
Abbreviations: SLN, sentinel lymph node; non-SLN, non-sentinel lymph node
3.3 Predictive factors of positive non-SLN patients
To identify predictive factors of positive non-SLN patients, chi-squared analysis was first performed between patients with and without positive non-SLN. The Breslow index (P < 0.001), the Clark level (P < 0.001), ulceration (P = 0.044), number of positive SLNs (P = 0.001), AJCC stage (P < 0.001), and N stage (P < 0.001) were found to be correlated with positive non-SLN. In the multivariable logistic regression analysis, the Breslow index (P = 0.020, HR, 1.978; 95% CI: 1.114-3.511 for 2-4 mm; P < 0.001, HR, 4.195; 95% CI: 2.081-8.459 for > 4 mm), Clark level (P = 0.012, HR, 2.304; 95% CI: 1.166-4.554), and number of positive SLNs (P = 0.031, HR, 1.754; 95% CI: 1.053-2.922) were independently related to positive non-SLN (Table 4).
| Logistic regression analysis | ||||||
|---|---|---|---|---|---|---|
| Variable | Negative non-SLN (n = 229) [cases (%)] | Positive non-SLN (n = 99) [cases (%)] | Pearson χ2 | P value | Hazard Ratio (95% CI) | P value |
| Age | 0.005 | 0.946 | Not included | |||
| < 60 | 142 (62.0) | 61 (61.6) | ||||
| ≥ 60 | 87 (38.0) | 38 (38.4) | ||||
| Gender | 1.886 | 0.170 | Not included | |||
| Male | 106 (46.3) | 54(54.5) | ||||
| Female | 123 (53.7) | 45 (45.5) | ||||
| Breslow index | 30.270 | < 0.001 | < 0.001 | |||
| ≤ 2 mm | 96 (41.9) | 15 (15.2) | Reference | |||
| > 2-4 mm | 74 (32.3) | 31 (31.3) | 1.978 (1.114-3.511) | 0.020 | ||
| > 4 mm | 59 (25.8) | 53 (53.5) | 4.195 (2.081-8.459) | < 0.001 | ||
| Clark level | 14.579 | < 0.001 | 0.012 | |||
| I-III | 77 (33.6) | 13 (13.1) | Reference | |||
| IV-V | 152 (66.4) | 86 (86.9) | 2.304 (1.166-4.554) | |||
| Ulceration | 4.054 | 0.044 | 0.913 | |||
| Absent | 134 (58.5) | 46 (46.5) | Reference | |||
| Present | 95 (41.5) | 53 (53.5) | 1.030 (0.611-1.734) | |||
| No. of positive SLNs | 10.233 | 0.001 | 0.031 | |||
| 1 positive | 156 (68.1) | 49 (49.5) | Reference | |||
| > 1 positive | 73 (31.9) | 50 (50.5) | 1.754 (1.053-2.922) | |||
| Location | 0.167 | 0.683 | Not included | |||
| Acral | 152 (66.4) | 68 (68.7) | ||||
| Cutaneous | 77 (33.6) | 31 (31.3) | ||||
| N stage | 124.848 | < 0.001 | Not included | |||
| 1a | 154 (67.2) | 3 (3.0) | ||||
| 2a | 65 (28.4) | 64 (64.6) | ||||
| 3a | 10 (4.4) | 32 (32.3) | ||||
| AJCC Stage | 30.349 | < 0.001 | Not included | |||
| IIIA | 84 (36.7) | 12 (12.1) | ||||
| IIIB | 52 (22.7) | 15 (15.2) | ||||
| IIIC | 93 (40.6) | 72 (72.7) | ||||
- * AJCC stage refers to the pathological staging system.
- Abbreviations: SLN, sentinel lymph node; non-SLN, non-sentinel lymph node; AJCC, American Joint Committee on Cancer; CI, confidence interval.
4 DISCUSSION
In this current multicenter study, using a large cohort of Chinese SLN-positive melanoma patients, we discovered that non-SLN status was an independent prognostic factor for cutaneous and acral melanoma patients, and that non-SLN-positive patients had worse DFS and OS even after immediate CLND than those with non-SLN-negative melanoma. The Breslow index, Clark level, and number of positive SLNs were independent predictive factors for non-SLN status.
Recently, the clinical significance of immediate CLND after SLNB in melanoma has been debated. The primary concern regarding CLND is the proportion of procedure-related complications, including lymphedema, with incidence reaching as high as 25% for axillary dissection and 48% for inguinal dissection [18], as well as seroma and wound infections following CLND. SLNB, on the other hand, has been reported with substantially lower complication rates, ranging from 5%-14% versus 23%-66% for those with CLND [19-23]. Several retrospective studies have reported no survival benefit from CLND in melanoma [2-6]. However, most of these studies were limited by a certain limit of selection bias and small sample sizes. The phase III study DeCOG-SLT, which randomized 483 patients, showed that CLND did not promote long-term distance metastasis-free survival, recurrence-free survival, nor improved the overall survival of SLN+ patients, compared to a cohort who underwent nodal observation [8]. The MSLT-II clinical trial, which comprised of 3531 patients, reported similar results, and although immediate CLND increased the rate of regional disease control and provided useful prognostic information, it did not increase the melanoma-specific survival among patients with SLN metastases [9, 10]. The DeCOG-SLT and MSLT-II clinical trials were well-designed and showed credible evidence that immediate CLND after SLNB did not benefit the patients’ survival. Nevertheless, the potential survival benefit associated with immediate CLND for all patients may have been diluted as the majority of the enrolled patients had no non-SLN metastases. In a meta-analysis by Delgado et al. [24] which included four randomized clinical trials (RCTs), the melanoma-specific survival (MSS) was higher after immediate CLND than after delayed CLND in patients with nodal metastasis (HR = 0.63, 95% CI: 0.35–0.74, P = 0.0004); suggesting time-dependent, disease-specific survival with early/immediate lymph node surgery. As oncologists could only obtain the non-SLN status after CLND, determining the influence of immediate CLND in non-SLN metastatic melanoma is difficult in real-world clinical practice. However, with a multicenter retrospective clinical study, we may be able to determine whether non-SLN metastatic melanomas require more aggressive treatment, including CLND.
A total of 220 (67.1%) acral and 108 (32.9%) cutaneous melanoma cases were included in this multicenter study. No significant difference in baseline characteristics was found between these two groups except for age, indicating that the patients had similar distributions for the number of positive SLNs and non-SLNs. Hence, the acral and cutaneous melanomas were integrated for further analysis. Surprisingly, up to 28% of the melanoma patients had positive SLNs. Of all the Chinese SLN+ melanoma patients, 37.5% were found to have more than one positive SLN, and 30.2% had non-SLN metastasis. In contrast, only 9%-18% of patients had more than one positive SLN and 18%-33.3% had non-SLN metastases in the MSLT-II and DeCOG-SLT clinical trials. Unfortunately, multiple SLN-positive and non-SLN metastases indicate poorer DFS and OS. In comparison, Asian melanoma patients tend to have a higher Breslow index, Clark level, ulceration rate, and especially, a higher proportion of positive SLNs and tumor burden than Western patients [25-30], which could be due to a higher proportion of the acral subtype and a less timely/late diagnosis among Asian patients. Considering all these factors, whether the conclusions of the MSLT-II and DeCOG-SLT are suitable for Asian melanoma is debatable. Hence, while exemption of immediate CLND may be practicable in non-Asian melanoma patients, in Asian patients, especially in those with acral subtype, multiple positive SLNs or non-SLN metastases, this should be considered more cautiously,
According to this present study, non-SLN status is an important prognostic factor and has a significant impact on surgical strategy. Thus, the preoperative prediction of non-SLN metastases is important for the following treatment strategy. Previously, several investigations have attempted to develop risk assessments using known clinical parameters to correctly identify patients with a high risk of non-SLN involvement [31-37]. Although certain factors, i.e. thicker primary and larger SN tumor size, were found to be statistically significant, few of these factors were sufficiently specific, and the conclusions varied. In this study, we first assessed the predictive factors of non-SLN metastases in Chinese cutaneous and acral melanoma patients, and found that the Breslow index, Clark level, and number of positive SLNs were independent predictive factors for non-SLN status. Hence, for patients with higher Breslow index, Clark level, and multiple positive SLNs, whose non-SLN are more likely positive and prognosis are poorer, the exemption of immediate CLND should be made more cautiously.
Several potential limitations exist in this study. First, although this study enrolled patients from four of the largest cancer centers in China, it was a retrospective study. Well-designed prospective, randomized clinical trials with a higher proportion of acral subtype and multiple positive SLNs cohort than MSLT-II and DeCOG-SLT may be needed for proper validation of our findings. Second, several potential non-SLN status predictors, such as the maximum diameter of the tumor and micrometastases in the SLN were not included in the current study. Previous studies have shown that the tumor burden of SLN (i.e. the maximum diameter, microanatomic location, extranodal extension, of the LNs) was correlated with the tumor burden of non-SLN in cutaneous melanoma and that non-SLN metastasis could be associated with a larger diameter of SLN metastases [34, 35, 37]. However, some studies have reported no or little possibility of non-SLN positivity for patients with less than 0.1 mm SLN-micrometastases [38, 39]. Third, although DFS is a commonly used indicator for patients’ prognosis, it does not differentiate between locoregional recurrent disease and distant metastases. Patients with locally recurrent disease and regional lymph node metastasis may still have a chance for elective radical surgery. Fourth, although most of the enrolled patients received chemotherapy and/or high dose of interferon as adjuvant treatment, they did not receive targeted therapy or PD-1/PD-L1/CTLA-4 targeted immune therapy. While an increasing number of adjuvant targeted and immune therapy clinical trials with positive results, such as the COMBI-AD [40], EORTC 18071 [41], and CheckMate 238 [42], are emerging, modern medical therapy may profoundly influence surgical strategy [43-45]. Recently, a retrospective analysis of patients with SLN-positive melanoma who received adjuvant anti-PD-1 therapy without CLND (post-MSLT-II trial) was reported at the 16th International Congress of Society of Melanoma Research (SMR), and no survival benefit was found in the CLND group. However, the selection bias in anti-PD-1 therapy with the CLND group and anti-PD-1 therapy without the CLND group was too obvious, and the follow-up time was not sufficiently long.
5 CONCLUSION
In conclusion, Chinese patients from this multicenter analysis seemed to have higher SLN and non-SLN involvement rates and a greater lymph node tumor burden than those reported in Western melanoma patients. Non-SLN-positive melanoma patients had worse DFS and OS even after immediate CLND than those with non-SLN-negative melanoma. Hence, more aggressive treatment, including CLND, may still be indispensable for non-SLN-positive melanoma. As non-SLN status cannot be confirmed until CLND is performed, the prediction of non-SLN status in patients with positive SLN has become quite important. The Breslow index, Clark level, and the number of positive SLNs were identified as important factors for predicting the status of non-SLN metastases and could be used to develop more personalized surgical strategies.
ACKNOWLEDGMENTS
We thank all the researchers from FUSCC, TJMUCH, BJCH, and ZJCH who have participated in the current study. We thank all the patients who have participated in the current study.
AUTHORSHIP
Conception/design: WS, YX, PP, WJY, and YC. Data collection and analysis: WS, YX, JLY, ZCL, and TL. Manuscript writing and polishing: WS, KH, PP, and YC. All authors read and approved the final manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Ethics Committee of FUSCC, TJMUCH, BJCH, and ZJCH, and each participant signed an informed consent document.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
FUNDING
This work was financially supported by the Shanghai Committee of Science and Technology, China (Grant No. 19411951700); the Shanghai Anti-cancer Association “Ao Xiang” project (Grant No. SACA-AX112); and the National Natural Science Foundation of China (Grant No. 81802636).
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




