Treatment and prognosis of iliac fossa soft tissue sarcoma: A single-center study
Abbreviations
-
- AJCC
-
- American Joint Committee on Cancer
-
- CI
-
- confidence interval
-
- FNCLCC
-
- Fédération Nationale des Centres de Lutte Contre le Cancer
-
- IFS
-
- iliac fossa sarcoma
-
- LRFS
-
- local recurrence-free survival
-
- MFH/UPS
-
- Malignant Fibrous Histiocytoma/ Undifferentiated Pleomorphic Sarcoma
-
- MPNST
-
- Malignant Peripheral Nerve Sheath Tumor
-
- OS
-
- overall survival
-
- RPS
-
- retroperitoneal sarcoma
-
- UICC
-
- Union for International Cancer Control
Dear Editor,
Primary iliac fossa sarcoma (IFS) is a special type of retroperitoneal sarcoma (RPS), accounting for ∼15% of all RPS cases [1]. The deep location, large size, and invasion to surrounding tissues and organs are the main causes of unresectability of IFS. All these characteristics are associated with an increased risk of positive surgical margin and a decreased feasibility of adjuvant therapy. In patients with multivisceral and/or vascular involvement, a multivisceral en bloc approach [2] with blood vessel replacement may be required to achieve a negative margin and to improve the quality of resection [3]. However, whether these surgical procedures improve prognosis in patients with IFS remain undefined. Moreover, whether aggressive procedures lead to acceptable functional impairment requires validation. In addition, previous studies have reported inconsistent results regarding the role of adjuvant radiotherapy in the treatment of RPS [4]. To date, the role of radiotherapy in the local control of IFS remains to be determined. Therefore, we analyzed the clinical features, treatment, and outcomes of IFS patients in an attempt to determine the significant prognostic factors and efficient therapeutics in real clinical practice.
A total of 83 patients, comprising of 45 males and 38 females, who were diagnosed with primary IFS at Fudan University Shanghai Cancer Center and Minhang Branch between August 2011 and December 2016 were evaluated. Their median age was 42 years (range, 18-70 years), and average tumor size was 12 cm (range, 5-21 cm). The most common histological subtype was liposarcoma (43.4%). There were 18 stage I cases and 65 stage II/III cases. The numbers of patients with grade 1, 2, and 3 tumors were 18, 31, and 34, respectively (supplementary Table S1).
A total of 44 patients with grade 2/3 tumors and multivisceral and/or vascular involvement received neoadjuvant chemotherapy using the MAID (mesna + adriamycin + ifosfamide + dacarbazine) or AIM (adriamycin + ifosfamide + mesna) regimen for 2 to 4 cycles (dosage in both regimens: intravenous administration of 2 g/m2 ifosfomide for 5 days, 400 mg/m2 mesna every 4 hours for three doses following ifosfamide, 30 mg/m2 adriamycin for 2 days, and 400 mg/m2 dacarbazine for 5 days every 3 weeks). Neoadjuvant radiotherapy was performed in 38 patients who demonstrated no tumor shrinkage after neoadjuvant chemotherapy, with half-dose irradiation of 20-40 Gy (1-2 Gy/fraction per day, 5 days per week for 4 successive weeks). The target area was the tumor and its surrounding 2-3 cm edge. According to RECIST 1.1, we reviewed the responses to neoadjuvant therapy in 44 patients: 14 achieved partial responses, 24 stable disease, and 6 progressive disease.
Thirty-nine patients underwent simple tumor resection as their initial treatment. Of the 44 patients with neoadjuvant therapy, only 6 with sufficient tumor shrinkage and 5 with disease progression underwent simple resection. Eighteen patients underwent visceral resection. The main resected organs were the bladder and colon. Twelve of 18 patients with vascular involvement underwent blood vessel replacement, and 4 of whom underwent both procedures. The other seven patients had partial tumor resection. Of the 83 patients, 63 underwent R0/R1 resection, and the others underwent R2 resection. Of the 26 patients who underwent aggressive procedures, the resection margins of were R0/R1 in 21 (80.8%) patients and R2 in 5 (19.2%) patients, which were similar to those in the 50 patients with simple resection [R0/R1 in 42 (84.0%) patients and R2 in 8 (16.0%) patients, P = 0.723]. Twenty-five patients experienced surgical complications: lower extremity edema (n = 12), wound healing difficulty (n = 8), infection (n = 4), and ureteral leak (n = 1). One (1.2%) patient died of major postoperative bleeding. Intraoperative bleeding was more significant in patients with aggressive procedures than in patients with simple resection (1100 mL vs. 450 mL, P < 0.001). There was no significant difference in the complication rates between these two groups (28.0% vs. 42.4%, P = 0.174). Moreover, postoperative masculoskeletal tumor society scores were similar in both groups (mean: 25 vs. 26, P = 0.349).
Adjuvant chemotherapy with the MAID or AIM regimen was performed in 55 patients according to tumor grade. Of the 65 patients with stage II/III tumors, 41 underwent adjuvant radiotherapy (median dose, 40 Gy; range, 20-65 Gy): 35 completed the full-course, whilst 6 withdrew due to intolerable radiation-related complications. Twenty-four patients refused adjuvant radiotherapy.
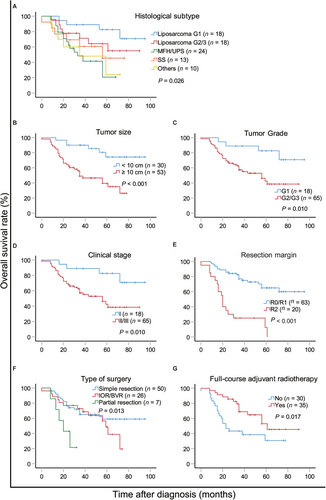
Thirty-seven patients died during the follow-up period (terminated in August 2019). The median overall survival (OS) was 61 months (range, 0-95 months). The 2- and 5-year OS rates were 73.5% [95% confidence interval (CI), 64.1%-82.9%] and 52.6% (95% CI, 40.4%-64.8%), respectively. Univariate analyses showed that tumor size (P < 0.001), grade (P = 0.010), clinical stage (P = 0.010), histological subtype (P = 0.026), type of surgery (P = 0.013), resection margin (P < 0.001), and adjuvant radiotherapy (P = 0.017) were significantly associated with OS (supplementary Table S2, Figure 1). Multivariate Cox regression analysis showed that tumor size (P = 0.007), resection margin (P < 0.001), and adjuvant radiotherapy (P = 0.003) were independent predictive factors of OS (supplementary Table S2).
Fifty-seven patients developed local recurrence. The 2- and 5-year local recurrence-free survival (LRFS) rates were 67.1% (95% CI, 56.9%-77.3%) and 34.7% (95% CI, 23.5%-45.9%), respectively. In univariate analysis, tumor size (P < 0.001), grade (P = 0.001), clinical stage (P = 0.001), histological subtype (P = 0.008), type of surgery (P < 0.001), resection margin (P < 0.001), and adjuvant radiotherapy (P = 0.044) were predictive for local control (supplementary Table S3, Figure 2). In multivariate analysis, tumor size (P = 0.005), grade (P = 0.018), type of surgery (P = 0.005), resection margin (P = 0.001), and adjuvant radiotherapy (P = 0.007) were identified as independent prognostic factors associated with local control (supplementary Table S3).
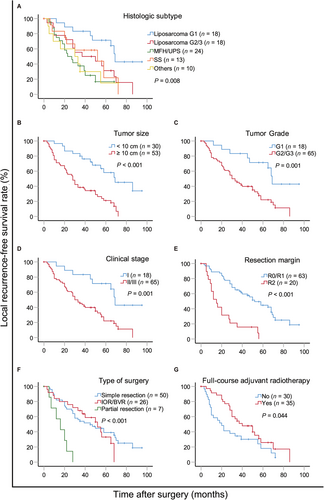
Surgery is the only curative treatment for primary IFS. Macroscopically complete resection is a favorable prognostic factor. Margin quality represents the best indicator of local control [5]. However, the involvement of adjacent organs and blood vessels makes it difficult to perform wide resection with satisfactory tumor-free margins. Bonvalot et al. [1] performed compartmental resection, in which the multivisceral removal and blood vessel replacement was a necessity to obtain a safe margin. However, Ikoma et al. [6] suggested that concomitant organ resection did not improve the prognosis of well-differentiated, primary retroperitoneal liposarcoma. In the present study, we found that macroscopically tumor-free margin should be the first aim of IFS surgeries as it is a predictor of LRFS and OS. Patients with IFS presented more vascular involvement (21.7%) compared with that of retroperitoneal space involvement (17.7%) as reported in literature [3]. Of the 18 patients with vascular invasion in the present study, their median OS was 56 months in the blood vessel replacement group and 20 months in the non-replacement group (P = 0.037). Blood vessel replacement improved the quality of surgery and ultimately prolonged OS. Patients undergoing aggressive procedures for tumor-free margin had similar short-term survival to patients with simple resection (P = 0.354), but the difference in 5-year OS (48.5 vs. 58.9 months) may be attributed to different tumor biological behaviors: the biological behavior of tumors in patients with visceral resection and/or blood vessel replacement is very aggressive. In addition, the difference in complication rates between these two groups was not significant, except that the amount of blood loss in the simple resection group was lower, indicating that the aggressive procedures were feasible and safe. We observed no impact of postoperative complications on OS, local recurrence, or distant metastases.
The role of radiotherapy in the treatment of RPS remains controversial [7]. It is difficult to evaluate the absolute benefits of neoadjuvant or adjuvant radiotherapy because treatment outcomes can be influenced by other factors. Recently, neoadjuvant radiotherapy is widely accepted. However, the effect of radiotherapy on IFS has not been evaluated. In the present study, the objective response rate of neoadjuvant radiotherapy was 21.1% (8/38). Adjuvant radiotherapy was associated with local control (supplementary Table S3, Figure 2D). Although a recent report indicated that radiotherapy had no significant impact on the OS or recurrence-free survival of patients with RPS [4], our results and those observed by Bonvalot et al. [1] favor the use of radiotherapy in IFS patients.
Currently, there is no level I evidence supporting the routine use of neoadjuvant or adjuvant chemotherapy and/or radiotherapy for resectable RPS [8]. Some regimens and agents are widely accepted to be effective in specific subtypes of PRS [9]. The role of chemotherapy in RPS remains controversial [10]. The present study indicated that neoadjuvant chemotherapy increased the resectability of high-grade tumors, whereas adjuvant chemotherapy showed an adverse effect on survival (supplementary Table S2), which may be explained by the imbalanced distribution of tumor grade between patients who did and did not receive adjuvant chemotherapy. Statistical analysis on the 65 patients with grade 2/3 tumors was not performed as only 10 did not receive adjuvant chemotherapy. Therefore, the role of adjuvant chemotherapy in IFS requires further investigation.
Given the retrospective nature of the present study and the small cohort from a single-center, conclusions should be drawn with caution. Although prognostic factors for RPS have been well studied, the present study focused on one subtype with a special location iliac fossa, in which a higher chance of vascular involvement was observed compared to those in RPS of other sites [3]. We demonstrated that R0/R1 resection, small tumor size, low grade, early-stage, thorough surgery, and adjuvant radiotherapy were predictors of prolonged OS and improved local control. Thus, we concluded that well-designed surgery without tumor rupture should be performed to achieve a safe margin for IFS, and that radiotherapy may play an important role for local control.
AVAILABILITY OF DATA AND MATERIALS
Some of the data generated or analyzed during this study are included in this published article. They can be shared. The original datasets used in this study are available from the corresponding author on reasonable request.
AUTHOR INFORMATION
CONTRIBUTIONS
WS, HZ and BZ were responsible for the statistical analysis and manuscript preparation; YC and XQ was responsible for the study concepts, design, data acquisition, quality control of data and algorithms, and manuscript editing and review. RZ, CW, WY, and BW were all responsible for the data acquisition and manuscript review. HZ was responsible for the study design and the data analysis and interpretation. All authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
We thank American Editor for professional editing services.
DECLARATIONS
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The Institutional Review Board of Fudan University Shanghai Cancer Center approved this study, all patients had given consent.
CONSENT FOR PUBLICATION
The study participants provided written consent for the publication of this information.
COMPETING INTERESTS
The authors declare that they have no competing interests.
FUNDING
This work was supported by the grants from National Natural Science Foundation of China (No. 81302342).The funders had no role in the study design, the data collection and analysis, the decision to publish, or the preparation of the manuscript.




