Consideration of the thoracic phenotype of cerebro-costo-mandibular syndrome
Abstract
Cerebro-costo-mandibular syndrome (CCMS) is a congenital condition with skeletal and orofacial abnormalities that often results in respiratory distress in neonates. The three main phenotypes in the thorax are posterior rib gaps, abnormal costovertebral articulation and absent ribs. Although the condition can be lethal, accurate diagnosis, and subsequent management help improve the survival rate. Mutations in the causative gene SNRPB have been identified, however, the mechanism whereby the skeletal phenotypes affect respiratory function is not well-studied due to the multiple skeletal phenotypes, lack of anatomy-based studies into the condition and rarity of CCMS cases. This review aims to clarify the extent to which the three main skeletal phenotypes in the thorax contribute to respiratory distress in neonates with CCMS. Despite the posterior rib gaps being unique to this condition and visually striking on radiographic images, anatomical consideration, and meta-analyses suggested that they might not be the significant factor in causing respiratory distress in neonates. Rather, the increase in chest wall compliance due to the rib gaps and the decrease in compliance at the costovertebral complex was considered to result in an equilibrium, minimizing the impact of these abnormalities. The absence of floating ribs is likely insignificant as seen in the general population; however, a further absence of ribs or vestigial rib formation is associated with respiratory distress and increased lethality. Based on these, we propose to evaluate the number of absent or vestigial ribs as a priority indicator to develop a personalized treatment plan based on the phenotypes exhibited.
1 INTRODUCTION
Cerebro-costo-mandibular syndrome (CCMS) is a rare congenital condition causing skeletal and orofacial abnormalities that often result in respiratory distress in neonates and may lead to early mortality without early intervention and respiratory management. The condition was first noted in 1966 by Smith et al. and over 90 cases have been reported since (Table 1). Most cases of CCMS are sporadic, however, familial occurrences have been reported, including families with an affected parent and child (Bacrot et al., 2015; Drossou-Agakidou et al., 1991; Flodmark & Wattsgard, 2001; James & Aftimos, 2003; Leroy et al., 1981; Lynch et al., 2014; Merlob et al., 1987; Morin et al., 2001; Tooley et al., 2016; van den Ende et al., 1998; Wilcox & Tatum, 2004). Although mutations in the spliceosomal gene SNRPB have been identified as the cause of CCMS (Bacrot et al., 2015; Lynch et al., 2014), the molecular mechanism for the phenotype is unknown. The common skeletal features that characterize this condition include striking posterior rib gaps, absent (missing) ribs and fusion of the costovertebral complex (Tooley et al., 2016; Figure 1). A combination of severe micrognathia, orofacial anomalies, and glossoptosis, called Pierre Robin sequence, are also common findings in patients (Tooley et al., 2016). The presentation of these features and severity of the condition varies between patients. The rib gap phenotype is the defining feature of CCMS, making it referred to as “rib-gap syndrome” or “rib-gap defect with micrognathia syndrome,” whereas the other skeletal abnormalities may be observed in other conditions.
| Total 84 articles, 143 cases | Included or excluded | References |
|---|---|---|
| Full text and images were not available | Excluded | (Özahi et al., 2002; Padovani et al., 1982; Surka et al., 1999) |
| Information insufficient to judge the phenotype (insufficient description, unclear image, no full-chest image, no information on the survival length) | Excluded | (Blaas et al., 1993; Farriaux & Poupard, 1978; Feldman & Heyman, 1987; Kang et al., 1992; Morin et al., 2001; Tanyeri et al., 2018; Winter & Bloom, 1999) |
| Causal mutations in other genes than SNRPB and hence not diagnosed as CCMS | Excluded | (Legare et al., 2017; Zeevaert et al., 2009) |
| Costovertebral joint defects without rib gap | Excluded | (Neelam & Sudhir, 2021) |
| No ribs or underdeveloped ribs in the fetus/too young gestational stage/premature birth | Excluded | (Fidalgo-Alvarez et al., 1988; Hennekam & Goldschmeding, 1998; Ibba et al., 1997; Kirk et al., 1999; Lale et al., 2011; Megier et al., 1998; Sahyoun et al., 1998; Song et al., 1993) |
| Reporting follow-up only/follow-up cases and new cases without sufficient information | Excluded | (Doyle, 1969; Schrander-Stumpel et al., 1996; van den Ende et al., 1998) |
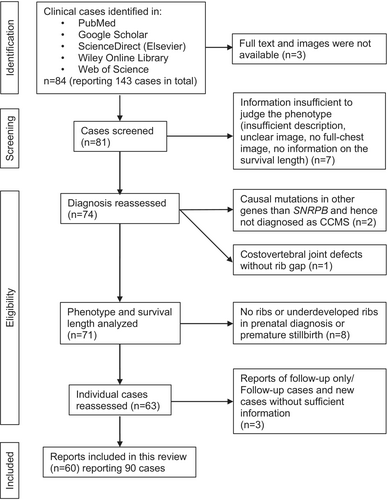
| Analyzed in this study (90 cases in 60 papers in total) | Included | (Abdalla et al., 2011; Bacrot et al., 2015; Beluffi et al., 2001; Bhat et al., 2020; Burton & Oestreich, 1988; Casas Gomez et al., 1996; Clarke & Nguyen, 1985; Cohnen et al., 1999; Drossou-Agakidou et al., 1991; Duval et al., 1998; Ech-Cherif El Kettani & Dafiri, 2004; Faure et al., 1978; Flodmark & Wattsgard, 2001; Frulio et al., 2007; Gurgey et al., 1985; Hanicka, 1979; Harris & Fellows, 1977; Hegde et al., 2011; Herman & Siegel, 1996; Hosalkar et al., 2000; James & Aftimos, 2003; Kemperdick & Lemburg, 1976; Kirk & Ades, 1998; Kringelbach & Henriksen, 1968; Kuhn et al., 1975; Langer Jr. & Herrmann, 1974; Leroy et al., 1981; Lim & Koh, 1992; Lynch et al., 2014; Matic et al., 2016; McNicholl et al., 1970; Meinecke et al., 1987; Merlob et al., 1987; Miller et al., 1972; Mohan & Mandalam, 1982; Mota et al., 2000; Nagasawa et al., 2010; Nicholls & Fletcher, 1973; Oestreich & Stanek, 2010; Ogasawara et al., 2014; Ogita et al., 2008; Ohtsuka et al., 2019; Ozkinay et al., 2001; Plotz et al., 1996; Ramaswamy et al., 2016; Schroer & Meyer, 1984; Shimogoori et al., 2010; Silverman et al., 1980; Simma et al., 1989; Smith et al., 1966; Smith & Sekar, 1985; So et al., 2010; Su et al., 2010; Tachibana et al., 1980; Tooley et al., 2016; Trautman et al., 1985; Walizadeh, 1978; Watson et al., 2014; Wilcox & Tatum, 2004; Williams & Sane, 1976) |
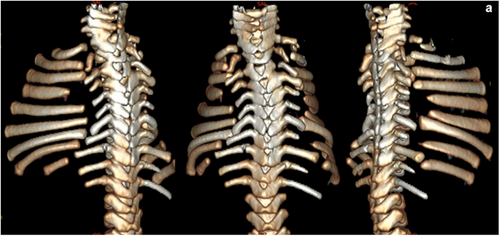
While rib gaps attract attention as the defining feature of CCMS, abnormal costovertebral articulations, which encompass a fusion of the costovertebral joint and absent transverse processes, are described only in some reports (Flodmark & Wattsgard, 2001; McNicholl et al., 1970; Tooley et al., 2016; Watson et al., 2014; Williams & Sane, 1976). This may be due to the abnormalities of the costovertebral joint being harder to identify and possible to overlook. Reduced numbers of ribs are observed in numerous case reports of CCMS with the most common description of this phenotype being the lack of bilateral twelfth ribs, although further rib agenesis is not uncommon (Bhat et al., 2020; Smith et al., 1966; Tooley et al., 2016). There is little discussion regarding the functional implications of this feature.
To date, the focus of much research into CCMS has been on the clinical or phenotypic basis for dysfunction. Clarifying how each of the phenotypes of the condition may affect the patient over an extended period is hindered by the rare incidence and limited long-term follow-up studies with patients (Bhat et al., 2020). As such, anatomically oriented studies are vital to clarify the significance of individual skeletal phenotypes of the condition.
This study aimed to clarify whether it was the posterior rib gaps, abnormalities of the costovertebral complex or the absent ribs that had the greatest impact on the severity of CCMS in neonates in anatomical aspects. By improving the understanding of how each skeletal phenotype contributes to the clinical severity of CCMS, it was anticipated that clinicians would be able to assess the presentation of the condition more accurately, make a well-informed prognosis and create a more personalized treatment plan based on the severity of the phenotypes exhibited by patients. To help understand the impact of each phenotype on prognosis, this article discusses anatomical consideration of thoracic phenotypes in CCMS and assesses it with a systematic review of past case reports.
2 THE UNIQUE FEATURES OF THE NEONATE RESPIRATORY SYSTEM
The neonatal respiratory system has fundamental structural differences from that of an adult with physiological differences in the developing respiratory muscles that collectively predispose infants to respiratory problems even in the absence of any abnormalities. The thoracic cage is ovoid at birth, becoming elliptical in shape with skeletal maturity (Canavese & Dimeglio, 2013). The rounder and more compressed shape of the ribcage in neonates facilitates a horizontal insertion of the ribs so the intercostal muscles cannot raise them effectively. As a result, the ribs of the neonate are already elevated to the near-horizontal angle, which could explain why the movement of the rib cage in infants contributes little to the tidal volume (Gaultier, 1995). This makes infants' respiration reliant on diaphragmatic breathing (Garcia-Martinez et al., 2016). Despite the reliance on the diaphragm in neonates to maintain respiration, the small mass and low oxidative capacity of the respiratory muscle fibers limit their strength and the ability of the muscles to resist fatigue (Goldsmith & Karotkin, 1988). The diaphragm and intercostal muscles of the neonate contain less type I muscle fibers (the slow endurance fibers) than the muscles of children or adults; this explains why the muscles are more prone to fatigue in neonates (Neumann & von Ungern-Sternberg, 2014). The fiber composition of the diaphragm muscle changes by age: the diaphragm of a premature infant contains approximately 10% type I fibers compared with 25% in a full-term infant, and 50%–55% in 7–8 months reaching the adulthood level (Anraku & Shargall, 2009; Muller & Bryan, 1979). It was also found in the fetal lamb that the fatigue resistance of the diaphragm increases steadily after birth, in line with the increase in type I fibers (Lavin et al., 2013). It was also shown in humans that, while diaphragm thickness is positively associated with body size, the transdiaphragmatic pressures in neonates are greater than that predicted by body size compared with adults, suggesting the large workload on the diaphragm (Kassim et al., 2015; Rehan & McCool, 2003). These findings demonstrate that breathing in neonates is very challenging both anatomically and physiologically.
In adults, due to changes in rib orientation with the anterior (ventral) ends of the ribs located more inferiorly, the volume of the thoracic cage can be increased by elevation of the ribs (Bastir et al., 2013). An adult-like thoracic shape with the angle of the slope of the rib is achieved by the end of the second postnatal year because of the development of an upright posture and subsequent redistribution of muscle tone in the infant (Openshaw et al., 1984). A more recent study using computed tomography (CT) data confirmed a marked increase in the antero-posterior and medio-lateral dimensions of the thorax by the age of three with authors attributing these changes to the rate of maturation of the respiratory system and the demands of locomotion with postural changes (Bastir et al., 2013). The change is also seen in CCMS patients by comparing chest radiographs at 2-week-old and 2-year-old in the same patient (Tooley et al., 2016).
A significant part of the work of the diaphragm in neonates is chest wall distortion due to the low resistance from the pliable rib cage (Neumann & von Ungern-Sternberg, 2014). The neonate thoracic cage is more cartilaginous than an adult's thorax. Therefore, it is highly compliant, resulting in paradoxical inward movement of the chest wall during inspiration (LoMauro & Aliverti, 2016). This is because the neonatal chest wall offers little outward recoil to opposing forces of the elastic properties of the lungs. Functional residual capacity is hence reduced as it relies upon the opposing forces of the lung and thoracic cage (Alapati & Shaffer, 2017). Thoracic compliance is three times higher than lung compliance, only equalizing by the end of the second postnatal year due to the rapid ossification (Papastamelos et al., 1995). This amount of compliance in the skeletal elements in relation to the lungs explains why there is a paradoxical retraction of the thorax on inspiration. The configuration of the adult rib cage is achieved in early infancy, with changes in rib orientation, muscle fiber types and ossification of the skeletal features (Anraku & Shargall, 2009; Bastir et al., 2013; Muller & Bryan, 1979). By the age of two, the ossification of the skeletal elements reduces paradoxical retraction, thereby eliminating rib cage distortion which improves respiratory efficiency (Papastamelos et al., 1995). The change in rib orientation increases the mechanical efficiency of the intercostal muscles, which, along with the decreased fatiguability of the diaphragm, facilitates the generation of greater respiratory forces.
The unique physiology of the neonate respiratory system means that any respiratory difficulties experienced after birth can have a severe impact and require immediate intervention and often assisted ventilation. It is not a surprise that the most severe respiratory difficulties associated with CCMS occur in the first year of life, after which the prognosis is favorable (Nagasawa et al., 2010). The physiological inadequacies of the neonate respiratory system mean that added stress due to abnormalities of CCMS can result in fatal respiratory distress.
3 SKELETAL ABNORMALITY IN CCMS: POSTERIOR RIB GAPS
Gaps in the posterior aspect of the ribs distinguish the disorder from other congenital syndromes with Pierre-Robin sequence (Bhat et al., 2020; McNicholl et al., 1970; Nagasawa et al., 2010; Oestreich & Stanek, 2010; Ramaswamy et al., 2016; Smith et al., 1966; Tooley et al., 2016; Watson et al., 2014). The gaps are characterized on radiographs as areas of discontinuity in ossified ribs and are described as affecting respiratory mechanics in a similar way to flail chest (Bhat et al., 2020; McNicholl et al., 1970; So et al., 2010; Watson et al., 2014; Williams & Sane, 1976). In existing CCMS reports, the posterior rib gaps are usually defined as the most functionally significant abnormality, rather than the other skeletal features of the condition: absent ribs or fused costovertebral complexes, which, rightly or wrongly, have not been the focus of investigation.
The prognosis for CCMS patients has generally improved over the decades, and the posterior rib gaps may not prove fatal in new cases (Tooley et al., 2016). This improvement in the prognosis means that it is reasonable to question the true significance of the rib gaps to the respiratory difficulties experienced by CCMS patients. As mentioned above, if a patient survives their first year with early intervention, then they have a vastly increased chance of survival (Nagasawa et al., 2010). This seems to suggest that there are either compensatory mechanisms in place or that the composition of the rib gaps changes over time, thereby reducing the impact of the gaps after infancy. Although the improved prognosis could simply be due to earlier diagnoses leading to early intervention and more effective aggressive treatment. There are no existing studies exploring the functional impact of the rib gaps, possibly due to the rarity of CCMS cases. A better understanding of the pathogenesis of the rib gaps would enable a more accurate prognosis after the identification of this phenotype in neonates.
3.1 The presentation of rib gaps in CCMS case reports
There is currently a single study in the literature that attempts to clarify if the location and frequency of rib gaps and absent ribs have an impact on the prognosis and severity of CCMS (Nagasawa et al., 2010). In this study, after assessing the majority of cases of CCMS worldwide, researchers found posterior rib gaps were most commonly located in the fifth rib on both sides of the thorax, with the occurrence of rib gaps declining with increased distance from the fifth rib (Nagasawa et al., 2010). It should be noted that lower incidence of rib gaps in the eleventh and twelfth ribs should be expected, as these ribs are most likely to be absent. Authors categorized the lethality of the condition based on the life span of the patient, with patients in the most lethal group surviving less than 1 month after birth. In this most lethal group, they found a significantly higher number of absent ribs when compared to the other groups. However, in the groups with survival of between 1 and 12 months or over a year, there was no significant difference in the number of rib deformities (Nagasawa et al., 2010). Moreover, there was no significant difference in the number of rib gaps between the three groups (Nagasawa et al., 2010). This appears to show that when the patient is under 1-month old, the absent-rib phenotype is more detrimental to their respiratory health than rib gaps. After the age of 1 month, there is a reduced likelihood of the condition being lethal to the patient.
As shown below in Section 6, we have conducted a further meta-analysis including newly reported cases. Both studies show similar results, despite the difference in the analytical criteria.
3.2 The composition of the rib gaps
In many case reports, it is suggested that the posterior rib gaps in CCMS consist of fibro-vascular tissue, and that the tissue eventually undergoes calcification (Bhat et al., 2020; Miller et al., 1972; Oestreich & Stanek, 2010; Silverman et al., 1980; Watson et al., 2014; Williams & Sane, 1976). However, due to the absence of many long-term follow-up studies, it is relatively unknown at what age this calcification begins to occur. One follow-up study reported a marked decrease in the size of the rib gaps in a patient (Williams & Sane, 1976). Based on the flail chest movement they observed in the neonatal patient which diminished by the age of five, the authors suggested that the rib gaps consisted of fibrous or mesenchymal tissue which later calcified and formed pseudoarthrosis (Williams & Sane, 1976).
There have since been similar reports of the eventual calcification of the tissue comprising the rib gaps. A report with radiographs from a newborn and 3 years of age showed evidence of narrowed rib gaps and pseudoarticulations (Ogita et al., 2008). In 2011, a 15-year-old patient presented with posterior rib gaps filled with a calcified, disorganized tissue (Abdalla et al., 2011). In 2016, Tooley et al. reported a case featuring chest radiographs at 2 weeks and 2 years of age, which demonstrates the changes in rib gaps in conjunction with the morphological development of the ribs over time. The authors also reported a case who was 15 years of age, displaying healed rib gaps (Tooley et al., 2016). The calcification and healing of rib gaps in childhood could provide an explanation for the severity of CCMS in neonates and the consequent improvement in respiratory symptoms over time.
Post-mortem histological studies revealed that the missing portion of the rib is filled by fibrovascular tissue (Oestreich & Stanek, 2010; Silverman et al., 1980), confirming previous descriptions (Miller et al., 1972; Smith et al., 1966). The fibrous tissue in the space between the two ends of the bone creates an area of greater compliance that contributes to respiratory distress (Watson et al., 2014). In the histological sections, the periosteum that spans the rib gap is very significant. The periosteum is a highly vascularized tissue layer that lines the outer surface of the bone, and its innermost layer consists of many osteoprogenitors that constantly build and repair the bone (Colnot et al., 2012). This appears to offer the potential that rib gaps would have the ability to heal over time. Further studies are needed to clarify the composition of the rib gaps at birth and throughout infancy. More frequent follow-up studies with patients could help clarify the timing of calcification.
The rib gaps of CCMS patients and the associated disruption to chest wall integrity are considered comparable to that of flail chest in some reports (McNicholl et al., 1970; Williams & Sane, 1976). Flail chest movements in adults are usually caused by blunt thoracic trauma and result from three or more ribs being fractured in more than one place (Bastos et al., 2008). This disrupts the integrity of the thoracic cage causing a section of the chest wall to move paradoxically, moving inward on inspiration and outward on expiration (Al-Qadi, 2018). This raises questions as to whether the effect of posterior rib gaps on respiratory mechanics can be likened to flail chest movements. The rib gaps described in CCMS occur only once in each affected rib rather than at multiple sites as described in reports of flail chest, therefore, simple flail chest movement is unlikely. It is possible that there are compensatory mechanisms in place to counteract the chest wall distortion in CCMS patients and stabilize the posterior thorax.
3.3 The compensatory effect of the back muscles
The rib gap develops in the posterior aspect of the rib, located between 0.5 and 2 cm lateral to the angle of the rib in neonates (Smith et al., 1966), and not in the distal and sternal parts of the rib. Volume-rendered images from a CT-scan of CCMS patients further confirmed the location (Bacrot et al., 2015; Tooley et al., 2016; Watson et al., 2014). The location of the rib gaps might be vital for suggesting how compensatory mechanisms could adjust for increased compliance in the posterior thorax. Despite many intrinsic muscles of the back not being classed as muscles with a primary respiratory function, they have attachment points along the posterior aspect of the rib (Figure 2). The attachment points of certain muscles on both the vertebrae and the ribs could provide an explanation for how stability is achieved between both sides of the rib gap, as these muscles could provide an anchoring effect on each side of the gap. The intrinsic muscles of the back that may potentially provide an anchoring effect are discussed below.
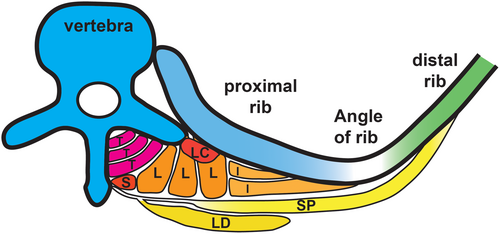
3.3.1 Serratus posterior
Due to the attachment points on the ribs, the serratus posterior muscles have previously been classed as inspiratory muscles (Moore et al., 2017; Snell, 2000; Vilensky et al., 2001). The serratus posterior superior and inferior elevate and depress the associated ribs, respectively (Moore et al., 2017). As there is no hypertrophy of the serratus posterior muscles in patients with chronic obstructive pulmonary disease (COPD), it was suggested that the muscles were not classed as muscles of respiration (Loukas et al., 2008). Nonetheless, due to the attachment points immediately lateral to the angle of the ribs, it is reasonable to assume the muscles contribute to stability surrounding the rib gap in the posterior aspect of the thorax.
3.3.2 Levatores costarum
The reported role of the levatores costarum muscles is to elevate the ribs, but their role in normal inspiration is not clarified (Moore et al., 2017). These muscles span between each rib, stabilizing the rib cage and perhaps contributing to synchronized movement of the ribs. Although levatores costarum muscles are suggested to be mostly tendinous with a small muscle belly (Bayoglu et al., 2017), a study using electromyography found that they were active during quiet breathing (Goldman et al., 1985). This suggests that even if they do not exert a great amount of movement onto individual ribs, these muscles have a role in stabilizing the thorax and contribute toward synchronized movement in CCMS patients as well.
3.3.3 Erector spinae muscles
Acting as extensors of the vertebral column, the erector spinae muscles are divided into three sections from lateral to medial; iliocostalis, longissimus, and spinalis. Iliocostalis and longissimus both have costal attachments at the proximal side of the angle of ribs, suggesting they may have a role in stabilizing the rib cage (Figure 2). Any suggested stabilizing effect of the erector spinae muscles on the ribcage is based on their attachment points and the architecture (Delp et al., 2001). It is suspected that the attachment of iliocostalis to the rib may be compromised by rib gaps in CCMS patients that may contribute to the postnatal progression of scoliosis (Tooley et al., 2016). Further research into muscle attachment in CCMS patients is needed to better understand their respiratory difficulties and the development of scoliosis.
Altogether, all thoracic intrinsic muscles that attach to vertebrae and ribs, that is, serratus posterior, levatores costarum, iliocostalis, and longissimus, have an attachment point on or nearby the angle of the rib and are suggested to contribute to the synchronized movement of the thorax during respiration. Such function is also anticipated in CCMS patients unless the attachment is compromised by the rib gap. Given that there is no significant correlation between the number of rib gaps and the prognosis of that patient (Nagasawa et al., 2010; see also Section 6), the compensatory mechanism by the muscles is speculated to lessen the impact of the gaps on respiratory mechanics and chest compliance in CCMS patients.
4 SKELETAL ABNORMALITY: FUSION OF THE COSTOVERTEBRAL COMPLEX
Abnormal costovertebral articulation is commonly reported in patients with CCMS, but the significance of this feature on respiratory mechanics is rarely discussed (Flodmark & Wattsgard, 2001;McNicholl et al., 1970; Tooley et al., 2016; Watson et al., 2014; Williams & Sane, 1976). The abnormalities include fusion of the ribs to the vertebral body and absent transverse processes (McNicholl et al., 1970; Tooley et al., 2016; Watson et al., 2014; Williams & Sane, 1976). Given the kinematics of the costovertebral joint (Beyer et al., 2014), it is suspected that the fusion at the joint and the absence of transverse processes of the vertebrae would limit the range of motion at this joint usually required to increase lung volume. It is possible that the posterior rib gaps create a point of flexibility and increase the chest wall compliance in the posterior thorax, thus counteracting the decrease in compliance caused by the fused costovertebral joints.
4.1 The normal anatomy and mechanics of the costovertebral complex
A typical rib articulates posteriorly through two joints at the vertebral column: the costovertebral joint between the head of the rib and the vertebral body/bodies, and the costotransverse joint between the tubercle of the rib and the transverse process of the vertebra, with the latter excluding the 11th and 12th ribs (Moore et al., 2017). Movement at the costovertebral joints in the second to sixth ribs causes the anterior ends of the ribs to rise expanding the antero-posterior diameter of the thorax (Ellis, 2008; Moore et al., 2017). Costotransverse joints at the seventh to tenth ribs generate elevation and depression of the lateral part of the ribs, increasing the transverse diameter of the thorax (Moore et al., 2017). These changes in the diameter of the thorax increase intrathoracic volume and consequent pressure changes facilitate inspiration and expiration (Moore et al., 2017).
4.2 The kinematics of the costovertebral joint and respiratory mechanics
The kinematic characteristics of the costovertebral joint have an important role in determining the mechanical behavior of the thorax (Andriacchi et al., 1974). Torsion on the longitudinal axis of the rib neck leads to a great range of motion of the whole rib (Duprey et al., 2010; Moore et al., 2017), that would need to be compensated for, given the fusion of this joint in CCMS. A more recent study found that there is a larger range of motion at the costovertebral joint at lower lung volumes (Beyer et al., 2016). Furthermore, above the middle of inspiratory capacity, the costovertebral joint has no significant effect on the range of motion at the joint (Beyer et al., 2016). Due to the decrease in chest wall compliance with increasing lung volumes (Wilson & De Troyer, 2004), the reliance is shifted to the diaphragm at higher lung volumes (Beyer et al., 2016). It should be noted that the neonates' horizontal orientation of the ribs resembles the latter condition with deep inhalation in adults, with their ribs already at the elevated position. Therefore, neonates greatly rely on the diaphragm regardless of the depth of breathing. This suggests that the fusion of the costovertebral joint in neonates and the subsequent failure of rib movement along the longitudinal axis of the rib neck may not impact as strongly as it would in adults.
4.3 Fetal respiratory movements and development
The fusion between the rib and vertebrae is now commonly described in CCMS case reports since the first description (Smith et al., 1966). We could hypothesize that due to this lack of articulation in the posterior thorax, the rib gaps may develop to compensate for the decreased compliance. This could explain how the chest wall is able to move despite the fusion and decreased compliance at the costovertebral complex. For this hypothesis to be considered, we assume that there is some respiratory movement in utero whilst the ribs were developing, as these early movements would necessitate the development of a method to overcome the fused articulations between the ribs and vertebrae.
In the 1970s, breathing movements in utero were first accepted as a marker for normal fetal lung development (Florido et al., 2008). Although fetal breathing movements do not contribute toward gas exchange, they help strengthen respiratory muscles and aid lung development (Niblock et al., 2020). It was investigated by resecting the phrenic nerve in fetal sheep and thus eliminating the diaphragmatic component of fetal “breathing” movements. This resulted in lung hypoplasia, suggesting that breathing movements have an important role in lung growth (Harding et al., 1993).
Unlike the posterior rib gaps, fusion at the costovertebral joint is not a unique feature to CCMS. Costovertebral fusion experienced in other conditions can reveal the impact of this abnormality if there are no compensatory factors in place. In the case of inflammatory disease ankylosing spondylitis, progressive worsening of the condition can result in fusion of the costovertebral joints, causing pain during breathing in patients (Pascual et al., 1992). Pain is often worsened by deep inspiration, for example, sneezing and coughing (Benhamou et al., 1993), agreeing with the aforementioned limited range of movement at the joint. However, there are limitations when comparing this condition to CCMS in neonates, as the neonatal thoracic cage is more highly compliant, whereas the progressive effects of ankylosing spondylitis will begin in later life. Back pain in ankylosing spondylitis patients is exacerbated during breathing movements, suggesting that there would need to be a mechanism for increasing compliance of the thorax in CCMS patients. This supports the hypothesis that the rib gaps develop in response to fetal respiratory efforts, allowing rib movement which would otherwise be impossible because of the fusion (or a failure of separation) between the ribs and vertebrae.
In summary, the motion at the costovertebral joint controls intrathoracic pressure particularly in the initial phase of breathing movements, which begins at the fetal stage. It is possible that the CCMS fetuses failed to form the continuous rib shafts due to the demand for high compliance at the posterior rib, in order to compensate for decreased compliance and fusion of the costovertebral joint. As such, the rib gap might have been necessary in utero to facilitate fetal respiratory movements. The impact of fusion between the ribs and vertebrae without a compensatory mechanism can be seen in ankylosing spondylitis, in which patients experience painful breathing movements due to their condition.
5 SKELETAL ABNORMALITY: ABSENT RIBS
Another common skeletal abnormality in CCMS cases is the absence of some ribs or rib agenesis (Bhat et al., 2020; McNicholl et al., 1970; Nagasawa et al., 2010; Smith et al., 1966; Tooley et al., 2016), although it is rarely the focus of investigation in CCMS reports. The most commonly reported absent ribs are the twelfth ribs, however further rib agenesis is not uncommon, such as one patient reported by Tooley et al. (2016) having as few as seven ribs on the right side and 10 ribs on the left. Nagasawa et al. (2010) reported an average of 6.4 rib defects (missing ribs) in the group of patients with the most lethal condition, compared with 1.1 and 1.4 rib defects in the groups with an increased survival rate. This suggests that with an increasing number of absent ribs, the prognosis for CCMS patients worsens. As the ribs serve as attachment points for many important muscles, particularly muscles of respiration, it is necessary to address how the absence of the most inferior ribs may affect the development of these muscles and their insertion points on the ribcage. Even though it is a more mobile shorter rib that is not connected to the sternum, the twelfth rib has numerous muscles attached including the diaphragm, quadratus lumborum, longissimus thoracis and iliocostalis (Cranfield et al., 1997).
5.1 The normal anatomy and function of the diaphragm and quadratus lumborum
5.1.1 Diaphragm
The diaphragm attaches to the lumbar vertebrae posteriorly via crura and medial arcuate ligaments, the 12th rib via the lateral arcuate ligaments, and the xiphoid process, lower 6 ribs and costal cartilage anterolaterally (Moore et al., 2017). By the synchronous action of crural and costal parts of the muscles, the gastrointestinal and respiratory functions of the diaphragm are coordinated (Pickering & Jones, 2002).
5.1.2 Quadratus lumborum
The quadratus lumborum is one of the main muscles comprising the posterior abdominal wall, located laterally to the lumbar transverse processes. It attaches superiorly to both the inferior border of the twelfth rib and the lumbar transverse processes and inferiorly to the iliolumbar ligament and iliac crest (Moore et al., 2017). The main actions of this muscle are lateral flexion and some extension of the spine, and due to its costal attachments, it is assumed to have a respiratory function of stabilizing the twelfth rib during inspiration (Phillips et al., 2008). A further fascicular study on the orientation of muscle fibers has revealed that the quadratus lumborum consists of three layers with distinct muscle fiber orientations, among which, just over half the number of fascicles were part of the costal muscle fibers attaching to the 12th rib (Phillips et al., 2008). The spine-supporting function was relatively minor compared to erector spinae and multifidus. Hence, it was suggested that the quadratus lumborum could be classified as a respiratory muscle that anchors the twelfth rib and provides stability for the diaphragm, thus aiding respiration (Phillips et al., 2008).
5.2 The clinical significance of quadratus lumborum and the diaphragm in CCMS cases
Clinical researchers studying the relationship between lumbar scoliosis, quadratus lumborum and twelfth rib length theorized that the quadratus lumborum was crucial for the movement of the 12th rib (Grivas et al., 2016). It was found that the twelfth rib was longer on the convex side in children with lumbar idiopathic scoliosis. There is not enough evidence to suggest whether the asymmetry in the twelfth rib preceded scoliosis or whether the rib length changed in response to increased mechanical demands, as bones remodel throughout life to adapt to their mechanical environment (Pearson & Lieberman, 2004). Nonetheless, the results demonstrate that there is an intrinsic relationship between the twelfth rib and quadratus lumborum. This supports the idea that the development and mechanics of quadratus lumborum could be significantly affected by the absence of the twelfth rib in CCMS patients. Equally important is the development of the diaphragm which also attaches to the twelfth rib. For function not to be compromised, a question is raised as to which bones these muscles attach and whether this may have a significant impact on causing respiratory distress in neonates with CCMS.
5.3 Rib agenesis in lumbocostovertebral syndrome
Due to the lack of anatomical focus in CCMS studies, it is challenging to determine if missing ribs may or may not contribute to respiratory distress in patients. Lumbocostovertebral syndrome (LCVS) is a rare congenital disorder that can cause a variety of malformations including lumbar hernia, rib and vertebral abnormalities and genitourinary defects (Akcora et al., 2008; Bhat et al., 2004; Sengar et al., 2011; Vagholkar & Dastoor, 2013; Wataya et al., 2016). Although the presentation of the phenotype differs slightly in LCVS, rib agenesis in CCMS and LCVS pose the same questions about muscle attachments in the absence of caudal ribs. The absence of the eleventh and twelfth ribs in LCVS predisposes patients with the condition to herniation of the lumbar region. It was suggested that the attachment of muscles to these ribs has a significant role in strengthening this area and if these ribs are absent, then the muscles are attenuated due to the compromised attachment (Vagholkar & Dastoor, 2013). Surprisingly, a study of one patient with seventh and eighth rib agenesis found that despite the presence of the ribs below this defect, the diaphragm was inserted on the sixth rib, above the defect (Sengar et al., 2011). In another study of one LCVS patient with missing eighth to twelfth ribs on the right side, although it is not clarified how the attachments of the diaphragm work in the absence of these ribs, the maximal inspiratory pressure was deemed normal, indicating good diaphragmatic muscle strength (Bhat et al., 2004). Given that patients with LCVS do not present with the same respiratory difficulties experienced by CCMS patients, this appears to suggest that in the absence of the twelfth rib in CCMS, the diaphragm will develop with attachment points on the nearest rib and that this will not compromise function.
5.4 The prevalence of missing ribs in the normal population
It has been demonstrated that there is some incidence of rib abnormalities within the general population not presenting respiratory distress. In 2008, 367 pregnant women were evaluated and 6.3% of the fetuses were found to have an abnormal number of ribs, with 4.1% having 11 ribs unilaterally (Hershkovitz, 2008). A more recent study of 188 pregnant women found that 4.2% of the fetuses had an abnormal number of ribs with no other demonstrable anomalies, although this contains supernumerary ribs as well (Khodair & Hassanen, 2014). In the general population, if the absence of the twelfth rib is not accompanied by other irregularities, then the prognosis for the individual is favorable (Kalelioglu et al., 2019). Generally, the prevalence of missing ribs in the adult population is not well studied as it is classed merely as a normal variant (Hershkovitz, 2008). Therefore, it is likely that the absence of the twelfth rib alone in CCMS is not the primary cause of respiratory distress.
5.5 Missing ribs in CCMS and the biomechanics of the floating ribs
A higher number of missing ribs is found in the group of CCMS patients with the most lethal condition (Nagasawa et al., 2010; see also Section 6). This appears against the idea that missing some ribs may not be crucial for respiratory function based on the phenotype in LCVS and asymptomatic missing ribs in the normal population (Kalelioglu et al., 2019). In this regard, we speculate that the number of missing ribs does not have a direct cause for respiratory distress in CCMS, but rather reflects the overall severity of the CCMS phenotype.
Research is needed to evaluate the mechanical contribution of the floating ribs as several studies have assessed rib cage biomechanics and thoracic stability without intact thoracic cadaveric specimens, making it difficult to evaluate the biomechanical role of the floating ribs in thoracic stability and respiratory efforts (Brasiliense et al., 2011; Mannen et al., 2015). It appears that further rib agenesis beyond the eleventh and twelfth ribs may be significant, as further missing ribs compromise the biomechanics of the ribcage. This led us to assess the missing rib phenotype in two methods: one is to assess the number of missing ribs out of the 12 pairs (total 24), whereas in another assessment missing ribs were evaluated among the 1st to 11th ribs (total 22) and 1st to 10th ribs (total 20) in Section 6.
6 META-ANALYSIS OF REPORTED CCMS CASES: A SYSTEMATIC REVIEW
To validate which of the thoracic skeletal abnormalities is most crucial in the prognosis of CCMS, case reports of CCMS were systematically collected and analyzed for the relation of the phenotype and survival length.
6.1 Methods
The literature search for clinical case reports was conducted using search engines of PubMed, Google Scholar, ScienceDirect, Wiley Online Library and Web of Science, with keywords of “cerebro-costo-mandibular syndrome,” “cerebrocostomandibular,” “CCMS” or “rib gap.” Of the total 84 collected articles reporting 143 cases in total (including follow-up studies of same patients and cases that may not be CCMS), 90 CCMS cases, in which follow-ups were counted as one individual case, were analyzed for the phenotype and survival lengths. The flowchart of the systematic review and individual articles included in and excluded from the analysis are shown in Figure 3 and Table 1, respectively. To focus on the functional impact of skeletal phenotype, we excluded cases of rib agenesis that were seen in premature stillbirths, even if they were diagnosed as CCMS. If the detailed phenotype was not described in the text, they were judged based on the diagnostic image by at least two individuals. All cases were divided into three groups according to the life span: less than 1 month, more than 1 month and less than 1 year, and lived longer than 1 year, in line with the classification by Nagasawa et al. (2010). The number of missing ribs, rib gaps and vestigial ribs was recorded for each case at the earliest available age. It should be noted that some cases were diagnosed at the adult age as a part of the family history analysis. As rib gaps are fused and become insignificant with age (Ogita et al., 2008; Williams & Sane, 1976), traces of the fusion of gaps (pseudoarticulation) were included. Nonetheless, it is plausible that the number of rib gaps may be underestimated in adult cases. Given that missing 12th ribs are found in the normal population without symptoms (Section 5.4), the number of missing ribs in the 1st–11th ribs (total 22 ribs) and 1st–10th ribs were also assessed. Normality of variance was assessed by Shapiro–Wilk test.
6.2 Results
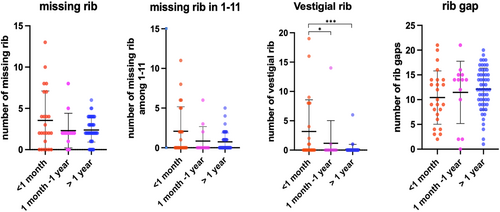
The average number of missing ribs, rib gaps and vestigial ribs in each of the three survival groups are shown in Table 2.
| Group (lethality) | Case number | Average | SD | Variance |
|---|---|---|---|---|
| Missing ribs (among 1st to 12th ribs) | ||||
| <1 month | 24 | 3.54 | 3.56 | 12.69 |
| 1 month <1 year | 13 | 2.31 | 2.10 | 4.40 |
| >1 year | 53 | 2.32 | 1.45 | 2.11 |
| Missing ribs (among 1st to 11th ribs) | ||||
| <1 month | 24 | 2.08 | 3.09 | 9.56 |
| 1 month <1 year | 13 | 0.85 | 1.82 | 3.31 |
| >1 year | 53 | 0.74 | 1.11 | 1.24 |
| Missing ribs (among 1st to 10th ribs) | ||||
| <1 month | 24 | 1.33 | 2.40 | 5.80 |
| 1 month <1 year | 13 | 0.54 | 1.20 | 1.44 |
| >1 year | 53 | 0.32 | 0.83 | 0.68 |
| Vestigial ribs | ||||
| <1 month | 24 | 3.13 | 5.42 | 29.42 |
| 1 month <1 year | 13 | 1.08 | 3.88 | 15.08 |
| >1 year | 53 | 0.13 | 0.83 | 0.69 |
| Rib gaps | ||||
| <1 month | 24 | 10.42 | 5.38 | 28.95 |
| 1 month <1 year | 13 | 11.46 | 6.29 | 39.60 |
| >1 year | 53 | 12.04 | 4.29 | 18.38 |
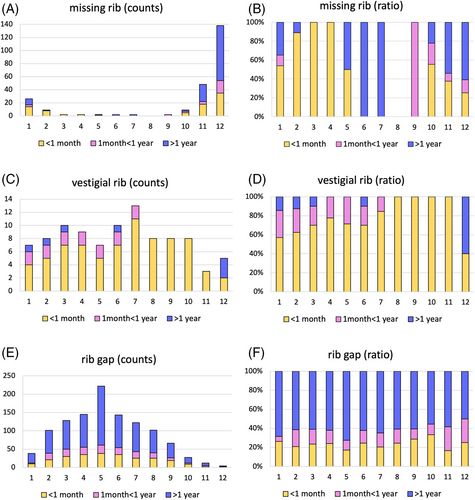
Shapiro–Wilk test showed that the number of missing ribs and vestigial ribs were not normally distributed, as was the number of rib gaps in the group of survival length >1 month and <1 year. Therefore, a statistical analysis was conducted with the Kruskal–Wallis test with Chi-square distribution, followed by post hoc multiple comparisons with Dunn's test. The scatter plots in Figure 4 demonstrate the distribution of cases in each group. No significant differences were found in missing ribs of all 1–12th, 1–11th, and 1–10th categories and rib gap phenotypes among the three survival groups (p > 0.05). The number of vestigial ribs was statistically significant between the group with less than 1-month survival and other groups (Figure 4).
To find out the impact of each rib defect at different axial levels, the frequency of the phenotype on individual ribs is demonstrated in Figure 5. The actual counts of individual ribs presented with the phenotype (90 cases, both sides, in total 180 ribs) and the ratio are shown in Figure 5A–F, respectively. Consistent with the report by Nagasawa et al. (2010), the incidence of a missing rib is the highest at the 12th rib, followed by the 11th rib and 1st rib (Figure 5A). To note, if a missing rib happens in the 1st–5th ribs, those were most likely seen in the shortest survival group (yellow in Figure 5B). As shown in statistical analysis in Figure 3, the vestigial ribs were most frequently seen in the less than 1-month survival group (yellow in Figure 5C,D). The rib gap phenotype is most frequently seen on the 5th rib (Figure 5E), which agrees with the previous report (Nagasawa et al., 2010). Regarding the prevalence of rib gaps at each axial level, there seemed no clear bias between the survival groups (Figure 5E,F). The result supports the idea that rib gaps do not necessarily work as a disadvantage for CCMS patients.
6.3 Discussion
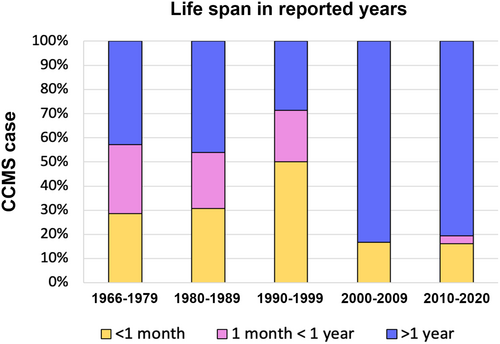
Since the last comprehensive meta-analysis by Nagasawa et al. (2010), a relatively large number of cases of CCMS have been reported in the last decade. This is likely thanks to the widespread knowledge of the syndrome and advanced clinical diagnosis. Identification of the responsible gene SNRPB might have also attracted attention and led to reports of many cases and analyses (Lynch et al., 2014; Tooley et al., 2016). The propagated knowledge may also have contributed to accurate diagnosis, appropriate clinical intervention and improved survival rate in individual cases. Our data show that about 80% of CCMS cases reported since 2000 showed survival of longer than 1 year, whereas, before 2000, 50%–70% of cases died within 1 year (Figure 6). In Nagasawa's report, the number of rib defects was shown to have a statistically significant impact on the survival length of less than 1 month. In our study, missing ribs and vestigial ribs were separately counted, which resulted in a refined outcome. In summary, the present study confirmed that the number of rib gaps is not related to the prognosis and, rather, the formation of the ribs to the full length is more clinically relevant.
7 GENERAL DISCUSSION
By exploring the extent to which the most common skeletal defects in CCMS contribute to the respiratory difficulties experienced in neonates, this paper provides insight into the anatomical basis for dysfunction in CCMS. By assessing the significance of the three skeletal phenotypes: posterior rib gaps, abnormalities of the costovertebral complex, and absent ribs, it is possible to make a more well-informed judgment of which of these phenotypes is most crucial in causing respiratory distress in neonates. In turn, this will provide a new anatomical basis for the examination of suspected CCMS in neonates.
In regard to absent ribs, the most severe cases of CCMS are caused by the absence of not only the floating ribs but also additional ribs as well (Nagasawa et al., 2010). The present study also confirmed it (Figure 5B). In addition, the vestigial ribs at the middle axial levels were seen predominantly in CCMS patients in the group of less than a month survival (Figure 5C,D). The eighth, ninth and tenth ribs, that would serve as an alternative attachment for the diaphragm, move to increase the transverse diameter of the thorax (Ellis, 2008), which could be significant in explaining why the absence or vestigial form of these ribs causes a more severe presentation of CCMS. It is also possible that additional muscle attachments to these ribs may restrict an increase in transverse diameter.
Further studies are advised to include evaluation of the upper respiratory tract and orofacial abnormalities that are common in CCMS. Watson et al. (2014) believed that the posterior rib gaps cause respiratory distress in neonates in the presence of upper airway obstruction, suggesting that the causes of respiratory distress are multifaceted, rather than only due to the rib gaps that characterize the condition. As the rib gaps are filled with fibro-vascular tissue at birth (Bhat et al., 2020; Silverman et al., 1980), they likely have the greatest impact on chest wall compliance in infancy. To ascertain whether the calcification of the gaps is significant in reducing respiratory distress, a study of patients with CT imaging at more frequent intervals throughout infancy with comparison to respiration capacity would be beneficial. If calcification of the rib gaps does not coincide with an improvement in respiratory health, then this suggests calcification of the rib gaps does not benefit respiratory mechanics.
The rib gaps are consistently seen in the posterior aspect with only a slight variation in the position (Bhat et al., 2020; Tooley et al., 2016; Watson et al., 2014). There are many muscles inserting on this area, that likely stabilize the posterior part of the thorax. There is a lack of experimental evidence that the muscles inserting near the rib angle, such as serratus posterior, levatores costarum, and erector spinae, have any significant respiratory function by acting on the rib cage. Nonetheless, the insertion points of these muscles on the posterior rib make it reasonable to assume that some stability is provided by the muscles between the two ends of the rib. This is supported by the calcification of the gaps, as there must be an assumed stability in this region for calcification to occur. From this, it is speculated that the stability between the ends of the rib means that the rib gaps are not the significant factor in causing respiratory distress in neonates as flail chest movements would be limited.
It is necessary to consider the functional effect of these skeletal phenotypes in CCMS patients. The fusion of the costovertebral joints alone would cause a significant decrease in compliance in the posterior thorax. It is reasonable to question how the continuation of the vertebrae into the rib without articulation facilitates respiratory movement. The posterior rib gaps may function to increase compliance as a compensatory mechanism to aid respiratory movement. This is likely in response to fetal respiratory efforts which are essential for lung development in utero (Niblock et al., 2020). With evidence of the eventual ossification of the rib gaps, further research should explore the mechanics of the posterior thorax in CCMS patients with fused costovertebral joints and ossified rib gaps. If this hypothesis is correct, then patients would experience increased respiratory difficulties with increased ossification of the rib gaps. Calcification of the rib gaps may fill the gaps with disorganized tissue—as identified in one study (Abdalla et al., 2011), which likely maintains high compliance.
Tooley et al. (2016) report an improvement in the prognosis of CCMS in recent decades and admit that posterior rib gaps may not prove fatal in new cases. If newly diagnosed patients have a higher chance of survival due to advanced medical intervention, then it is difficult to make a comparison between modern cases and patients from decades past. This is one limitation of meta-analyses which include CCMS patients across decades since the discovery, as the data may be skewed by lower survival rates in earlier cases. Therefore, when including historical case reports, the advancement of technologies and healthcare in more recent cases should be considered.
Today, patients with suspected CCMS have opportunities for early diagnosis due to developments in genetic testing procedures. In the last decade, significant progress into the genetic basis for CCMS has been accomplished (Bacrot et al., 2015; Lynch et al., 2014). Nevertheless, affected infants still face significant morbidity and mortality. Because of this, Tooley et al. (2016) suggested CCMS be considered in infants presenting with severe micrognathia or Pierre Robin sequence, by routine chest radiographs, and parents of children diagnosed with CCMS should be offered genetic testing for future pregnancies. Through early diagnosis and genetic counseling, health practitioners will be able to manage parents' expectations of the health of their children and the outlook for future pregnancies. A combination of genetic testing and increased anatomical consideration of the phenotype will enable a more accurate assessment of the condition for future cases.




