Apoptosis and its therapeutic implications in neurodegenerative diseases
Abstract
Neurodegenerative disorders are characterized by progressive loss of particular populations of neurons. Apoptosis has been implicated in the pathogenesis of neurodegenerative diseases, including Parkinson disease, Alzheimer disease, Huntington disease, and amyotrophic lateral sclerosis. In this review, we focus on the existing notions relevant to comprehending the apoptotic death process, including the morphological features, mediators and regulators of cellular apoptosis. We also highlight the evidence of neuronal apoptotic death in Parkinson disease, Alzheimer disease, Huntington disease, and amyotrophic lateral sclerosis. Additionally, we present evidence of potential therapeutic agents that could modify the apoptotic pathway in the aforementioned neurodegenerative diseases and delay disease progression. Finally, we review the clinical trials that were conducted to evaluate the use of anti-apoptotic drugs in the treatment of the aforementioned neurodegenerative diseases, in order to highlight the essential need for early detection and intervention of neurodegenerative diseases in humans.
1 INTRODUCTION
Neurodegenerative disorders are characterized by progressive loss of particular populations of neurons. They can be categorized based on the principal clinical presentation, anatomic distribution of the neuronal loss, or the chief molecular abnormality (Dugger & Dickson, 2017). The neuropathological assessment at autopsy is the present gold standard basis for the diagnosis of a particular neurodegenerative disease (Bruzova et al., 2021). Neurodegenerative disorders are characterized by particular protein aggregations (Soto & Pritzkow, 2018; Wells et al., 2019). On the other hand, neurodegenerative disorders are associated with many pathological processes in common, such as oxidative stress, neuroinflammation, and programmed cell death (Cenini et al., 2020; Guzman-Martinez et al., 2019). There are many types of programmed cell death, including apoptosis (Chi et al., 2018). Apoptosis has been implicated in the pathogenesis of neurodegenerative diseases (Chi et al., 2018).
2 APOPTOSIS
Apoptosis takes place both physiologically and pathologically (Kaczanowski, 2016; Tuzlak et al., 2016). It occurs physiologically as a homeostatic mechanism to conserve cell populations in tissues during development, and it is necessary for the formation of the proper circuitry during the development and maturation of the nervous system (Kupcho et al., 2019; Wong & Marin, 2019). Pathologically, apoptosis takes place when cells are injured by disease or injurious agents and during aging (Kaczanowski, 2016). Apoptosis is correlated with cytosolic calcium increase, and DNA fragmentation (Banfalvi, 2017; Majtnerova & Rousar, 2018; Xu et al., 2019).
Apoptosis is an energy-dependent biochemical mechanism involving the genetically determined removal of cells, and it is characterized by specific morphological features (Cikla-Suzgun & Kucukguzel, 2019). Characteristically, the organelle integrity is preserved within an intact plasma membrane while apoptotic alterations take place during the apoptotic process (Erekat, 2018a). Additionally, apoptosis is characterized by cell shrinkage, chromatin condensation, and formation of apoptotic bodies that contain their own content of fragmented chromatin and organelles (Banfalvi, 2017). Eventually, the apoptotic bodies are engulfed by phagocytes, which degrade them preventing the occurrence of any inflammatory reaction (Banfalvi, 2017; Erekat, 2017).
2.1 B-cell lymphoma 2 (Bcl-2) family proteins
The Bcl-2 family consists of proteins that regulate apoptosis. It includes more than 20 various members that can be divided into pro-apoptotic and anti-apoptotic members (Edlich, 2018; Kale et al., 2018). Pro-apoptotic Bcl-2 members include Bcl-2-associated X protein (BAX) and Brassinosteroid Insensitive 1 (BRI1)-Associated Kinase (BAK). On the other hand, anti-apoptotic Bcl-2 members include Bcl-2 and B-cell lymphoma-extra large (Bcl-xL) (Edlich, 2018). Bcl-2 family members are characteristically small proteins (20–30 kDa), and they are recognized based on the presence of at least one Bcl-2 homology 3 (BH3) domain (Kale et al., 2018; Warren et al., 2019). For instance, one BH3 domain only is present in BH3-only members, such as BH3 interacting-domain death agonist (BID) and Bcl-2-associated death promoter (BAD). On the other hand, two to four BH3 domains are found in multidomain members, such as BAX and Bcl-2 (Ferdek & Jakubowska, 2017; Ngoi et al., 2020).
Normally, pro-apoptotic Bcl-2 proteins exist as monomers in the cytosol or are slackly correlated with the outer mitochondrial membrane (Subburaj et al., 2015). In order to function, pro-apoptotic multidomain Bcl-2 proteins need to be activated by binding with a BH3-only Bcl-2 protein (Liu et al., 2016). Similarly, BH3-only Bcl-2 members need multidomain Bcl-2 members to trigger apoptosis (Renault et al., 2014). Indeed, binding of BH3-only members with multidomain members is very essential for inducing apoptosis by stimulating structural alterations and other events that ultimately lead to the permeabilization of the outer mitochondrial membrane (Bloch et al., 2021; Rose et al., 2021). On the other hand, pro-apoptotic BH3-only members, such as p53 upregulated modulator of apoptosis (PUMA), bind anti-apoptotic Bcl-2 proteins neutralizing them and subsequently inducing apoptosis (Chen et al., 2018; Vavrova & Rezacova, 2014). Additionally, Bcl-2 family members regulate the permeability transition (PT)-pore during apoptosis (Bloch et al., 2021; Morris et al., 2021). For instance, BAX is conformationally altered, and it consequently inserts itself into the outer mitochondrial membrane opening mitochondrial PT-pore (Bloch et al., 2021; Pena-Blanco & Garcia-Saez, 2018; Rathore et al., 2017). Thus, the PT-pore connecting the inner and outer mitochondrial membranes is opened causing depolarization of the mitochondria, release of small molecules into the cytosol, and enlarged mitochondrial osmosis with subsequent rupture of the outer mitochondrial membrane (Rathore et al., 2017). Hence, apoptosis-promoting molecules can be discharged from the mitochondria (Rathore et al., 2017).
On the other hand, anti-apoptotic Bcl-2 proteins, such as Bcl-2 and Bcl-xL, can suppress apoptosis by counteracting pro-apoptotic Bcl-2 protein function through oligomerization (Maji et al., 2018). Furthermore, anti-apoptotic Bcl-2 proteins probably inhibit apoptosis by exerting more direct effects, such as preserving the integrity of the mitochondrial membrane (Anilkumar et al., 2020; Pecot et al., 2016). Moreover, both pro- and anti-apoptotic Bcl-2 members are interrelated with the function of endoplasmic reticulum, and thus playing roles in calcium homeostasis (Fouque et al., 2016; Vervliet et al., 2016).
2.2 Caspases
Caspases are cysteine-aspartic proteases, which constitute a family of important signaling molecules that have multiple functions according to their subtype and the organ involved (McIlwain et al., 2015). For instance, caspases are necessary for maintaining homeostasis via controlling apoptosis (Van Opdenbosch & Lamkanfi, 2019). Based on the catalytic cysteine residues in the active site of the caspase, caspases hydrolyze the peptide bonds in certain reactions cleaving the key intracellular substrates causing cell dissociation during apoptosis (Van Opdenbosch & Lamkanfi, 2019).
Caspases are synthesized in the cell as inactive zymogens called pro-caspases, which have no significant protease activity (Parrish et al., 2013). However, they can be activated upon their cleavage subsequent to their dimerization, which is triggered by various adapter proteins that bind to specific sites in the procaspase (Kim et al., 2014; Marek, 2013; Parrish et al., 2013). Caspases are categorized into inflammatory caspases (caspases 1, 4, 5, and 12) and apoptotic caspases (Ke et al., 2016; McIlwain et al., 2013). Based on their mechanism of action and their position in the apoptotic signaling pathways, apoptotic caspases are subdivided into initiator caspases (caspase 2, 8, 9, and 10) and executioner or effector caspases (caspases 3, 6, and 7) (Boice & Bouchier-Hayes, 2020; Erekat, 2018a; Wang, Hu, et al., 2020). Different initiator caspases mediate different apoptotic pathways (Van Opdenbosch & Lamkanfi, 2019). Caspase-9 mediates the intrinsic apoptotic pathway, whereas the extrinsic apoptotic pathway is mediated by caspase-8 (Erekat, 2019; Van Opdenbosch & Lamkanfi, 2019).
Upon apoptotic stimuli, initiator caspases become dimerized, cleaved and subsequently activated (Erekat, 2018a; Van Opdenbosch & Lamkanfi, 2019). Once activated, initiator caspases activate the executioner caspases, which initiate the morphological and biochemical characteristics of apoptotic degradation stage, including DNA fragmentation, cell shrinkage, and membrane blebbing (Erekat, 2018a; Van Opdenbosch & Lamkanfi, 2019). Additionally, activated executioner caspases activate cytoplasmic endonucleases and proteases, which degrade nuclear material, and cytoskeletal and nuclear proteins, respectively (Erekat, 2018a; Van Opdenbosch & Lamkanfi, 2019).
2.3 Apoptotic pathways
Apoptosis occurs via two main pathways, which are the intrinsic and the extrinsic pathways that are regulated at multiple levels (Erekat, 2018a). Intrinsic apoptotic pathway is also called mitochondria-mediated pathway, whereas the extrinsic apoptotic pathway is also known as cell death receptor-mediated pathway. Both intrinsic and extrinsic apoptotic pathways are regulated by the inhibitors of apoptosis proteins (IAPs) (Derakhshan et al., 2017; Kiraz et al., 2016).
The intrinsic apoptotic signaling pathway consists of mitochondria-initiated events that are triggered by non-cell death receptor-mediated stimuli, which release intracellular signals performing directly on cell targets either positively or negatively (Chaabane et al., 2014; Yee et al., 2014). Stimuli which operate in positive mode include toxins, hyperthermia, free radicals, hypoxia, radiation, and viral infections (Kalimuthu & Se-Kwon, 2013). In contrast, negative stimuli include loss or absence of certain hormones, growth factors and cytokines that suppress death programs exciting apoptosis (Pan et al., 2021).
Thus, the intrinsic apoptotic pathway (Figure 1) is mediated by mitochondria, which contain key apoptogenic factors such as cytochrome c (Pena-Blanco & Garcia-Saez, 2018). The intrinsic pathway is regulated by members of the Bcl-2 family (Edlich, 2018; Zhang & Saghatelian, 2013). Pro- and anti-apoptotic Bcl-2 proteins are translocated to the mitochondria to control the release of the apoptogenic factors (Edlich, 2018). The pro-apoptotic Bcl-2 proteins trigger the permeabilization of the outer mitochondrial membrane causing cytochrome c to be discharged from the mitochondrial intermembranous space (Suhaili et al., 2017). Consequently, it participates in the formation of a multimeric Apaf-1/cytochrome c complex by binding with apoptotic protease activating factor 1 (Apaf-1) in the presence of ATP (Elena-Real et al., 2018). Subsequently, Apaf-1/cytochrome c complex engages procaspsase-9 producing the apoptosome (Elena-Real et al., 2018). As a consequence, procaspase-9 is cleaved resulting in its activation and subsequent dissociation from the apoptosome (Erekat, 2018a). Once activated, caspase-9 cleaves the executioner caspases-3, -6, and/or -7 resulting in their activation (Van Opdenbosch & Lamkanfi, 2019).
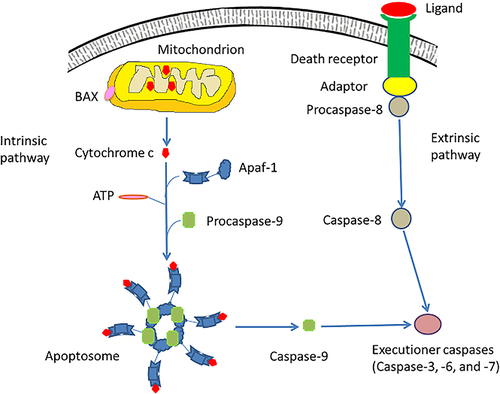
The extrinsic apoptotic pathway (Figure 1) depends on cell surface death receptors, such as those for tumor necrosis factor (TNF), which are regulated by the expression levels of triggering ligands (Bertheloot et al., 2021; Leonard & Johnson, 2018; O'Reilly et al., 2016). The cell surface death receptors activate procaspases through ligand binding (Leonard & Johnson, 2018). Such death receptors are members of the TNF receptor superfamily that have “death domain”, which is a cytoplasmic domain consisting of approximately 80 amino acids (O'Reilly et al., 2016). Death domains have critical role in conveying the death signal from the cell surface to the intracellular signaling pathways (O'Reilly et al., 2016). Thus, when cell surface death receptor-ligand binding occurs, cytoplasmic adapter proteins are recruited, so that they connect with procaspase-8 through dimerization of the death effector domain (O'Reilly et al., 2016). Subsequently, the death-inducing signaling complex (DISC) is formed causing the auto-catalytic activation of procaspase-8. Once activated, caspase-8 stimulates the executioner caspases, which mediate the execution phase of apoptosis (Erekat, 2018b; O'Reilly et al., 2016). Ligands that excite cell surface death receptors include cytokines, such as TNF-α, interferon gamma (IFNγ), and transforming growth factor beta 1 (TGF-β1) (O'Reilly et al., 2016).
3 APOPTOSIS IN NEURODEGENERATIVE DISEASES
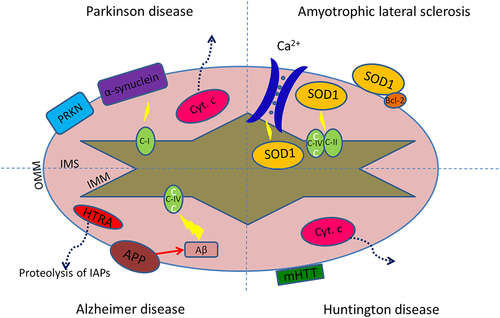
Chronic neurodegenerative diseases are progressive, and most information about them is collected from post-mortem data at the endpoints of the disease process when many other aspects might have interfered with cell death. Thus, human-derived tissue and animal models of neurodegenerative diseases have been very important to model the commencement of the neurodegenerative disease pathology and the various stages of disease, and to subsequently investigate the underlying molecular mechanisms. The evidence for the occurrence of apoptosis in Parkinson disease, Alzheimer disease, Huntington disease, and amyotrophic lateral sclerosis has been provided by many studies, as summarized by Table 1. Additionally, Figure 2 illustrates mitochondria-mediated apoptotic pathway, which has been thoroughly investigated in the aforementioned neurodegenerative diseases (Guo et al., 2013; Huang et al., 2019; Scheuing et al., 2014).
| Neurodegenerative disease | Evidence of apoptosis | Reference |
|---|---|---|
| Parkinson disease | DNA fragmentation detected by TUNEL labeling | Erekat, 2018a; Ma et al., 2018; Balakrishnan et al., 2021; Teng et al., 2020 |
| Overexpression of BAX | Balakrishnan et al., 2021; Dong et al., 2020 | |
| Activation of caspase-9, and -3 | Balakrishnan et al., 2021; Dong et al., 2020; Ma et al., 2018 | |
| Activation of caspase-8 | Balakrishnan et al., 2021 | |
| Reduced Bcl-2 levels | Moujalled et al., 2021; Balakrishnan et al., 2021; Dong et al., 2020 | |
| Alzheimer disease | DNA fragmentation detected by TUNEL labeling, and BAX overexpression | Du et al., 2020 |
| Reduced Bcl-2 expression | Du et al., 2020; Kim et al., 2020; Wang, Pu, et al., 2020; Wang, Hu, et al., 2020 | |
| Activation of caspase-3 | Du et al., 2020; Park et al., 2020; Wang, Pu, et al., 2020; Wang, Hu, et al., 2020 | |
| Huntington disease | DNA fragmentation detected by TUNEL labeling, and BAX overexpression | Ji et al., 2015; Teles et al., 2008 |
| Reduced Bcl-2 expression | Ji et al., 2015 | |
| Activation of caspase-3 | Ji et al., 2015; Maglione et al., 2006 | |
| Activation of caspase-8, and -9 | Maglione et al., 2006 | |
| Amyotrophic lateral sclerosis | DNA fragmentation detected by TUNEL labeling | Shinoe et al., 2001 |
| Caspase-9 activation | Patel et al., 2010; Vaz et al., 2015 | |
| BAX overexpression and reduced Bcl-2 expression | Patel et al., 2010 |
3.1 Apoptosis in Parkinson disease
Parkinson disease is a progressive neurological disorder that is featured by dopaminergic neurodegeneration in the substantia nigra pars compacta (Erekat, 2018a; Youssef et al., 2019). Parkinson disease is usually idiopathic; and can be genetic in rare cases only (Correia Guedes et al., 2020; Stoker & Greenland, 2018). Familial forms of Parkinson disease are associated with mutations in genes, such as leucine-rich repeat kinase 2 (LRRK2), Parkin 2 (PARK2), Parkin 7 (PARK7), Phosphatase and tensin homolog (PTEN)-induced kinase 1 (PINK1) and α-synuclein genes (Balestrino & Schapira, 2020; Correia Guedes et al., 2020). The functions of the corresponding proteins may interconnect with components of the mitochondria-mediated apoptotic pathway given that they are both located on the outer mitochondrial membrane (Borsche et al., 2021; Larsen et al., 2018; Moujalled et al., 2021).
Parkinson disease is pathologically characterized by Lewy bodies that result from the aggregation of α-synuclein, which is localized presynaptically particularly in the nerve terminals (Sorrentino & Giasson, 2020). Additionally, pathogenic processes in Parkinson disease, such as reduced activity of mitochondrial complex I and augmented formation of reactive oxygen species, have been shown to result from the buildup of wild type α-synuclein in dopaminergic neurons (Burre et al., 2018; Reeve et al., 2015). Besides, α-synuclein is localized to the mitochondrial membrane, subsequent to the overexpression of A53T mutant or wild-type, leading to oxidative stress and cytochrome c discharge into the cytoplasm stimulating mitochondria-mediated apoptosis (Rocha, De Miranda, & Sanders, 2018; Salehi et al., 2021).
Apoptosis has been implicated as the mechanism of dopaminergic neuronal loss in Parkinson disease, as illustrated by the identification of apoptotic cells and DNA fragmentation in the substantia nigra of Parkinson diseased patients in post-mortem and in vitro and in vivo studies (Balakrishnan et al., 2021; Erekat, 2018a; Ma et al., 2018; Teng et al., 2020). In addition to that, overexpression of active caspase-8, -9, and -3, as well as increased levels of pro-apoptotic proteins, such as BAX, and reduced anti-apoptotic Bcl-2 levels in the dopaminergic neurons of Parkinson disease in post-mortem and in vitro studies confirmed the role of apoptosis in the pathogenesis of Parkinson disease (Balakrishnan et al., 2021; Bose & Beal, 2016; Dong et al., 2020; Ma et al., 2018; Moujalled et al., 2021).
Dopaminergic neurons are selectively vulnerable to reduced activity of mitochondrial complex I and subsequent mitochondrial dysfunction, because dopamine metabolism results in increased reactive oxygen species generation and the subsequent inhibition of mitochondrial respiration (Haddad & Nakamura, 2015). Subsequently, the mitochondrial PT-pore is opened resulting in cytochrome c discharge into the cytosol, where it triggers the mitochondria-mediated apoptotic pathway, which has been suggested as the chief mechanism of dopaminergic neuronal death in Parkinson disease (Huang et al., 2019; Liu et al., 2018). Indeed, dopamine exposure has been shown to induce the activation of caspase-9 and -3 and the subsequent apoptosis (Vauzour et al., 2014). Dopamine-induced apoptosis is inhibited in response to experimental conditions, such as the addition of antioxidants, and the overexpression of the anti-apoptotic Bcl-2 protein (Shan et al., 2019; Venderova & Park, 2012; Wu et al., 2018).
Thus, many mitochondrial toxins, such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), rotenone, and 6-hydroxydopamine (6-OHDA), prevent mitochondrial complex I resulting in impaired mitochondrial function. Consequently, formation of reactive oxygen species is enhanced leading to apoptosis and subsequent degeneration of dopaminergic neurons (Konnova & Swanberg, 2018). Furthermore, mitochondrial dysfunction has been shown to trigger the mitochondria-mediated apoptotic pathway in humans and in animal models of Parkinson disease, and thus increasing the vulnerability of dopaminergic neurons to degeneration (Su et al., 2021).
3.2 Apoptosis in Alzheimer disease
Alzheimer disease is a devastative neurodegenerative disorder in the elderly, and it is the leading cause of dementia (Cao et al., 2019; Idda et al., 2018). It is pathologically characterized by the buildup of amyloid β-containing neuritic plaques, neurofibrillary tangles and dystrophic neurites, which contain hyperphosphorylated tau (He et al., 2018; Khan et al., 2021; Pires et al., 2019). Alzheimer disease can be familial in rare autosomal dominant cases that are correlated with mutations in the amyloid precursor gene, and in the presenilin-1 and -2 genes (Nicolas et al., 2018; Rocha, Costa, et al., 2018; Tiedt et al., 2018). Increased formation of the mitochondrial reactive oxygen species can elevate β-amyloid protein levels, which inhibit the mitochondrial respiratory chain complex IV causing its dysfunction (Guo et al., 2013). The amyloid precursor protein may be translocated to the outer mitochondrial membrane, where it is cleaved by γ-secretase complexes that contain presenilin 1 to form β-amyloid (Guo et al., 2013). Presenilin 1 augments the proteolytic activity of high temperature requirement protein A2 (HTRA2) (Guo et al., 2013), which is then translocated to the cytosol, where it causes degradation of IAPs (Zheng et al., 2021).
Apoptosis might be implicated in the pathogenesis of Alzheimer disease, but the evidence for its role in neuronal cell death in Alzheimer disease is limited (Moujalled et al., 2021; Obulesu & Lakshmi, 2014). Brains of Alzheimer diseased patients have demonstrated DNA fragmentation that is detected by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining, reduced expression of anti-apoptotic Bcl-2, overexpression of pro-apoptotic BAX, and the presence of activated caspase-3 in neurons with neurofibrillary tangles, indicating the involvement of apoptosis in the pathogenesis of Alzheimer disease (Du et al., 2020; Kim et al., 2020; Park et al., 2020; Wang, Pu, et al., 2020).
Amyloid precursor protein is an extensively expressed transmembrane protein in neurons, and its cleavage results in the formation of C31, which is a carboxy-terminal peptide that induces apoptosis (Park et al., 2020; Roher et al., 2017). Additionally, in vitro studies have shown that exposure to β-amyloid increases oxidative stress and decreases energy availability. Thus, it increases the cellular susceptibility to death by inducing apoptosis, which leads to caspase activation, membrane blebbing and chromatin condensation (Turunc Bayrakdar et al., 2014; Zhang et al., 2017). Accordingly, it is postulated that β-amyloid triggers neuronal apoptosis by oxidative mechanism, through which β-amyloid peptides induce an early and concurrent formation of H2O2 and 4-hydroxynonenal (HNE). Subsequently, c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (p38MAPK) are activated, and nuclear alterations distinctive of apoptosis are apparent (Cheignon et al., 2018). Consequently, activated caspases cleave the microtubule-binding protein tau, which produces a product that aggregates more quickly and extensively into tau filaments, compared with wild-type tau, eventually leading to β-amyloid-induced apoptosis and associated neuronal loss (Chen et al., 2017; Tapia-Rojas et al., 2019). However, antioxidants have been shown to inhibit the neuronal apoptosis and MAPK activation following exposure to β-amyloid (Jurcau, 2021).
Mutant presenilin proteins increase neuronal vulnerability to different insults, such as exposure to β-amyloid or glutamate, and energy depletion, in association with the induction of apoptosis (Honarnejad et al., 2013). However, agents that prevent oxidative stress inhibit the apoptotic induction correlated with the mutant human presenilin-1. Thus, presenilin-1 is suggested to be implicated in apoptosis by causing impaired mitochondrial function and oxidative stress in the Alzheimer diseased brain (Zeng et al., 2015). Indeed, alterations in the levels of expression and activities of antioxidant enzymes have been shown in Alzheimer diseased brain (Peter et al., 2015; Pocernich & Butterfield, 2012).
3.3 Apoptosis in Huntington disease
Huntington disease is an autosomal dominant neurodegenerative disorder, which is characterized by progressive impairment of coordinated voluntary movements, and behavioral and cognitive decline (Dickey & La Spada, 2018; Testa & Jankovic, 2019). It results from a mutation of abnormally expanded CAG trinucleotide repeats in a gene encodig polyglutamine repeats in a protein, called huntingtin. Consequently, inclusion bodies are formed leading to the degeneration of GABAergic neurons in the neostriatum (Bogomazova et al., 2019; Ekman et al., 2019). Mutant huntingtin is shown to induce neuronal apoptosis, and to be a substrate for caspase-3 that cleaves it producing increasingly neurotoxic huntingtin fragments (Ghosh et al., 2020; McColgan & Tabrizi, 2018; Pantiya et al., 2020). Subsequently, wild-type huntingtin is depleted leading to some features of the disease (McAdam et al., 2020). Mutant huntingtin disrupts the equilibrium between pro-apoptotic and anti-apoptotic molecules, and it can interact with mitochondria causing its dysfunction and subsequent abnormalities, such as energy depletion and increased cytochrome c release, which induce apoptosis (Scheuing et al., 2014).
Additionally, huntingtin interacting protein 1 (HIP-1) binds a polypeptide named HIP-1 protein interactor (Hippi) producing a complex that can activate caspase 8 (Bhattacharyya et al., 2008). The formation of the pro-apoptotic Hippi-Hip-1 complex is enhanced in Huntington disease due to the mutated huntigtin-associated increase in the concentration of the free cellular HIP-1 (Bhattacharyya et al., 2008).
Apoptosis is implicated in the pathogenesis of Huntington disease (Jodeiri Farshbaf & Ghaedi, 2017). DNA fragmentation, detected by TUNEL, has been shown in post-mortem Huntington diseased brain tissue (Waldvogel et al., 2012). Additionally, cytochrome c discharge, BAX overexpression, and active caspase-3, -8, and -9 have been illustrated in the brain of Huntington diseased patients and in experimental models of Huntington disease (Ji et al., 2015).
3.4 Apoptosis in amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis is recognized by the progressive and specific loss of motor neurons in the cortex and the ventral horn of the spinal cord. Consequently, all extremities, respiratory and sip musculature is progressively paralyzed leading to death within 3–5 years after the onset of the disease (McAlary et al., 2020). Familial and sporadic forms of the disease have been identified (Zarei et al., 2015). A mutation in the gene encoding superoxide dismutase has been recognized in 20% of patients with familial amyotrophic lateral sclerosis (Guissart et al., 2020). Superoxide dismutase normally acts as free radical scavenger, and thus imposing anti-oxidative and cytoprotective impact (Rosa et al., 2021).
The pathology of the disease possibly involves calcium overload and oxidative stress resulting in impaired mitochondrial function and the subsequent stimulation of the mitochondria-mediated apoptosis (Smith et al., 2019). Indeed, overexpression of mutant superoxide dismutase 1 damages electron transport chain activities and reduces mitochondrial calcium-loading capability. Superoxide dismutase 1 has been localized to the outer mitochondrial membrane, intermembraneous space, and matrix (Guo et al., 2013). Mutant superoxide dismutase 1 causes cytochrome c discharge inducing apoptosis (Guo et al., 2013). Additionally, it stimulates abnormal formation of mitochondrial reactive oxygen species, and it binds the anti-apoptotic protein Bcl-2 degrading it, and thus promoting apoptosis (Guo et al., 2013).
Implication of apoptosis in the pathogenesis of amyotrophic lateral sclerosis has been suggested by the illustration of the DNA fragmentation, Caspase 9 activation, cytochrome c discharge, BAX overexpression, and reduced Bcl-2 expression in post-mortem tissue and transgenic mouse models of amyotrophic lateral sclerosis (Patel et al., 2010; Sathasivam & Shaw, 2005; Shinoe et al., 2001; Vaz et al., 2015).
4 THERAPEUTIC IMPLICATIONS
Given the accumulating evidence that supports the implication of apoptosis in the pathogenesis of various neurodegenerative diseases (Erekat, 2018a; Ma et al., 2018; Moujalled et al., 2021), treatments that impede the sequence of events involved in the apoptotic pathways may serve as potential therapeutic approaches in rescuing neurons from cell death that is implicated in the pathogenesis of the various neurodegenerative diseases, as summarized in Table 2.
| Neurodegenerative disease | Therapeutic implications | Reference |
|---|---|---|
| Parkinson disease | Minocycline inhibits mitochondrial cytochrome c release increasing the expression of anti-apoptotic BCL-2 | Cankaya et al., 2019 Kim & Suh, 2009 |
| Caspase inhibition has been shown to rescue dopaminergic neurons from death in mice | Eberhardt et al., 2000 | |
| Propargylamine derivative CGP 3466 (dibenzo[b,f]oxepin-10-ylmethyl-methyl-prop-2-ynyl-amine) is a drug that ultimately results in the upregulation of the anti-apoptotic Bcl-2 and reduced expression of the pro-apoptotic Bax and active-caspase-3 | Maruyama et al., 2002 Erekat, 2018a |
|
| Hesperidin exerts neuroprotection, chiefly by antioxidant mechanisms, reducing caspase-3 and -9 activity in rats | Justin Thenmozhi et al., 2017 | |
| Alzheimer disease | Hesperidin exerts neuroprotection, chiefly by antioxidant mechanisms, decreasing the expression of BAX | Justin Thenmozhi et al., 2017 |
| Oral intake of antioxidants, such as vitamin E, has been reported to slow the progression of Alzheimer disease | Sinyor et al., 2020; Veurink et al., 2020 | |
| The antioxidant drug called Ebselen could suppress apoptosis in diseased mice | Martini et al., 2019 | |
| Huntington disease | Minocycline inhibits mitochondrial cytochrome c release increasing the expression of anti-apoptotic BCL-2 | Kim & Suh, 2009 |
| Caspase inhibition has been shown to slow the progression of the disease in a transgenic mouse model | Sanchez Mejia & Friedlander, 2001 | |
| Amyotrophic lateral sclerosis | Minocycline inhibits mitochondrial cytochrome c release increasing the expression of anti-apoptotic BCL-2 | Kim & Suh, 2009 |
| caspase inhibition interfered significantly with the progression of the disease delaying neuronal death and extended the survival of mice | Rohn, 2010 | |
| Bcl-2 overexpression and the deletion of proapoptotic BAX and BAK have been shown to exert neuroprotection in diseased mice by blocking the apoptosis of motor neurons of the lumbar spinal cord | Reyes et al., 2010 Moujalled et al., 2021 |
4.1 Inhibition of mitochondrial cytochrome c release
Minocycline is a second generation tetracycline that inhibits mitochondrial cytochrome c release, which is an essential step for the progression of the mitochondria-mediated apoptotic pathway (Firsov et al., 2018). It has been demonstrated to increase the expression of anti-apoptotic Bcl-2, and to display broad neuroprotection in experimental models of neurodegenerative diseases, such as Parkinson disease, Huntington disease and amyotrophic lateral sclerosis (Cankaya et al., 2019; Garrido-Mesa et al., 2013).
4.2 Caspase inhibition
Since apoptosis is mediated by caspase activation, caspase inhibition has been suggested as a potential anti-apoptotic therapeutic approach in neurodegenerative diseases that occur via apoptosis. Indeed, the administration of caspase inhibitors interfered significantly with the progression of the disease delaying neuronal death and subsequent onset of symptoms in mice with amyotrophic lateral sclerosis extending their survival (Rohn, 2010). Similarly, caspase inhibition has been shown to slow the progression of the disease in a transgenic mouse model of Huntington disease (Sanchez Mejia & Friedlander, 2001). Additionally, caspase inhibition has been shown to rescue dopaminergic neurons from death in mice with MPTP-induced Parkinson disease. However, rescued dopaminergic neurons were dysfunctional with disrupted nigrostriatal terminals (Eberhardt et al., 2000). Nevertheless, this issue was avoided by simultaneous administration of glial cell line-derived neurotrophic factor (GDNF) and caspase inhibition. Thus, combinations of caspase inhibition and specific growth factors have been suggested as potential therapeutic approach in Parkinson disease (Eberhardt et al., 2000).
4.3 Overexpression of the anti-apoptotic protein Bcl-2
Propargylamine derivative CGP 3466 (dibenzo[b,f]oxepin-10-ylmethyl-methyl-prop-2-ynyl-amine) is a drug that ultimately results in the upregulation of the anti-apoptotic Bcl-2 and reduced expression of the pro-apoptotic BAX and active caspase-3 (Maruyama et al., 2002). CGP 3466 has been reported to rescue dopaminergic neurons from death and to subsequently inhibit the development of motor symptoms in rodent models of Parkinson disease by preventing mitochondria-mediated apoptosis (Erekat, 2018a). Additionally, Bcl-2 overexpression and the deletion of pro-apoptotic BAX and BAK have been shown to exert neuroprotection in mice with amyotrophic lateral sclerosis by blocking the apoptosis of motor neurons in the lumbar spinal cord delaying the onset of the symptoms (Moujalled et al., 2021; Reyes et al., 2010).
4.4 The use of antioxidants
The use of antioxidants has been considered as a therapeutic strategy in an attempt to enhance cellular capacity to oppose apoptotic triggers (Feng & Wang, 2012; Fujita et al., 2012). For instance, Hesperidin, which is a flavonoid glycoside frequently found in citrus fruits, has been shown to exert neuroprotection, chiefly by antioxidant mechanisms, improving mitochondrial function and reducing apoptosis in animal models of neurodegenerative diseases. Indeed, it has been shown to reduce caspase-3 and -9 activity improving the behavioral alterations in 6-OHDA model of Parkinson disease. Additionally, it has been illustrated to decrease the expression of BAX ameliorating the cognitive dysfunction and reversing memory loss in a rat model of Alzheimer disease (Antunes et al., 2021; Justin Thenmozhi et al., 2017). Furthermore, the oral intake of antioxidants, such as vitamin E, has been reported to slow the progression of Alzheimer disease (Sinyor et al., 2020; Veurink et al., 2020). Besides, the administration of Ebselen, which is an antioxidant drug with glutathione peroxidase-like action, could attenuate apoptosis causing neuroprotection in a mouse model of Alzheimer disease (Martini et al., 2019).
Thus, as discussed above, caspase inhibition may cause a switch from apoptosis to necrosis, suggesting that blockade of apoptosis may lead to necrosis in cell culture models of Parkinson disease (Hartmann et al., 2001). Additionally, although genetic deletion of BAX inhibited the neuronal apoptosis in mouse model of Parkinson disease, the rescued dopaminergic neurons demonstrated marked neuronal atrophy, and Parkinson disease-related behavioral deficits were not improved (Kim et al., 2011). Therefore, although apoptosis is the endpoint in the pathogenesis of various neurodegenerative diseases, the inhibition of apoptosis has been shown to sometimes result in rescued neurons that are dysfunctional or in a switch from neuronal apoptosis to necrosis. Thus, it is still to be cautiously assessed whether or not blockade of neuronal apoptosis can be harmless and therapeutically beneficial in the neurodegenerative diseases.
5 CLINICAL TRIALS
Based on the results illustrated in preclinical studies in animal models and human-derived tissues, anti-apoptotic drugs have been developed and shown to be promising in the treatment of neurodegenerative diseases (Wilkins & Morris, 2017). Consequently, several anti-apoptotic drugs have been tested in clinical trials investigating their neuroprotective effects in patients with various neurodegenerative diseases (Wilkins & Morris, 2017). Nonetheless, such clinical trials have been plagued by limitations, such as different stages and burden of disease (Sarkar et al., 2016; Scheuing et al., 2014; Wilkins & Morris, 2017). Consequently, further clinical studies should be conducted taking in consideration early detection and intervention of the neurodegenerative disease. Thus, clinical trials conducted to evaluate the use minocycline and coenzyme Q10 (CoQ10), as examples of such anti-apoptotic drugs, in the treatment of neurodegenerative diseases, are reviewed in this section, as summarized in Table 3.
| Neurodegenerative disease | Clinical trial phase and findings | Reference |
|---|---|---|
| Minocycline | ||
| Parkinson disease | Phase II: considerations are warranted for definitive phase III trials to assess whether it can modify the long term progression of Parkinson disease | Investigators, 2008 |
| Alzheimer disease | Phase II: the progress of cognitive or functional impairment was not delayed | Howard et al., 2020 |
| Huntington disease | Phase II: futility criteria were reached warranting no additional study | Huntington Study Group, 2010 |
| Amyotrophic lateral sclerosis | Phase III: harmful effect on patients | Gordon et al., 2007 |
| CoQ10 | ||
| Parkinson disease | Phase III: it failed to confirm any clinical benefit of CoQ10 compared to placebo, and was ended when a futility criterion was reached | Parkinson Study Group et al., 2014 |
| Alzheimer disease | Phase III: it failed to slow cognitive decline in patients | Thal et al., 2003 |
| Huntington disease | Phase III: it could not validate the use of CoQ10 as a treatment to slow functional decline in Huntington disease | McGarry et al., 2017 |
| Amyotrophic lateral sclerosis | Phase II: it illustrates inadequate evidence to justify phase III | Kaufmann et al., 2009 |
5.1 Minocycline
In Parkinson disease, a double-blind phase II clinical trial involving 200 untreated patients was conducted, and it included chronic consumption of minocycline at 200 mg/d in 66 patients for 18 months (Investigators, 2008; LeWitt & Taylor, 2008). Its results warranted considerations of minocycline for definitive phase III trials to assess whether it can modify the long term progression of Parkinson disease (Investigators, 2008).
In Alzheimer disease, 554 patients with a diagnosis of mild Alzheimer disease were enrolled in a double-blind randomized clinical trial (Howard et al., 2020). It illustrated that chronic consumption of 200 mg/d or 400 mg/d of minocycline in 183 patients with mild Alzheimer disease for 24 months did not delay the progress of cognitive or functional impairment during that particular period (Howard et al., 2020).
In Huntington disease, 114 patients with Huntington disease were enrolled in phase II randomized clinical trial, in which 87 patients received minocycline (200 mg/d) (Huntington Study Group, 2010). However, this study reached futility criteria warranting no additional study of minocycline (Huntington Study Group, 2010).
In amyotrophic lateral sclerosis, 412 patients with amyotrophic lateral sclerosis were enrolled in a randomized placebo-controlled phase III clinical trial. Chronic consumption of minocycline in escalating doses of up to 400 mg/day for 9 months resulted in harmful effect on patients with amyotrophic lateral sclerosis (Gordon et al., 2007).
5.2 Coenzyme Q10
Coenzyme Q10 is an antioxidant in the inner mitochondrial membrane that has an anti-apoptotic activity. It prevents the activation of the mitochondrial PT, and thus obstructing BAX binding to mitochondria.
In Parkinson disease, 600 patients were enrolled in phase III randomized, placebo-controlled, double-blind clinical trial in order to assess the impact of the administration of 1200 mg/d or 2400 mg/d of CoQ10 on the deceleration of clinical decline in early Parkinson disease (Parkinson Study Group et al., 2014). It failed to confirm any clinical benefit of CoQ10 compared to placebo, and was ended when a futility criterion was reached (Parkinson Study Group et al., 2014).
In Alzheimer disease, 536 subjects were enrolled in phase III double-blind, placebo-controlled, randomized clinical trial. And it illustrated that chronic consumption of 120, 240, or 360 mg tid of the synthetic CoQ10, which is called idebenone, in patients with Alzheimer disease for 1 year failed to slow cognitive decline (Thal et al., 2003).
In Huntington disease, 609 patients with early-stage Huntington disease were enrolled in a randomized, double-blind, placebo-controlled phase III clinical trial that included chronic administration of 2400 mg/d CoQ for 60 months. However, its data could not validate the use of CoQ10 as a treatment to slow functional decline in Huntington disease (McGarry et al., 2017).
In amyotrophic lateral sclerosis, 185 patients with amyotrophic lateral sclerosis were enrolled in a two-stage, bias-adjusted, randomized, placebo-controlled, double-blind, phase II study. Chronic consumption of CoQ10 at 2700 mg daily for 9 months illustrated inadequate evidence to justify phase III (Kaufmann et al., 2009).
6 CONCLUSIONS
Neuronal apoptotic pathway is implicated in the pathogenesis of various neurodegenerative diseases based on in vitro, in vivo, and human post-mortem reports. Clarification of the agents and factors that stimulate the apoptotic process in the various neurodegenerative diseases can result in an improved comprehension of the succession of events that cause apoptosis in the various neurodegenerative diseases. Accordingly, it would be probable to ascertain the possible factors that can be used as therapeutic targets to halt or delay the advancement of the disease and to identify the individuals who are predisposed to evolving a neurodegenerative disorder at the initial stages of the disease.




