Feasibility assessment of shear wave elastography to lumbar back muscles
A Radioanatomic Study
Abstract
Low back pain is often associated with tensional changes in the paraspinal muscles detected by palpatory procedures. Shear wave elastography (SWE), recently introduced, allows the stiffness of muscles to be assessed noninvasively. The aim of this work was to study the feasibility of using SWE on the three main lumbar back muscles (multifidus, longissimus, and iliocostalis) in vivo after analyzing their muscular architecture ex vivo. We determined the orientation of fibers in the multifidus, longissimus, and iliocotalis muscles in seven fresh cadavers using gross anatomy and B-Mode ultrasound imaging. We then quantified the stiffness of these three muscles at the L3 level ex vivo and in 16 healthy young adults. Little pennation was observed in the longissimus and iliocostalis, in which the direction of fibers was almost parallel to the line of spinous processes. The multifidus appeared as a multiceps and multipennate muscle. Given the random layering of millimetric fascicles, tendons, and fatty spaces, the multifidus had multiple fiber orientations. Muscular fascicles and fibers were oriented from 9° to 22° to the line of spinous processes. The shear moduli related to stiffness were 6.9 ± 2.7 kPa for the longissimus, 4.9 ± 1.4 kPa for the iliocostalis, and 5.4 ± 1.6 kPa for the multifidus. SWE is a feasible method for quantifying the stiffness of the lumbar back muscles. Clin. Anat. 30:774–780, 2017. © 2017Wiley Periodicals, Inc.
INTRODUCTION
Chronic low back pain (LBP) is one of the most common and costly medical problems, especially in the middle-aged and elderly (Hoy et al., 2010). Pain arises from the intervertebral discs, zygapophyseal joints and ligaments, or paraspinal muscles (Deyo and Weinstein, 2001). Musculoskeletal disturbances associated with LBP, such as muscle fiber changes, impaired blood flow, decreased activity, and muscle atrophy, induce changes in tissue texture and muscle biomechanics (Fryer and Gibbons, 2004). Clinically, LBP is often associated with tensional changes in the paraspinal muscles detected by manual palpation and confirmed by strain elastography (Chan et al., 2012). However, soft tissue paraspinal palpatory procedures have low reliability (Seffinger et al., 2004).
A new noninvasive imaging method called shear wave elastography (SWE) allows the biomechanical properties of muscular tissue to be assessed (Levinson et al., 1995; Gennisson et al., 2013; Klauser et al. 2014). SWE provides a quantifiable spatial representation of stiffness in the form of an elastogram. The basic principle of SWE is (1) to create a shear wave using an ultrasound push beam, (2) to map the longitudinal wave propagation in the tissue using ultrasonography (US), and (3) to trace the wave back to the mechanical properties of the tissue using inversion algorithms (Arda et al., 2011). SWE can demonstrate changes in muscles stiffness related to stretching, contraction, manual therapy procedures, and muscle manipulation and in many muscular diseases (Nordez and Hug, 2010; Hug et al., 2015; Lacourpaille et al., 2015). It is not only reliable and reproducible but also strongly influenced by technical parameters such as the angulation between the ultrasonographic probe and the muscle fiber and by the anatomical characteristics of muscles such as pennation, fascia, or cross-sectional area (Lacourpaille et al., 2012; Dorado Cortez et al., 2015). Thus, skeletal muscle applications using SWE require knowledge of muscle anatomy so that the probe is placed parallel to the muscle fiber orientation. Regarding the importance of biomechanical changes in the paraspinal muscles related to LBP, SWE seems a promising tool for quantifying stiffness in those muscles.
The aim of this study was to investigate the potential of SWE imaging for quantifying the stiffness of the three main paraspinal muscles: the multifidus, longissimus, and iliocostalis. Our investigation comprised two steps. Taking into account the complexity of the anatomy of the paraspinal muscles, we first determined their fascicular anatomy in cadavers to optimize the ultrasound probe positions for SWE imaging. We then investigated the feasibility of quantifying the stiffness of the normal back muscles in vivo at rest using SWE.
MATERIALS AND METHODS
The study protocol was approved by the local ethics committee and consistent with the Declaration of Helsinki. Written consent was obtained from all volunteers before inclusion in the study. The scientific committee of the Paris School of Surgery ensured that the anatomical subjects were obtained by body donations made during their lifetimes, with written informed consent from those donors on file.
In Vitro Experiments
Specimens
Seven fresh adults cadavers (time after death <10 days; mean age: 79 years; four females) were examined anatomically. All subjects were Caucasians. The bodies had been donated to the Paris School of Surgery (Assistance Publique des Hôpitaux de Paris, APHP). None of the cadavers revealed evidence of previous surgical procedures, spine deformation, or traumatic lesions of the lumbar region.
Experimental protocol
Muscles were collected 5–10 days after death. The temperature of the muscles was 6–12°C. In four cadavers, the right lumbar muscles were removed en bloc separately after the subcutaneous soft tissues and the thoracolumbar fascia (TLF) had been removed. The muscles were analyzed using B-mode ultrasound and elastography at the L3 level. The other three cadavers (right sides) were dissected and observed grossly to establish their pennation and the orientation of their fibers with respect to the line of spinous processes.
Gross anatomy
The cadavers were positioned in prone position. The upper limbs were placed along the body. A large linear skin incision was made from the spinous process of T5 to the sacral hiatus, and then the skin was widely retracted laterally. After the thick underlying fatty layer had been dissected, the superficial plane of the paraspinal compartment was exposed, covered by the aponeurosis of the erector spinae (ESA) and sheathed in the TLF. After resection of the serratus posterior inferior and the TLF, the longissimus, iliocostalis, and multifidus were individualized, then severed and retracted laterally. In each muscle we studied the disposition of the fibers, and we measured the pennation of each in the lumbar region using a protractor.
In Vivo Experiment
Participants
Sixteen healthy right-handed volunteers participated in this study (mean age: 23 years; nine females; BMI <25). The participants were informed of the purpose of the study and the methods used. They were either sedentary or physically active and had no history of back pain. None of the cadavers revealed any evidence of previous surgical procedures, spine deformation, or traumatic lesions in the lumbar region.
Experimental protocol
After 5 minutes of rest in prone position, muscle stiffness was measured at the L3 level in the right multifidus, longissimus, and iliocostalis using ultrasound mode B and elastography. The L3 level was identified as the vertebra just above the bi-iliac line and confirmed with B-mode.
Stiffness Measurements
Instrumentation
The muscle shear modulus (SM) was measured using an AixPlorer ultrasonic scanner (version 6.1.1, SuperSonic Imagine, Aix en Provence, France). All ultrasound examinations were performed by the same operator (M.C.) with 10 years' experience in radiology. For the ex vivo experiment, the scanner was used in the muscle preset coupled with a linear probe (15–4 Hz).
For the ex vivo experiment, we first tried to measure stiffness in lumbar back muscles using the linear probes (15–4 Hz and 10–2 Hz), but stiffness was not recorded in the deep parts of the muscles owing to the cumulative attenuation effect of the skin (which is particularly thick in the back), fat, TLF, and ESA. We then used a convex probe (SuperCurved 6–1, SuperSonic Imagine, Aix-en-Provence, France) preset in the general mode, which can search deeper layers than the linear probe. The “penetration” mode was selected to deepen the penetration of the shear waves.
A generous amount of coupling gel was applied to the muscle surface or ESA in the specimens and to the skin surface of the participants to minimize the influence of transducer pressure on the measurements. During acquisition of stiffness measurements, the transducer was kept motionless until the elastography map stabilized, which took 5–15 sec. For each target level the probe orientation was aligned—if possible—with the direction of the muscle fibers as confirmed using mode B.
Data analysis
The software (Q-BoxTM) installed in the ultrasound system was used to measure the mean shear modulus across circular regions of interest (ROI). For each pixel of the ROI, Young's modulus (YM) was deduced from YM = 3ρv2 (where ρ, density, is assumed to be constant (1000 kg m−3) in human soft tissues; v, shear wave velocity). The device yields the YM by applying the constant 3, assuming that soft tissues are isotropic, unlike skeletal muscle (Royer et al., 2011; Taniguchi et al., 2015). Hence, we derived the shear modulus by dividing the YM obtained by 3. The ROI (10 mm diameter) were positioned in the center of the muscle. Care was taken to avoid including the ESA and TLF, fat, vessel, or muscle located too close to the vertebra within this circular region because this could affect the accuracy of the elasticity measurement. Differences between the ex vivo and in vivo results were assessed using a Student's t test.
RESULTS
Gross Anatomy
On first inspection, the TLF appeared as a large triangular fibrous sheet covering the lumbar back muscles (Fig. 1). It was thick and had no weak point except at the passage of the vascular pedicle laterally for each spinal level.
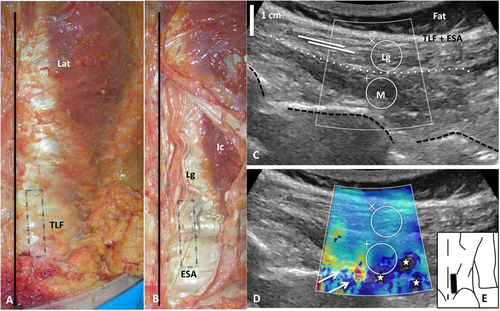
A, B: Gross anatomy of the right TLF and erector spinae aponeurosis (ESA). The black line and the black rectangle show, respectively, the line of spinous processes and the probe position. Lat = latissimus dorsi. C: Ultrasound image of right paraspinal muscles (in vivo) with B-mode imaging on which the TLF and ESA, the longissimus (Lg) and the multifidus (M) were observed when the probe was positioned with a small tilt on the skin, almost parallel to the line of spinous processes. The white dashed line shows the boundary between M and Lg. The black dashed line corresponds to the transverse processes. White lines illustrate the direction of the muscles fibers of the longissimus. No preferential fiber orientation was seen in the multifidus. D: Ultrasound image of paraspinal muscle elastogram. White stars represent failures in stiffness measurement due to an attenuation effect and white arrows show bone artifacts. E: Posterior view of the back, showing the probe position (black rectangle). [Color figure can be viewed at wileyonlinelibrary.com]
The TLF was separated from the deeper plane by a thin layer. The deeper plane was constituted by the ESA, which covered the longissimus medially and the iliocostalis laterally along a large proportion of their length (Fig. 1). The ESA was continuous and composed of longitudinal fibers along which the muscle fibers were attached obliquely. Under L4–L5, the ESA was the tendon common to both the iliocostalis and longissimus muscles.
The longissimus and iliocostalis—also named erector spinae—had voluminous bellies, where the muscular fibers were oriented longitudinally, almost parallel to the line of spinous processes. The mean pennations (±standard deviation) with the inner part of the ESA were 8° (±3) and 4° (±1) for the longissimus and the iliocostalis, respectively (Figs. 2 and 3). Near the paraspinal groove, the inner parts of the erector spinae were also attached to the mammillary processes with thin fascicles, where the mean pennations (±standard deviation) were 21° (±3°) and 60° (±6) for the longissimus and iliocostalis muscles, respectively.
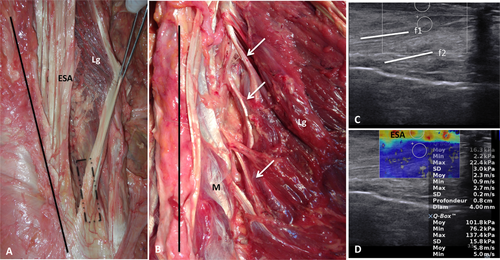
A, B: Gross anatomy of the right longissimus (Lg), covered by the ESA. We found little pennation between the Lg and the ESA (A). White arrows show the longissimus fascicles attached to the mammillary processes (B). The black line and the black rectangle show, respectively, the line of spinous processes and the probe position. M = multifidus. C: Ultrasound image of right longissimus (ex vivo) with B-mode imaging. Muscular fibers (f1 and f2) had little pennation with the ESA. D: Elastogram of the longissimus (ex vivo). Stiffness was greater (warm colors) in the superficial part of the Lg and in the ESA than in the deepest part of the Lg (cold colors). [Color figure can be viewed at wileyonlinelibrary.com]

A: Gross anatomy of the right iliocostalis (Ic). ESA= erector spinae aponeurosis, Lg= longissimus. The black line and the black rectangle show, respectively, the line of spinous processes and the probe position. B, C: Ultrasound image of right iliocostalis with B-mode imaging (B) and elastogram (C) in ex vivo experiment. Muscular fibers (white lines f1 and f2) had little pennation with the ESA and little angulation with the skin. D, E: Ultrasound image of right iliocostalis with B-mode imaging (D) and elastogram (E) in in vivo experiment. We observed attenuation effect (holes) and artifacts in the depth of the muscle (white arrows). Dashed lines represent the skin. F: Posterior view of the back showing the probe position (black rectangle). [Color figure can be viewed at wileyonlinelibrary.com]
Above L4, the multifidus was found in the deep part of the erector spinae, from which it was separated by a fat-filled space and by the thin aponeurosis of the multifidus (Fig. 4). Below L4, the multifidus was superficial, covered only by the ESA, with few smooth attachments on it but no fatty space. On first inspection, the multifidus represented a homogenous muscular mass with a triangular shape. It comprised many millimetric tendinous and fleshy fascicles originating from the spinous processes to the mammillary processes located 1–3 spinal levels above. The muscular organization was unclear and the multifidus appeared as a multiceps and multipennate muscle. Multifidus fascicles were arranged in three or four layers from superficial to deep with few or no cleavage planes between them. Some interdigitations attached fascicles between them. For each lumbar level, the muscular fascicles and fibers were oriented from 9° to 22° to the line of spinous processes.
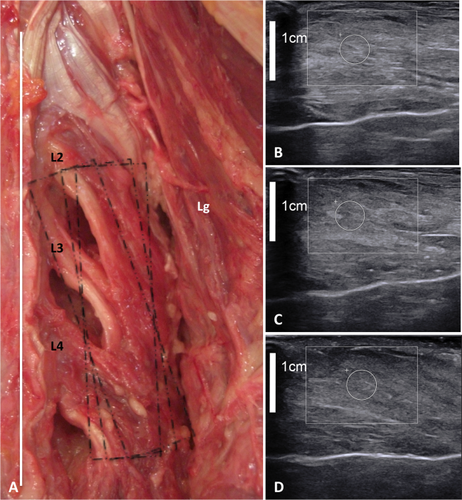
A: Gross anatomy of the right mutifidus (M). Lg = longissimus. The white line and the black rectangles show, respectively, the line of spinous processes and the three different probe positions. B–D: Ultrasound image of right multifidus with B-mode imaging with different probe rotations (angle between 0° and 30° with the line of spinous processes) in the ex vivo experiment. Regardless of the probe position, no preferential muscle fiber orientation was seen in the multifidus. [Color figure can be viewed at wileyonlinelibrary.com]
Mode-B Analysis
The orientation of the fibers of the superficial and largest part of the erector spinae was easily identified with B-mode ultrasound in both the ex vivo and in vivo experiments (Figs. 1-3). As in the anatomical description, most of the muscle fibers ran almost parallel to the midline with little pennation with the ESA. In depth, fascicles attached to the mammillary processes were not identified.
For the multifidus, it was not possible to identify the proper orientation of the fibers for ex vivo and in vivo experiments because of the thinness of the fascicles and their arrangement (Figs. 1 and 4). In vivo, the multifidus (especially the inner and medial part of the muscle) in the upper lumbar spine was thin, deep, and covered by a succession of tissues (from deep to superficial: fat, longissimus, ESA, fat, TLF, fat, subcutaneous tissues, and skin). In the in vivo experiment, the orientation of the probe could not be perpendicular to the skin because the muscle slid sideways to the spinous processes, and we needed to tilt the probe toward the vertebra. For both experiments, the architecture of the multifidus was not recognizable in the lumbo-sacral part. As seen by gross anatomy, the architecture of the multifidus was complex, with randomly alternating hyperechogenic tendon and aponeurosis, hypoechogenic muscle tissue, and hyperechogenic fat. Whatever the rotation of the probe at an angle between 0° and 25° to the line of spinous processes, only few muscular fibers were identified in their entirety, others not appearing in the same plane as the probe. The analysis of muscle fiber directions by both gross anatomy and US allows the anatomy and the organization of the lumbar back muscles to be established accurately as a radio-anatomical model for SWE.
Stiffness Analysis
SWE allowed an elastogram to be obtained, and the stiffness of the three muscles measured (Figs. 1-4). For both experiments, the outer part of the longissimus covered by the ESA was stiffer than the rest of the muscle part. In the ex vivo experiment and in vivo in the longissimus, the elastogram was completed in the Q-Box and stiffness could be measured throughout the muscle. The intramuscular stiffness pattern was homogeneous. In the deep part of the iliocostalis and in the multifidus in vivo the elastograms were incomplete, with “holes” and artifacts.
In vivo, the mean SM were 6.9 ± 2.7 kPa for the longissimus, 4.9 ± 1.4 kPa for the iliocostalis, and 5.4 ± 1.6 kPa for the multifidus. Ex vivo, the mean SM were 7.7 ± 3.1 kPa (in vivo +11%) for the longissimus, 6.9 ± 1.5 kPa (in vivo +40%) for the iliocostalis, and 5.1 ± 1.7 kPa (in vivo + 6%) for the multifidus. There were no significant difference between the ex vivo and in vivo measurements of the three muscles.
DISCUSSION
In this study, we demonstrate that gross anatomy analysis combined with US allows the limits of paraspinal muscle SWE to be defined and ultimately muscle stiffness in the lumbar region to be measured.
To the best of our knowledge, this article is the first to publish stiffness data for the three main paraspinal muscles using SWE (MEDLINE 1966-Avril 2017: English language; search terms: Iliocostalis, Longissimus, Multifidus, Elastography, Shear wave elastography, Stiffness). Few studies have explored the stiffness of paraspinal muscles. Moreau et al. (2016) found a SM of 8.6 kPa (shear modulus) at level L2–L3 and 6.9 kPa at level L4–L5 in the multifidus at rest in prone position using SWE, and Chan et al. (2012) found a YM of 36 kPa, which corresponds to SM = 12 kPa, using strain elastography. By extension to other trunk muscles, mean stiffness using SWE ranged from 3.5 kPa for the internal oblique to 22 kPa for the external oblique (Hirayama et al., 2015; Tran et al., 2016). Such variability could result from significant interindividual and intermuscular stiffness differences. In the literature concerning SWE, large interindividual variations in mean stiffness are described but no significant factor has been proved responsible for them (such as sex, age, physical activity, or muscle biology) (Arda et al., 2011; Eby et al. 2015; Akagi et al., 2016; Chino and Takahashi, 2016).
Stiffness measurement using SWE is highly dependent on the angle between the probe (i.e., the direction of propagation of the shear wave) and the muscular fiber, owing to the anisotropy of muscle tissue (Koo and Hug, 2015; Miyamoto et al., 2015). Stiffness values are greater in the direction parallel to the muscle fibers than perpendicular to them. As described in previous studies, paraspinal muscles are polyarticular, deep, and multipennate (Kalimo et al., 1989; Winckler, 1974). Gross anatomy analysis was useful for finding details of the probe orientation on the three muscles and establishing the limits of the method for these complex muscles (Hatta et al., 2016). There was little pennation in the longissimus and the iliocostalis. The fiber orientation was easily identified and was almost parallel to the line of spinous processes and to the skin. Concerning the multifidus, there were multiple fiber orientations given the random layering of millimetric fascicles, tendons, and fatty spaces. Such geometrical complexity and tissue heterogeneity meant that the probe was rotated relative to some fascicles. We observed that the fascicle angle with the line of spinous processes was <25°. Although the probe angle relative to the fascicle has a significant effect on the shear modulus in human muscles, the difference is negligible if the probe angle is no >20°. SWE remains a valid tool for determining the mechanical properties of pennate muscles along the fascicle direction under those conditions (Miyamoto et al., 2015). Deep location and surrounding tissues can influence stiffness in two ways; first by applying an external force, and second by disturbing the stiffness measurement by attenuation or artifacts, especially above L4 (Yoshitake et al., 2016). ROI size and ROI position, particularly in relation to underlying bone, influence stiffness measurements and could be important in the depth of the spinal groove (Sasaki et al., 2014; Ates et al., 2015; Ewertsen et al., 2016).
We observed greater mean stiffness ex vivo than in vivo, especially in the iliocostalis (+40%), though there was no significant difference between the two experiments. In a similar study comparing SWE in vivo and ex vivo in the supraspinatus, Itoigawa et al. (2015) observed greater stiffness in cadavers than in healthy participants. The stiffness difference could be explained by several differences within samples. Young, healthy, and active persons constituted the in vivo sample. The ex vivo sample comprised old persons whose muscles suffered from fatty degeneration, atrophy and postmortem changes. Knowing the high incidence of LBP, arthrosis, and inactivity with aging, we cannot exclude the possibility that the cadavers also suffered from one or more of these spine diseases. Previous studies on appendicular muscles failed to highlight reproducible and significant stiffness changes with aging and the relationship between stiffness and fat infiltration remains unclear (Rosskopf et al., 2016). In the supraspinatus, stiffness decreased with increasing fat content (Goutallier stage 0–III) and increased in the final stage of fatty infiltration (Goutallier stage IV) (Rosskopf et al., 2016). Temperature is known to influence stiffness ex vivo significantly (Sapin-de Brosses et al., 2010). The lack of significant difference between ex vivo and in vivo stiffness could be explained by the small sample sizes or high interindividual stiffness differences.
In clinical work, qualitative manual palpation is widely used to assess paraspinal muscle stiffness (Fryer and Gibbons, 2004). The relationship between decreased paraspinal muscle activity, decreased strength, changes in muscle fiber composition or atrophy, and LBP could be responsible for the palpable paraspinal muscle changes detected manually (Fryer and Gibbons, 2004). However, most spinal palpatory diagnostic procedures are unreliable (Seffinger et al., 2004). More recently, one study using strain elastography showed that patients with chronic LBP have multifidus muscles with less elasticity than younger control subjects without LBP (Chan et al., 2012). Since strain elatography is related to manual compression it is operator dependent. SWE is a promising objective tool for analyzing stiffness changes induced by LBP because of its reproducibility. Animal studies combining a traditional materials testing machine and SWE ultrasound measurement have validated the SWE technique throughout the functional range of motion of skeletal muscle (Eby et al., 2013).
There are some limitations to this study. First, we acknowledge that the small sample size limits our interpretation of muscle elasticity. However, no previous study has analyzed the performance of SWE in measuring the stiffness of lumbar back muscles. Second, the rheological fit model used to characterize stiffness with SWE assumes the muscle to be isotropic and purely elastic, but it is not. More accurate rheological fit and tri-dimensional analysis of the wave propagation could allow the anisotropic and viscoelastic properties of the muscle to be respected, especially in muscles with complex anatomy such as the paraspinal muscles. Future investigations with larger cohorts will aim at confirming the value of SWE.
CONCLUSION
The combination of gross anatomy analysis and US allows the anatomy and the organization of the lumbar back muscles to be established accurately as a radioanatomical model for SWE. Using this radioanatomical model, SWE assesses muscle stiffness in the lumbar back muscles and highlights their biomechanical properties responsible for gait. In the future, SWE could be a useful objective tool for analyzing biomechanical changes related to LBP.




