Chain conformation and bioactivity of water-soluble polysaccharide extracted from Rhizoma Panacis Japonici
Abstract
A water-soluble α-(1→4)-D-glucan heteropolysaccharide with 37% degree of branch extracted by base from Rhizoma Panacis Japonici, coded as RPS3, was fractionated into six fractions by the method of nonsolvent addition. Their weight-average molecular mass (Mw), polydispersity index (Mw/Mn), and radius of gyration (〈s2〉z1/2) were determined with laser light scattering (LLS) and size exclusion chromatography combined with LLS. The structure of the fraction was determined by methylation analyses and 13C NMR. The dependences of intrinsic viscosity ([η]) and 〈s2〉z1/2 on Mw were established as [η] = 0.71 Mw0.27 ± 0.01 (cm3/g) and 〈s2〉z1/2 = 1.53 Mw0.27 ± 0.02 (nm) in the Mw range from 5.62 × 104 to 3.05 × 106 (g/mol) for RPS3 in 0.15M NaCl aqueous solution at 25°C. On the basis of the current theory of the polymer solution, the fractal dimension (df), unperturbed chain dimension (A), and characteristic ratio (C∞) were calculated to be 3.0, 1.48 Å, and 15.1, respectively. The results revealed that the RPS3 chains existed as spherical conformation in the aqueous solution. Transmission electron microscope further provided the evidence of the sphere shape of the RPS3 and its fractionated molecules in water. In vitro cytotoxicity assay indicated that the fractions could inhibit the tumor cells and showed no harm to normal cells at low dose. The bioactivity was relative with molecular mass of the samples. © 2010 Wiley Periodicals, Inc. Biopolymers 93: 383–390, 2010.
This article was originally published online as an acceptedpreprint. The “Published Online” date corresponds to the preprint version. You can request a copy of the preprint by emailing the Biopolymers editorial office [email protected]
INTRODUCTION
Some polysaccharides are known to exhibit antitumor activities and immune stimulating effects, which are related to their chemical structures, molecular masses, and chain conformations in solutions.1-6 In our previous work, a water-soluble α-(1→4)-D-glucan heteropolysaccharide with 37% degree of branch (DB), coded as RPS3, was extracted by basic solution from Rhizoma Panacis Japonici (RPJ).7 Characterization of branched heteropolysaccharide is more complicated than a linear one. However, its molecular structure usually determines some specific properties and suggests its possible application in various areas such as food industry, pharmaceuticals, and biomaterials. It is essential to characterize the chemical structure and molecular parameters of the water-soluble polysaccharide for understanding its application as drug and biomaterials. To study on the relationship of bioactivity to molecular mass and chain conformation of the polysaccharide, it is necessary to obtain a series of fractions of the polysaccharide with different molecular mass and narrow molecular mass distribution.8-12 Comparing with preparation chromatography, reprecipitation is a simple and cheap method suitable for fractionating polymer with wide molecular mass distribution. The solvent and precipitant for the precipitation method can be used at room temperature and pressure or at supercritical state.13-15
In our previous work, it has been proved that RPS3 is a polysaccharide with wide molecular mass distribution, so it is possible to be fractionated by reprecipitation.16 However, fractionation of RPS3 and the solution properties have never been reported. In this work, RPS3 was fractionated by the method of nonsolvent addition with water as solvent and methanol as precipitant to prepare a series of polysaccharide fractions with narrow molecular mass distribution. Their structures were determined by methylation analyses and 13C NMR. Moreover, their molecular mass and size were characterized by using laser light scattering (LLS), size exclusion chromatography (SEC) combined with laser light scattering (SEC-LLS), and their intrinsic viscosities were measured by viscometer. The conformation parameters of the polysaccharide were deduced from the data of LLS and viscometry by applying the current theory of polymer solutions. The molecular morphology of the polysaccharide was observed by transmission electron microscopy (TEM). Furthermore, cell interaction of the polysaccharide fractions with different molecular mass was studied by fluorescence spectroscopy to valuate the relationship of the molecular mass to bioactivity.
EXPERIMENTAL SECTION
Materials
RPJ was purchased from Enshi (Hubei Province, China). 5-Fluorouracil (5-FU) was purchased from Sigma-Aldrich (China). 3-(4,5)-Dimethylthiazol-2,5-di-phenytetrazolium bromide (MTT) was purchased from Invitrogen Corporation (USA). All other chemical reagents were of analytical grade and commercially available. They were used as received.
Preparation of RPS3 and Fractions
Water-soluble polysaccharide RPS3 was prepared as Refs.7 and16. It was fractionated by addition of methanol as precipitant at room temperature according to the nonsolvent addition method. Briefly, RPS3 was first dissolved in water at concentration of 2% and stirred for 24 h to obtain transparent solution, and then methanol was added into the RPS3 solution dropwise under stirring at 25°C until the solution turned milky. The turbid mixture was centrifuged at 9000 rpm and the precipitated product was washed by methanol three times to obtain the first fraction. The supernate was subjected to the next step of fractionation by adding more volumes of methanol. In this way, the RPS3 was divided into six fractions coded as RPS3-1, RPS3-2, RPS3-3, RPS3-4, RPS3-5, and RPS3-6. Each fraction was dissolved in water again and precipitated by additional methanol repeatedly. The fractions were finally vacuum dried to obtain white powder.
Characterization of Molecular Parameters
GC-MS total ion current (TIC) chromatograms of methylation analyses and 13C NMR spectra of the fractions were performed as described in Ref.7. GC-MS was carried out on a GCT system (Macro-Mass, Waters, USA) with a capillary GC column (HP-5MS, 30 m × 0.32 mm ID × 0.25 μm, Agilent, USA) using helium (He) as carrier gas. 13C NMR spectra of the polysaccharides were measured on a NMR spectrometer (Inova-600, Varian, USA) in D2O at 25°C.
The LLS intensity of the polysaccharide solution was measured at 25°C with a multiangle LLS (MALLS, Dawn EOS, Wyatt Technology Corporation, USA) equipped with a GaAs3 semiconductor laser (λ = 690 nm) at angles (θ) of 43, 52, 60, 69, 80, 90, 100, 111, 121, and 132°. The polysaccharide solutions with concentration (c) in the range from 0.2 to 1.0 mg/ml in 0.15M NaCl aqueous solution were prepared, and optical clarification of the solution was achieved by filtration through 0.2 μm syringe filters (Whatman, England) before injecting into the scattering cell.
The size-exclusion chromatography combined with laser light scattering (SEC-LLS) measurements were carried out on the above MALLS with a differential refractive index detector (RI, Optilab DSP, Wyatt Technology Corporation, USA) and a pump (P1000, Thermo Separation Products, USA) with a SEC column (Asahipak GF-710HQ, 7.5 mm ID × 30 cm, Shodex, Japan). The polysaccharide solutions were prepared at a concentration of 1.0 mg/ml in 0.15M NaCl aqueous solution, and the injection volumes were 100 μl. The eluant was 0.15M NaCl aqueous solution purified by 0.2 μm membrane (Whatman, England) and degassed. The flow rate was 1.0 ml/min. The value of refractive index increments (dn/dc) 0.145 ml/g16 was used in LLS and SEC-LLS determination.
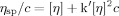 (1)
(1)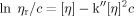 (2)
(2)Morphology of the polysaccharide aqueous solution at a concentration of 1 mg/ml was observed on a high-resolution transmission electron microscope (TEM, JEM-2010FEF, JEOL, Japan). Electron microscope copper grids were first coated with a thin film of carbon and dipped in the sample solution, dried under room condition for half hour, then vacuum dried for half hour. TEM images were taken at an accelerating voltage of 200 kV.
In Vitro Cytotoxicity Assay
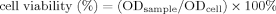 (3)
(3)The statistical calculations between two sets of data were carried out using Student's t-test software and differences were considered significant if P < 0.01. The following basic numerical characteristics were used during the statistical processing of the results: arithmetic mean, standard deviations, and standard deviations are displayed as error bars in the figures.
RESULTS AND DISCUSSION
Fractionation
Figure 1 shows the Zimm plot and SEC-LLS chromatograms for RPS3 in 0.15M NaCl at 25°C. The weight average molecular mass (Mw) was found to be 1.24 × 106 and the molecular mass distribution was wide as reported previously.16 The SEC-LLS chromatograms of the RPS3 fractions are shown in Figure 2. Despite small shoulder peak appeared at RPS3-1, RS3-5, and RPS3-6, the six fractions were eluted at different elution volumes with symmetrical and relatively narrow peaks. Clearly, the largest molecular mass fraction RPS1 was first eluted, then the second RPS2, and finally RPS3-6. The SEC chromatogram of each fraction was separated well, as a result of the wide molecular mass distribution for the original polysaccharide. This indicated that the RPS3 sample was fractionated successfully in molecular mass by such a method, and the efficiency of the fractionation was good. Figure 3 shows the GC-MS TIC chromatograms of methylation analyses and 13C NMR spectra of two of the fractions. It showed that the TIC peaks of the fractions were similar as that of RPS3 and the 1→4-glucose content of RPS3-2 and RPS3-3 were 58.3% and 55.4%, respectively, which were close to that of RPS3. Moreover, the NMR spectra were almost the same as RPS3, only the peak at 79 ppm changed slightly narrow and shifted a little to higher field. It suggested that the RPS3 fractions remained the structure distribution of the unfractionated RPS3 heteropolysaccharide. It was proved that the nonsolvent addition method was suitable for the fractionation of wide distribution heteropolysaccharide.
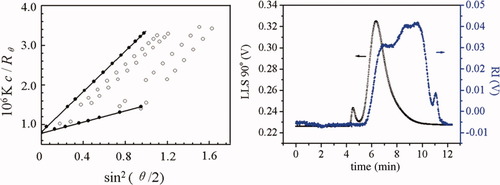
Zimm plot (left) and SEC-LLS chromatograms (right) for RPS3 in 0.15M NaCl at 25°C.
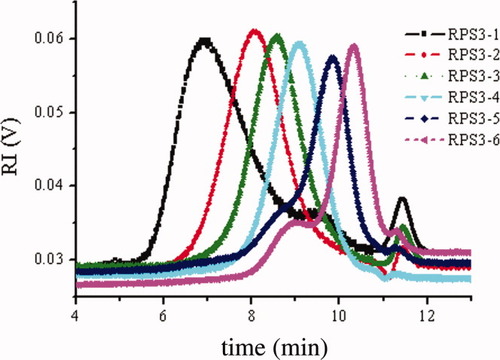
The SEC-LLS chromatograms of RPS3 fractions in 0.15M NaCl at 25°C.
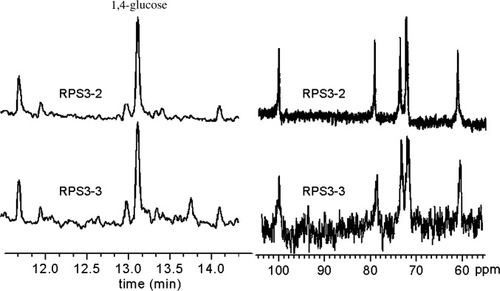
The GC-MS total ion current (TIC) chromatograms of methylation analyses (left) and 13C NMR spectra of the fractions (right).
Figure 4 shows the plots of ηsp/c vs. c and ln ηr/c vs. c for the polysaccharide fractions. By extrapolating the linear relationships of ηsp/c vs. c and ln ηr/c vs. c to an infinite dilution, the experimental values of [η] of the fractions were obtained. The results of the Mw, 〈s2〉z1/2, polydispersity index (Mw/Mn), and [η] are summarized in Table I. The Mw/Mn values of the fractions were smaller than 2.0. This meant that the molecular mass distribution of RPS3 fractions was relatively narrow. Therefore, the fractions can be used to study on the solution properties of RPS3 in the Mw range of 5.62 × 104 to 3.05 × 106.
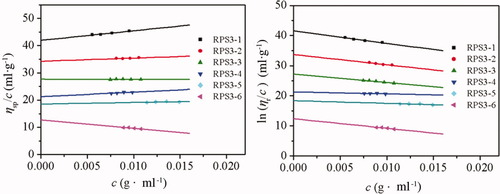
Plots of ηsp/c vs. c (left) and lnηr/c vs. c (right) of RPS3 fractions in 0.15M NaCl at 25°C.
| Sample | Mw × 10−4 (g/mol) | 〈s2〉z1/2 (nm) | Mw/Mn | [η] (ml/g) |
|---|---|---|---|---|
| RPS3 | 124 | 50.8 | 4.7 | 33.0 |
| RPS3-1 | 305 | 84.7 | 1.5 | 41.8 |
| RPS3-2 | 132 | 70.0 | 1.5 | 34.0 |
| RPS3-3 | 60.9 | 62.1 | 1.5 | 27.4 |
| RPS3-4 | 20.8 | 43.3 | 1.6 | 21.4 |
| RPS3-5 | 10.4 | 37.1 | 1.9 | 17.3 |
| RPS3-6 | 5.62 | 28.0 | 1.9 | 13.6 |
Molecular Parameters
The power law function 〈s2〉z1/2 = k Mw ν can be estimated from many experimental points in the SEC-LLS chromatogram for RPS3 or the LLS data for its fractions. Figure 5 shows plots of 〈s2〉z1/2 versus Mw for RPS3 and the fractions in double logarithmic coordinates, respectively. The two fitting lines were almost parallel. From the straight line fitting of the experimental points by SEC-LLS for RPS3, the relation of 〈s2〉z1/2 to Mw was established as 〈s2〉z 1/2 = 0.44 Mw0.33 ± 0.01. Moreover, the relationship from the straight line of 〈s2〉z1/2 versus Mw for RPS3 fractions by LLS in the Mw range of 5.62 × 104 to 3.05 × 106 was obtained as 〈s2〉z 1/2 = 1.5 Mw0.27 ± 0.02. The ν values obtained from two methods were accordant to be at average value of 0.30 ± 0.03. According to the theory of polymer solutions, the exponents of 0.33, 0.50–0.60, and 1.0 reflect the polymer molecular shape as sphere, random coil, and rigid rod, respectively.17-19 The low ν value of RPS3 confirmed that RPS3 indicated a compact globular chain conformation.
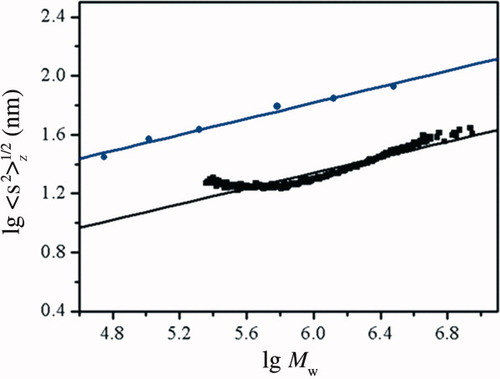
Plots of log 〈s2〉z1/2 vs. log Mw for RPS3 (line) by SEC-LLS and for RPS3 fractions by LLS (dot) in 0.15M NaCl at 25°C.
The exponent value (α) of Mark-Houwink equation is usually related to the shape and the nature of macromolecules in solution. In general, α value of 0.5 suggests that the polymer molecules behave as a dense sphere, whereas that in the range of 0.6–0.8 indicates a flexible chain.20, 21 The value of α depends on the DB as well. The typically α values vary between 0.34 and 0.20 for hyperbranched glycopolymers with high DB values, the bigger the DB value, the smaller the α value.22 Figure 6 shows the [η] dependence on the Mw for RPS3 fractions in 0.15M NaCl at 25°C. The Mark-Houwink equation obtained from the fitting line for RPS3 fractions in the Mw range from 5.62 × 104 to 3.05 × 106 was established as [η] = 0.71 Mw0.27 ± 0.01. The experimental α value of 0.27 for RPS3 was noticeably low, which suggested a relative compact sphere-like structure. The low α value in Mark-Houwink equation for RPS3 was in good agreement with that in 〈s2〉z 1/2 = 1.5 Mw0.27 ± 0.02 equation, as a result of its branched structure. It is similar to the low α value of Mark-Houwink equation (α = 0.30) of a hyperbranched polysaccharide from Pleurotus tuber-regium in 0.25M LiCl/Me2SO solution.23
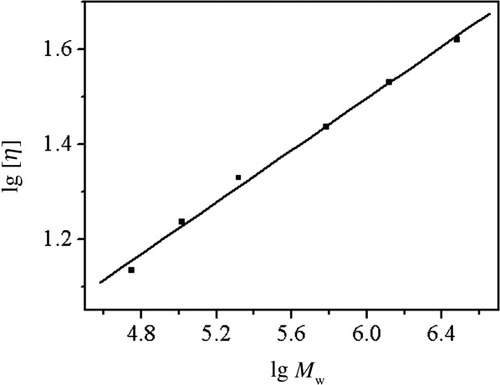
[η] dependences on Mw forRPS3 fractions in 0.15M NaCl at 25°C.
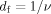 (4)
(4)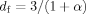 (5)
(5)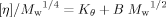 (6)
(6)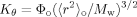 (7)
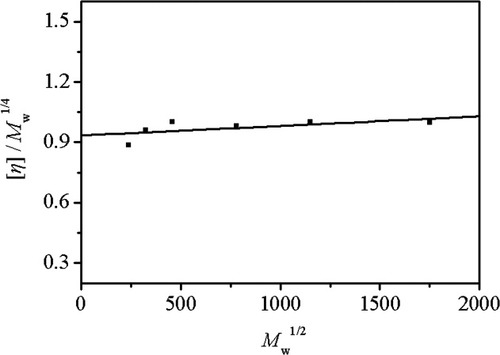
(7)
Zimm-Kilb plot for RPS3 fractions.
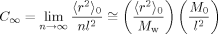 (8)
(8)Molecular Morphology
The conformation parameters of RPS3 deduced from the polymer solution theory indicated that it existed as spherical chain conformation in aqueous solution. To provide direct evidence of the polysaccharide chain conformation, TEM was used to observe the molecular morphology. Figure 8 shows the TEM image of RPS3 and fraction RPS3-3 in dilute aqueous solution. The spherical shapes of the RPS3-3 fraction were the same as RPS3 in dilute aqueous solution. The diameters of the particle of RPS3 visualized by TEM were in the range of 20–100 nm with the average diameter 50 nm, and the diameter of the RPS3-3 molecules was almost the homogeneous to be 60 nm, which were in good agreement with that determined by LLS. The molecular morphology observed by TEM proved directly that the macromolecules of RPS3 and its fraction existed as spherical conformation in aqueous solution. The shape of RPS3-3 fraction was the same as RPS3, also indicating that the nonsolvent addition method did not change the conformation of the fraction during fractionation. Therefore, the conformation parameters for the complex hyperbranched polysaccharides in the aqueous solution were reliable.
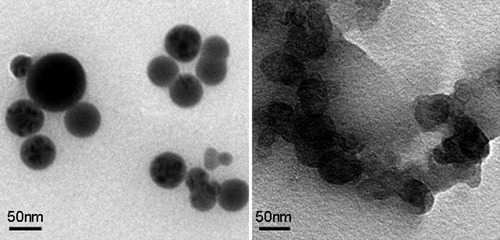
TEM image (bar = 50 nm) of RPS3 (left) and fraction RPS3-3 (right) in water.
Interaction of RPS3 and Fractions to Cells In Vitro
The cytotoxicities of RPS3 and its fractions were evaluated on the COS-7 and HepG2 cells by MTT assay. Figure 9 shows the influence of dose of RPS3 and its fractions on the proliferation of COS-7 cells, the relative errors were less than 10%. The cell viability of COS-7 cells in RPS3 and its fractions were significantly higher than that of 5-FU, indicating the low cytotoxicity of RPS3 and its fractions, it was especially true at doses less than 100 μg/ml, above that concentration some of the fractions resulted in about 20% loss of cell COS-7 viability. This suggested that the polysaccharide cytotoxicity to COS-7 had dose dependence. Compared with the fractions, RPS3 exhibited less proliferation on COS-7 cells, suggesting that polysaccharide with narrow molecular mass distribution was benefit to cell growth of COS-7. The cell viability of COS-7 of the RPS3 fractions having Mw from 10 × 104 to 60 × 104 was higher than others. Figure 10 shows the influence of dose of RPS3 and its fractions on the proliferation of HepG2 cells. There was obvious dose dependence of polysaccharide fractions on the cell viability of HepG2. Compared with 5-FU, RPS3 and its fractions at different doses had little cytotoxicity to HepG2 cells too. Except RPS3-6 with the smallest molecular mass, the fraction with smaller molecular mass was less cytotoxic to HepG2 cells. RPS3-1 and RPS3-2 appeared to exert a cytotoxic effect on HepG2 cells, i.e., they limit proliferation. Interestingly, this did not appear to be the case in COS-7 cells. It is meaningful that the different responses to the two cell lines at dose less than 10 μg/ml, namely, the COS-7 cell viability increase with the dose decrease, whereas the HepG2 cell viability decrease. It could be concluded that the fractions could inhibit the tumor cells and showed no harm to normal cells at low dose and this bioactivity increased in a moderate range of molecular mass, which was accordant to polysaccharide from Pleurotus tuber-regium and polysaccharide from Poria cocos.33, 34
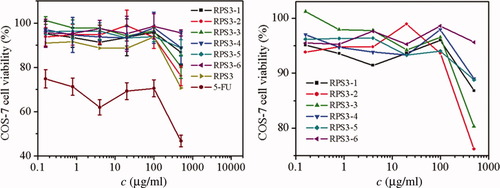
The influence of RPS3 and its fractions on COS-7 cell viability. Right: zoom in.
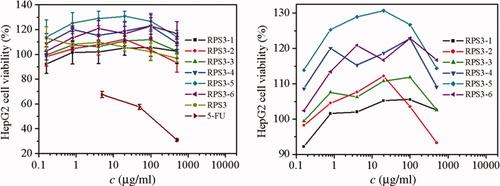
The influence of RPS3 and its fractions on HepG2 cell viability. Right: zoom in.
CONCLUSIONS
A water soluble α-(1→4)-D-glucan heteropolysaccharide, coded as RPS3, extracted by base from RPJ, had wide molecular mass distribution. It was fractionated into six fractions by nonsolvent addition method, successfully. The chemical structure and spherical chain conformation of the fractions were the same as original RPS3, indicating the fractionation did not break the polysaccharide structure. The spherical chain conformation of the polysaccharide was resulted from its highly branched structure. The interaction of RPS3 and its fractions to two cell lines in vitro showed that the polysaccharide could inhibit the tumor cells and show no harm to normal cells at low dose, and this bioactivity increased in a moderate range of molecular mass.