Conformational preferences and prolyl cis–trans isomerization of phosphorylated Ser/Thr-Pro motifs
Abstract
The conformational study on Ac-pSer-Pro-NHMe and Ac-pThr-Pro-NHMe peptides has been carried out using hybrid density functional methods with the implicit solvation reaction field theory at the B3LYP/ 6-311++G(d,p)//B3LYP/6-31+G(d) level of theory in the gas phase and in solution (chloroform and water). For both pSer-Pro and pThr-Pro peptides in the gas phase and in chloroform, the most preferred conformation has the α-helical structure for the pSer/pThr residue, the down-puckered polyproline I structure for the Pro residue, and the cis prolyl peptide bond between the two residues, in which two hydrogen bonds between the phosphate oxygens with the backbone NH groups seem to play a role. However, the trans conformations that have a single hydrogen bond of the phosphate oxygen with either of two backbone NH groups become most preferred for both peptides in water. This is because the hydration free energy of the anionic oxygen of the phosphate group is expected to dramatically decrease for the cis conformation upon formation of the hydrogen bond with the backbone NH groups. These calculated results are consistent with the observations by NMR and IR experiments, suggesting the existence of hydrogen bonds between the charged phosphoryl group and the backbone amide protons in solution. The calculated cis populations of 14.7 and 14.2% and rotational barriers of 19.87 and 20.57 kcal/mol to the cis-to-trans isomerization for pSer-Pro and pThr-Pro peptides in water, respectively, are consistent with the observed values for pSer-Pro and pThr-Pro containing peptides from NMR experiments. However, the hydrogen bond between the prolyl nitrogen and the following amide NH group, which was suggested to be capable of catalyzing the prolyl isomerization, does not play a role in stabilizing the preferred transition state for the pSer/pThr-Pro peptides in water. Instead, the amide hydrogen of the NHMe group is involved in a bifurcated hydrogen bond with the anionic oxygen and phosphoester oxygen of the phosphate group. © 2009 Wiley Periodicals, Inc. Biopolymers 93: 330–339, 2010.
This article was originally published online as an accepted preprint. The “Published Online” date corresponds to the preprint version. You can request a copy of the preprint by emailing the Biopolymers editorial office at [email protected]
INTRODUCTION
Post-translational modification by phosphorylation of the side chains of serine, threonine, and tyrosine residues of proteins is a ubiquitous regulatory mechanism in both eukaryotes and prokaryotes.1-3 Intracellular phosphorylation by protein kinases provides a mechanism for the cell to switch on or off many diverse processes such as metabolic pathways, kinase cascade activation, membrane transport, gene transcription, and motor mechanisms.2 Approximately 30% of cellular proteins contain covalently bound phosphate, and abnormal levels of protein phosphorylation are known to cause major diseases such as cancer, diabetes, and rheumatoid arthritis.1
Phosphorylation on serine or threonine residues preceding proline (i.e., the Ser/Thr-Pro sequence) of proteins is one of the major cellular signaling mechanisms for the control of a series of cell cycle events.4-7 The biological significance of these motifs has been further highlighted by the discovery of the peptidyl-prolyl cis/trans isomerase (PPIase) Pin1.8 Pin1 preferentially isomerizes the peptide bond between phosphorylated Ser/Thr and Pro residues (i.e., the pSer/pThrPro bonds) with up to 1300-fold selectively compared to unphosphorylated bonds in certain proteins,8, 9 whereby the prolyl isomerization catalytically induces conformational changes in proteins following phosphorylation to regulate protein function.4-7 In particular, these phosphorylation mechanisms might play a role in cell growth control and diseases such as cancer and Alzheimer's.4-7 Most Pin1-type PPIases also have the WW domain, a protein-interacting module present in various proteins.10-12 It has been suggested that the WW domain targets the enzyme to its substrates, and the PPIase domain can induce the conformational changes and carry out the necessary function of the enzyme.4-7, 10-12 In the crystal structure of Pin1 complexed with the dipeptide Ala-Pro, the side chains of Lys63, Arg68, and Arg69 residues form a basic cluster at the entrance to the Pro-binding site and a sulfate is associated with it as a counter ion, which suggests that Pin1 prefers an acidic residue preceding proline in substrates.13 In addition, a hydrophobic pocket composed of Phe134, Met130, and Leu122 residues provides the binding site for the cyclic side-chain of the substrate proline, and the peptidyl-prolyl bond undergoing catalyzed cis–trans isomerization is surrounded by the side chains of Cys113, His59, His157, and Ser154 residues.13
The conformational changes associated with the phosphorylation of Ser and Thr residues of peptides have been studied by NMR,14-18 CD,18-20 IR,18, 20 VCD,18, 20 and fluorescence resonance energy transfer21 experiments. In particular, the observations by NMR16-18 and IR20 experiments suggest the existence of hydrogen bonds between the charged phosphoryl group and the backbone amide protons in solution. The phosphorylation of two Ser residues within a 17-residue peptide alters their ability to adopt α-helical conformation in a position-dependent manner.19 Ser and Thr dipeptides and a tetrapeptide GSSS adopt a mixture of polyproline II and β-strand conformations.18, 20 A limited number of molecular dynamics simulation have been carried out to investigate the phosphorylation-induced conformational changes of peptides.20, 22-25 There is an ab initio study on For-pSer-NH2 complexed with Mg2+ at the HF/3-21G level of theory in the gas phase, in which the pSer residue is suggested to be more flexible than the Ser residue by the denser conformational energy levels of the former than the latter.26 In addition, hydrogen bonds between the charged phosphoryl group and the backbone amide protons are found to contribute to stabilizing low-energy structures.
The cis-to-trans isomerization rates of the pSer/pThrPro bond of a series of peptides determined by a protease-coupled assay were reported to be about 2- to 8-fold slower than those of the nonphosphorylated analogs.15 Furthermore, the analysis of the cis–trans isomerization of the side-chain phosphorylated peptides as a function of pH showed that both the cis content and rate constant of prolyl cis-to-trans isomerization were lower for the dianionic state of the phosphothreonine-containing peptides when compared with the monoprotonated state.15 From molecular dynamic simulations on the unphosphorylated and phosphorylated TSPI motifs, it was reported that the phosphorylation slows slightly down the rate of the cis–trans isomerization of the SerPro bond.22
Although previous experimental and computational works have focused on understanding how the phosphorylation affects the structures and rotational barriers of the Ser/Thr-Pro motifs, the conformational preferences of feasible local minima and transition states and the prolyl cis–trans isomerization for phosphorylated Ser/Thr-Pro motifs are little known to date. We report here the results on N-acetyl-N′-methylamides of pSer-Pro and pThr-Pro (Ac-pSer-Pro-NHMe and Ac-pThr-Pro-NHMe) calculated using hybrid density functional methods with the implicit solvation reaction field theory to investigate the conformational preferences and the cis–trans isomerization for phosphorylated Ser/Thr-Pro motifs in the gas phase and in solution.
METHODS
Chemical structures and torsional parameters for Ac-X-Pro-NHMe (X = pSer and pThr) peptides are defined in Figure 1. All ab initio HF and density functional calculations were carried out using the Gaussian 03 package.27 Here, each backbone conformation of the peptides is represented by a capital letter depending on its values of φ and ψ for the backbone.28 Conformations C, E, A, F, and D are equivalent to the γ-turn (C7eq), extended (C5), α-helical (αR), polyproline-like (PII or β), and β2 structures in the literature, respectively. The trans and cis conformations for the XPro peptide bond are denoted by “t” and “c”, respectively. The down- and up-puckered conformations of the proline ring are defined as those of which the Cγ atom and the C′O group of the prolyl residue lie on the same and opposite sides, respectively, of the plane defined by the three atoms Cδ, N, and Cα (see Figure 1), which are represented by “d” and “u,” respectively. Usually, the down- and up-puckered conformations of the Pro residue have positive and negative values of the endocyclic torsion angle χ1, respectively. The degree of puckering is described by the puckering amplitude χm of Altona and Sundaralingam,29 which is the maximum value attainable by endocyclic torsion angles of the ring. In addition, the conformations for the side-chain torsion angles of the pSer and pThr residues having minima near 180°, 60°, and −60° are denoted by “t,” “g+,” and “g−” for trans, gauche+, and gauche− conformations, respectively. Thus, for example, the conformational letter code Ag+g−/cFd for the pSer-Pro dipeptide denotes that the backbone conformation of the pSer residue is A with side-chain conformations g+ and g− for torsion angles χ11 and χ12, respectively, and the backbone conformation of the Pro residue is F with the cis prolyl peptide bond and the down puckering.
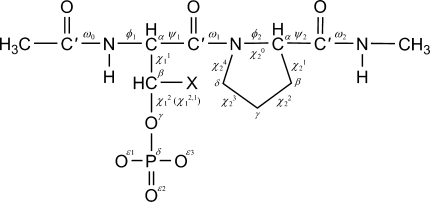
Definition of torsion angles and structural parameters for the pSer/pThr-Pro peptide: X = H and CH3 for pSer and pThr residues, respectively.
The 171 and 75 local minima optimized using the ECEPP/3 force field30 for Ac-Ser-Pro-NHMe and Ac-Thr-Pro-NHMe, respectively, started from the conformations generated by combining local minima of each residue,30 were used as initial points for optimizations at the HF/6-31G(d) level of theory. The larger number of local minima for Ac-Ser-Pro-NHMe than Ac-Thr-Pro-NHMe obtained by the ECEPP/3 force field may be ascribed to the larger number of local minima for Ac-Ser-NHMe than Ac-Thr-NHMe (see Table XIV of Ref.30). All local minima optimized at the HF/6-31G(d) level of theory were then reoptimized at the HF/6-31+G(d) level of theory. The local minima of the Ac-Ser-Pro-NHMe and Ac-Thr-Pro-NHMe peptides optimized at the HF/6-31+G(d) level of theory with the hydroxyl groups of the serine and threonine residues replaced by the phosphate group were used as starting points for the optimization of Ac-pSer-Pro-NHMe and Ac-pThr-Pro-NHMe, respectively, at the same level of theory. The geometries of ethyl phosphate and isopropyl phosphate optimized at the HF/6-31+G(d) level of theory were used as initial geometries for the phosphate groups of the pSer and pThr residues, respectively. Then, all local minima of the pSer and pThr residues at the HF/6-31+G(d) level of theory were reoptimized at the B3LYP/6-31+G(d) level of theory. In addition, X-ray structures of 17 pSer-Pro and 12 pThr-Pro motifs of proteins were used as starting points for the optimization at B3LYP/6-31G(d) level of theory and then reoptimized at the B3LYP/6-31+G(d) level of theory, as described below.
Although we took care of all feasible transition states with the backbone torsion angles ω1 = +116.4°, +118.0°, −65.1°, and −69.8° as initial structures, as found for the Ac-Pro-NHMe peptide,31 only two transition states ts1 and ts2 for both pSer-Pro and pThr-Pro dipeptides with ω1 ≈ +115° were located after the optimization at the B3LYP/6-31+G(d) level of theory. The first two cis conformations Ag+g−/cFd and Ag+g−/cDd for the pSer-Pro dipeptide and Ag+g−/cFd and Bg+g−/cBd for the pThr-Pro dipeptide were used as starting points for the optimization of transition states. Each transition state was checked by the intrinsic reaction coordinate (IRC) method32, 33 whether it connects the reactants and products, that is, the trans and cis conformers. However, as in most cases, the IRC calculation did not step all the way to the minimum on either side of the path.34 Further optimizations were carried out starting from the reactants and products obtained by the IRC method to reach the two minima that the transition state connects.
Single-point energies were calculated at the B3LYP/6-311++G(d,p) level of theory for all local minima and transition states located at the B3LYP/6-31+G(d) level of theory. Vibrational frequencies were calculated for all stationary points at the B3LYP/6-31+G(d) level of theory, which were used to compute enthalpies and Gibbs free energies with a scale factor of 0.98 at 25°C and 1 atm. The scale factor of 0.98 was chosen to reproduce experimental frequencies for the amide I band of N-methylacetamide in Ar and N2 matrixes.35 Each transition state was also confirmed by checking whether it has one imaginary frequency after frequency calculations at the B3LYP/6-31+G(d) level of theory. The zero-point energy correction and the thermal energy corrections were employed in calculating the Gibbs free energy of each conformation at 25°C. Here, the ideal gas, rigid rotor, and harmonic oscillator approximations were used for the translational, rotational, and vibrational contributions to the Gibbs free energy, respectively.36, 37
We used the implicit solvation conductor-like polarizable continuum model (CPCM) self-consistent reaction field method,38, 39 implemented in the Gaussian 03 package,27 to compute solvation free energies (ΔGs) at the B3LYP/6-31+G(d) level of theory with the UAKS cavity, which are the united atom topological model radii optimized for the density functional PBE0/6-31G(d) level of theory.40, 41 The solvation free energy is the sum of the electrostatic free energy and the nonelectrostatic energy terms.42 The latter is composed of the cavitation, dispersion, and repulsion energy terms. For CPCM-UAKS calculations, the default average areas of 0.2 Å2 for tesserae were used. Solvation free energies of Ac-pSer-Pro-NHMe and Ac-pThr-Pro-NHMe peptides were calculated for their local minima and transition states optimized at the B3LYP/6-31+G(d) level of theory in the gas phase. Solvents considered here are nonpolar chloroform and polar water, whose dielectric constants are 4.9 and 78.4 at 25°C, respectively. Recently, the CPCM-UAKS calculations for a number of neutral and charged organic molecules at the B3LYP/6-31+G(d) level of theory provided hydration free energies in agreement with available experimental data.43 In addition, the observed pKa value of the Cys residue was well reproduced at the B3LYP/6-311++G(d,p)//CPCM B3LYP/6-31+G(d) level of theory.44 In the gas phase, the relative free energy (ΔGg) for each conformation was computed by the sum of the relative electronic energy (ΔEe), the thermal contribution, and the entropic contribution. In solution, the relative total free energy (ΔG) was taken as the sum of the relative free energy (ΔGg) and the relative solvation free energy (ΔΔGs).
RESULTS AND DISCUSSION
Conformational Preferences in the Gas Phase
We obtained 27 and 21 local minima for pSer-Pro and pThr-Pro peptides, respectively, and two transition states for each peptide at the B3LYP/6-31+G(d) level of theory in the gas phase. Their backbone torsion angles and thermodynamic properties at the B3LYP/6-311++G(d,p)//B3LYP/6-31+G(d) level of theory in the gas phase are listed in Tables I and II, respectively. The torsion angles for side chains and the puckering amplitude for the prolyl ring of each conformation for pSer-Pro and pThr-Pro peptides are presented in Supporting Information Tables SI and SII, respectively.
| Conformera | Backbone Torsion Anglesb | Thermodynamic Properties | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pSer | Pro | Gas Phase | Chloroform | Water | |||||||||
| φ1 | ψ1 | ω1 | φ2 | ψ2 | ω2 | ΔEec | ΔHgd | ΔGge | ΔΔGsf | ΔGg | ΔΔGsf | ΔGg | |
| Ag+g−/cFd | −74.5 | −39.7 | −15.3 | −110.0 | 131.7 | 179.8 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 4.28 | 4.28 |
| Ag+g−/cDd | −79.6 | −32.1 | −6.5 | −110.5 | 74.7 | 170.3 | 2.97 | 3.43 | 2.81 | 0.23 | 3.04 | 4.36 | 7.17 |
| Fg+g−/tBu | −98.0 | −171.7 | 166.6 | −78.2 | 1.4 | 176.1 | 8.45 | 8.65 | 8.71 | −6.60 | 2.11 | −8.71 | 0.00 |
| Etg−/tAd | −141.8 | 158.2 | −175.8 | −75.7 | −15.0 | −178.7 | 9.26 | 9.22 | 8.83 | −5.38 | 3.44 | −6.02 | 2.81 |
| Fg+g−/tBd | −96.7 | −175.7 | 165.9 | −85.3 | 12.3 | 172.5 | 8.78 | 8.88 | 8.89 | −6.14 | 2.76 | −7.77 | 1.12 |
| Etg−/tAu | −141.0 | 162.7 | −177.8 | −68.5 | −22.5 | −177.3 | 10.60 | 11.18 | 10.35 | −6.57 | 3.78 | −9.05 | 1.30 |
| Fg+g+/tAd | −96.0 | 176.2 | −177.9 | −82.4 | −12.1 | 175.8 | 10.77 | 11.10 | 10.38 | −5.55 | 4.83 | −9.32 | 1.06 |
| Etg+/cCd | −130.7 | 150.8 | 10.1 | −84.2 | 121.1 | −179.9 | 11.71 | 11.69 | 10.40 | −6.11 | 4.29 | −5.20 | 5.20 |
| Etg+/cCu | −136.3 | 151.9 | 4.1 | −71.6 | 121.6 | −179.2 | 11.89 | 11.86 | 10.45 | −5.76 | 4.69 | −4.47 | 5.98 |
| Etg+/tAd | −128.5 | 143.3 | 176.8 | −74.1 | −28.0 | −178.6 | 10.64 | 10.87 | 10.65 | −5.47 | 5.18 | −5.85 | 4.80 |
| Fg+g+/tAu | −94.1 | −178.6 | 176.0 | −62.8 | −29.7 | 179.1 | 10.76 | 11.12 | 10.77 | −6.13 | 4.64 | −10.42 | 0.35 |
| Etg+/tAu | −115.2 | 140.9 | 174.3 | −70.2 | −30.7 | −178.2 | 13.60 | 13.86 | 12.92 | −6.48 | 6.43 | −8.19 | 4.73 |
| Eg−g−/cFd | −144.4 | 164.0 | 13.0 | −82.4 | 146.2 | 178.8 | 14.65 | 14.52 | 13.26 | −6.27 | 6.98 | −4.63 | 8.62 |
| Eg+g+/tAu | −119.4 | −143.3 | 173.1 | −59.3 | −18.8 | −178.5 | 12.28 | 12.98 | 13.49 | −3.34 | 10.15 | −4.58 | 8.91 |
| Eg−g−/cFu | −149.5 | 163.5 | 5.3 | −63.9 | 144.9 | −179.8 | 14.80 | 14.82 | 13.67 | −6.05 | 7.62 | −4.37 | 9.30 |
| Fg−g+/cBd | −62.7 | 139.5 | 3.8 | −95.0 | 28.8 | 174.0 | 15.13 | 15.07 | 16.17 | −11.60 | 4.57 | −15.77 | 0.40 |
| Cg−g+/tCu | −62.1 | 124.6 | −178.7 | −83.2 | 66.4 | −174.6 | 17.92 | 18.40 | 16.26 | −11.77 | 4.49 | −14.99 | 1.27 |
| Cg−g+/tCd | −61.4 | 128.2 | −169.6 | −88.4 | 62.4 | −175.5 | 17.85 | 17.94 | 17.47 | −13.13 | 4.34 | −16.84 | 0.63 |
| Fg−g+/tBd | −62.5 | 135.5 | −178.3 | −98.3 | 9.9 | 171.7 | 19.96 | 19.93 | 18.50 | −13.42 | 5.07 | −18.13 | 0.37 |
| Fg+g−/cBd | −67.4 | 145.4 | 0.8 | −91.2 | 28.8 | 173.9 | 18.68 | 18.45 | 19.45 | −12.29 | 7.17 | −16.11 | 3.34 |
| Fg−g−/cBd | −53.4 | 135.4 | −1.4 | −97.6 | 31.2 | 172.8 | 20.33 | 20.34 | 21.11 | −9.35 | 11.76 | −8.70 | 12.41 |
| Fg−g−/tCd | −60.3 | 147.3 | −174.2 | −82.6 | 57.2 | −175.7 | 25.38 | 26.19 | 22.69 | −10.93 | 11.75 | −14.82 | 7.87 |
| Ftg+/cCd | −73.5 | 143.4 | −3.4 | −101.4 | 51.1 | 174.5 | 24.47 | 24.92 | 23.39 | −11.07 | 12.31 | −13.63 | 9.76 |
| Etg−/cBd | −142.2 | 156.6 | −2.6 | −92.7 | 1.3 | 175.4 | 28.61 | 29.07 | 27.38 | −11.90 | 15.48 | −16.35 | 11.02 |
| Ftg−/cBd | −68.3 | 151.4 | 2.7 | −93.8 | 27.4 | 171.1 | 29.27 | 29.80 | 27.83 | −12.00 | 15.82 | −16.37 | 11.46 |
| Fg−t/tBd | −76.8 | 172.1 | 159.3 | −87.7 | −1.5 | 175.7 | 30.00 | 31.05 | 28.80 | −10.07 | 18.73 | −16.14 | 12.67 |
| Fg+g+/cBd | −85.1 | 176.9 | −8.0 | −99.9 | 1.8 | 173.6 | 30.78 | 30.55 | 29.32 | −10.03 | 19.28 | −16.89 | 12.43 |
| ts1 | −107.6 | −43.4 | 118.9 | −128.8 | −1.3 | 180.0 | 18.26 | 17.18 | 17.99 | 1.31 | 19.30 | 3.82 | 21.81 |
| ts2 | −105.7 | −161.6 | 130.4 | −136.5 | 107.6 | −174.7 | 21.37 | 20.11 | 21.13 | −1.75 | 19.38 | −0.86 | 20.27 |
- a See the text for definition. For example, the first conformational letter code Ag+g−/cFd denotes that the backbone conformation of the pSer residue is A with side-chain conformations g+ and g− for torsion angles χ11 and χ12, respectively, and the backbone conformation of the Pro residue is F with the cis prolyl peptide bond and the down puckering.
- b Torsion angles are defined in Figure 1; units in degrees.
- c Relative electronic energies in kcal/mol.
- d Relative enthalpy changes in kcal/mol at 25°C.
- e Relative Gibbs free energy changes in kcal/mol at 25°C in the gas phase.
- f Relative solvation free energy changes in kcal/mol.
- g Relative Gibbs free energy changes in kcal/mol in solution.
| Conformera | Backbone Torsion Anglesb | Thermodynamic Properties | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pThr | Pro | Gas Phase | Chloroform | Water | |||||||||
| φ1 | ψ1 | ω1 | φ2 | ψ2 | ω2 | ΔEec | ΔHgd | ΔGge | ΔΔGsf | ΔGg | ΔΔGsf | ΔGg | |
| Ag+g−/cFd | −74.7 | −38.5 | −16.4 | −108.5 | 131.3 | 179.7 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 6.16 | 6.16 |
| Bg+g−/cBd | −110.1 | 12.8 | 23.4 | −101.3 | 2.3 | 176.6 | 6.52 | 6.06 | 6.93 | −3.20 | 3.73 | 1.76 | 8.69 |
| Fg+g+/tAu | −96.4 | −178.7 | 177.3 | −63.9 | −28.9 | 178.6 | 7.39 | 7.93 | 7.56 | −5.61 | 1.95 | −7.56 | 0.00 |
| Etg+/cCu | −137.3 | 149.6 | 3.8 | −69.5 | 122.8 | −179.6 | 9.95 | 10.00 | 8.28 | −5.79 | 2.49 | −2.73 | 5.55 |
| Ag+t/cDd | −79.7 | −51.4 | −15.6 | −118.2 | 106.6 | −179.9 | 10.23 | 10.25 | 8.32 | −0.66 | 7.66 | 3.97 | 12.30 |
| Etg+/tAd | −126.2 | 139.0 | 177.2 | −73.4 | −26.5 | −178.7 | 9.15 | 9.27 | 8.98 | −5.00 | 3.98 | −3.25 | 5.73 |
| Fg+g−/tBu | −98.4 | −174.5 | 167.5 | −80.7 | 6.4 | 174.9 | 9.34 | 9.52 | 9.67 | −6.63 | 3.04 | −6.53 | 3.14 |
| Fg+g−/tBd | −97.2 | −178.0 | 165.9 | −86.2 | 16.1 | 171.3 | 9.60 | 9.72 | 9.93 | −5.89 | 4.03 | −5.61 | 4.32 |
| Etg−/tAd | −137.1 | 151.9 | −170.7 | −83.8 | −12.4 | −179.0 | 11.05 | 11.10 | 10.25 | −5.32 | 4.93 | −4.16 | 6.10 |
| Eg+g+/tAu | −113.8 | −142.5 | 172.9 | −61.3 | −16.9 | −178.3 | 9.46 | 10.07 | 10.88 | −3.43 | 7.45 | −1.58 | 9.30 |
| Etg−/tAu | −138.1 | 158.0 | −175.1 | −70.1 | −25.5 | −177.2 | 11.83 | 11.96 | 11.35 | −5.80 | 5.55 | −5.89 | 5.46 |
| Cg−g−/cCu | −30.6 | 122.9 | 11.4 | −84.4 | 111.5 | −174.4 | 11.54 | 12.06 | 11.86 | −4.10 | 7.76 | 2.15 | 14.01 |
| Cg−g+/tBd | −59.1 | 125.9 | −172.6 | −101.6 | 8.4 | 172.4 | 15.18 | 14.54 | 12.61 | −10.06 | 2.55 | −10.61 | 1.99 |
| Fg−g+/cBd | −58.5 | 133.9 | 6.9 | −97.4 | 26.7 | 173.9 | 12.27 | 12.30 | 13.39 | −11.08 | 2.31 | −12.57 | 0.82 |
| Cg−g+/tCd | −62.9 | 123.8 | −168.1 | −88.2 | 62.5 | −175.9 | 14.52 | 14.71 | 14.63 | −12.33 | 2.30 | −14.09 | 0.54 |
| Etg−/cFd | −144.5 | 162.2 | 14.7 | −79.7 | 144.8 | 179.1 | 15.40 | 15.54 | 14.68 | −5.88 | 8.80 | −2.68 | 11.99 |
| Etg−/cFu | −148.2 | 164.2 | 3.0 | −58.2 | 143.8 | −179.9 | 15.11 | 15.35 | 14.69 | −6.01 | 8.68 | −2.31 | 12.39 |
| Cg−g+/tCu | −65.1 | 122.5 | −176.7 | −82.9 | 67.1 | −175.0 | 15.26 | 15.46 | 15.56 | −11.98 | 3.58 | −13.89 | 1.66 |
| Cg−g−/tCd | −37.2 | 114.7 | −164.6 | −89.7 | 61.9 | −175.7 | 17.90 | 17.57 | 16.28 | −8.74 | 7.54 | −7.28 | 9.00 |
| Fg+g−/cBd | −67.8 | 146.3 | 0.1 | −90.5 | 28.3 | 174.0 | 20.14 | 19.88 | 20.94 | −11.51 | 9.43 | −11.68 | 9.27 |
| Etg−/cBd | −138.7 | 150.5 | −1.3 | −90.3 | 0.7 | 173.7 | 29.61 | 29.98 | 27.79 | −10.64 | 17.14 | −12.59 | 15.19 |
| ts1 | −95.0 | −51.3 | 121.1 | −124.8 | −12.9 | −179.2 | 18.05 | 16.91 | 18.27 | 0.92 | 19.19 | 5.98 | 24.25 |
| ts2 | −106.9 | −160.4 | 128.9 | −138.9 | 108.0 | −174.5 | 19.81 | 18.60 | 19.46 | −1.21 | 18.25 | 1.93 | 21.39 |
- a, b, c, d, e, f, g −g See footnotes a−g of Table I.
In the gas phase, the most preferred conformation is Ag+g−/cFd with the cis prolyl peptide bond for both pSer-Pro and pThr-Pro peptides, which are shown in Figures 2a and 3a, respectively. This conformation Ag+g−/cFd has the α-helical structure for the pSer/pThr residue and the down-puckered polyproline I structure for the Pro residue, in which two hydrogen bonds between the phosphate oxygens with the backbone NH groups seem to play a role. These hydrogen bonds are those between the anionic Oε1 of the phosphate group and the NH of the pSer/pThr residue and between the anionic Oε3 of the phosphate group and the NH of the C-terminal NHMe group, where Oε1 and Oε3 are the phosphate anionic oxygens in gauche+ and trans orientations to Cβ about the OγPδ bond, respectively (see Figure 1). The optimized distances of d(Oε1…HN)pSer/pThr and d(Oε3 pSer/pThr…HNNHMe) are 1.58 and 1.63 Å for the pSer-Pro peptide and 1.59 and 1.60 Å for the pThr-Pro peptide, respectively. In addition, the favorable electrostatic interactions of the phosphoester Oγ with two NH groups of the pSer/pThr residue and the C-terminal NHMe group having the distances of d(Oγ…HN)pSer/pThr and d(Oγ pSer/pThr…HNNHMe) equal to 2.51 and 2.50 Å for the pSer-Pro peptide and 2.46 and 2.54 Å for the pThr-Pro peptide, respectively, appear to contribute to stabilize this conformation Ag+g−/cFd. Although there are no observed evidences for this conformation Ag+g−/cFd to be most preferred for the pSer/pThr-Pro motifs in the gas phase to date, the results obtained by NMR16, 18 and IR20 experiments and an ab initio study26 on small pSer/pThr-containing peptides indicate that the hydrogen bonds between the phosphate group and the backbone amide NH groups may play a role in determining the preferred conformations even in aqueous solution.
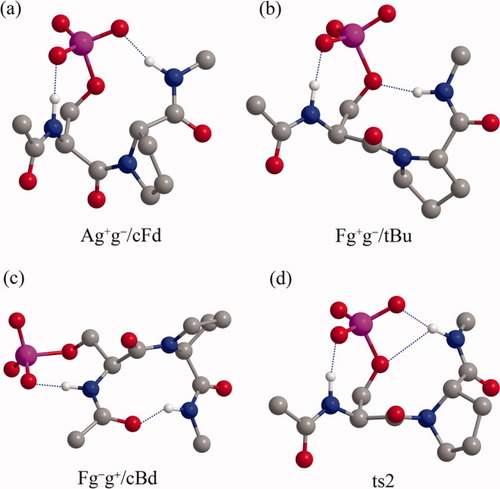
The preferred conformations of Ac-pSer-Pro-NHMe optimized at the B3LYP/6-311++G(d,p)//B3LYP/6-31+G(d) level of theory in the gas phase and using the implicit solvation CPCM method in water. Hydrogen bonds are represented by broken lines. All hydrogens are omitted for clarity, except for amide hydrogens.
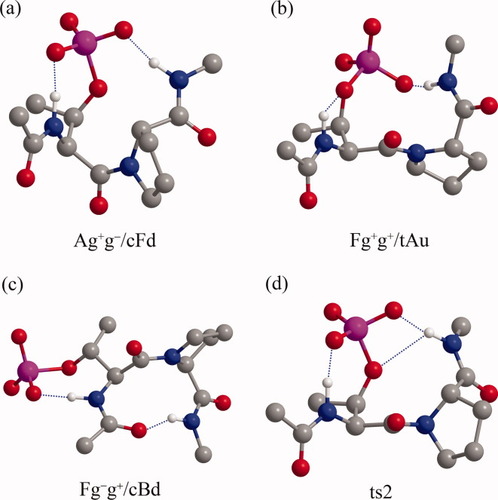
The preferred conformations of Ac-pThr-Pro-NHMe optimized at the B3LYP/6-311++G(d,p)//B3LYP/6-31+G(d) level of theory in the gas phase and using the implicit solvation CPCM method in water. Hydrogen bonds are represented by broken lines. All hydrogens are omitted for clarity, except for amide hydrogens.
The second preferred conformation is Ag+g−/cDd having the cis prolyl peptide bond with ΔGg = 2.81 kcal/mol for the pSer-Pro peptide and Bg+g−/cBd with ΔGg = 6.93 kcal/mol for the pThr-Pro peptide in the gas phase. It should be noted that the first two preferred conformations with the cis prolyl peptide bond for both pSer-Pro and pThr-Pro peptides are in common stabilized by the hydrogen bond between the phosphate group and the C-terminal NH groups, which drive the shift of the backbone torsion angle ψ of the Pro residue by ∼+30° from its mean value of about −80°.31
The most preferred conformations with the trans prolyl peptide bond are Fg+g−/tBu for the pSer-Pro peptide (Figure 2b) and Fg+g+/tAu for the pThr-Pro peptide (Figure 3b), whose ΔGg are 8.71 and 7.56 kcal/mol, respectively, but these two conformations are quite similar each other except for the χ12 torsion angle about the CβOγ bond of the phosphate group. The followed conformations are Etg−/tAd and Fg+g−/tBd with ΔGg = 8.83 and 8.89 kcal/mol, respectively, for the pSer-Pro peptide, and Etg+/cCu, Ag+t/cDd, Etg+/tAd, Fg+g−/tBu, and Fg+g−/tBd with ΔGg = 8.28, 8.32, 8.98, 9.67, and 9.93 kcal/mol, respectively, for the pThr-Pro peptide.
The most preferred trans conformations Fg+g−/tBu for the pSer-Pro peptide and Fg+g+/tAu for the pThr-Pro peptide have the polyproline II structure for the pSer/pThr residue and the distorted 310- or αR-helical structure for the Pro residue with the up puckering. The hydrogen bonds between the phosphate oxygen Oε1 and the NH group of the pSer residue with the distance of d(Oε1…HN)pSer = 1.61 Å and between the phosphoester Oγ and the NH of the C-terminal NHMe group with the distance of d(Oγ…HNNHMe) = 1.88 Å appear to contribute to stabilize the structure of the pSer residue in the conformation Fg+g−/tBu. On the other hand, the structure of the pThr residue in the conformation Fg+g+/tAu seems to be ascribed to the hydrogen bonds between the phosphoester Oγ and the NH group of the pThr residue with the distance of d(Oγ…HN)pThr = 2.42 Å and between the phosphate oxygen Oε3 and the NH of the C-terminal NHMe group with the distance of d(Oε3…HNNHMe) = 1.62 Å. Therefore, the less stability of the trans conformations Fg+g−/tBu and Fg+g+/tAu for pSer-Pro and pThr-Pro peptides, respectively, against the cis conformation Ag+g−/cFd can be ascribed to the replacement of the stronger hydrogen bond between one of the phosphate anionic oxygens and the backbone NH group by the weaker hydrogen bond between the phospoester Oγ of the phosphate group and the backbone NH group.
We located two transition states ts1 and ts2 for the cis–trans isomerization of the pSer/pThr-Pro peptide bond in the gas phase. However, transition states ts1 and ts2 are both for the clockwise rotation through ω1 ≈ +120° about the prolyl peptide bond, whereas the clockwise and anticlockwise rotations through ω1 ≈ +120° and −60°, respectively, are feasible for the AcPro peptide bond of the proline dipeptide.31 Although ts1 and ts2 have similar values of the torsion angle ω1 about the prolyl peptide bond, they have the different backbone conformation and puckering.
In the ts1 conformation of the pSer-Pro peptide, there are a bifurcated hydrogen bond of the phosphate Oε1 with two backbone NH groups having the distances of d(Oε1…HN)pSer = 1.59 Å and d(Oε1…HNNHMe) = 1.75 Å, and another hydrogen bond between the prolyl nitrogen and the following NH group with the distance of d(NpSer…HNNHMe) = 2.35 Å that plays a role in stabilizing the transition states of the proline dipeptide.31 However, two hydrogen bonds of the phosphate Oε1 and Oε3 with two backbone NH groups and another hydrogen bond between the prolyl nitrogen and the following NH group appear to be responsible for stabilizing the ts1 conformation of the pThr-Pro peptide, whose distances are d(Oε1…HN)pThr = 1.58 Å, d(Oε3…HNNHMe) = 1.74 Å, and d(NpThr… HNNHMe) = 2.40 Å, respectively.
The ts2 conformation for both peptides has in common three hydrogen bonds between the phosphate Oε1 and the NH of the pSer (or pThr) residue, between the phosphate Oε3 and the NH of the NHMe group, and between the phosphoester Oγ and the NH of the NHMe group, whose distances are d(Oε1…HN)pSer = 1.61 Å, d(Oε3…HNNHMe) = 1.69 Å, and d(Oγ…HNNHMe) = 2.40 Å for the pSer-Pro peptide, respectively, and d(Oε1…HN)pThr = 1.59 Å, d(Oε3…HNNHMe) = 1.66 Å, and d(Oγ…HNNHMe) = 2.46 Å for the pThr-Pro peptide, respectively (Figures 2d and 3d, respectively). It should be noted that the hydrogen bond between the prolyl nitrogen and the following NH group does not exist in the ts2 conformation for the two peptides.
Conformational Preferences in Solution
The relative solvation free energies (ΔΔGs) and relative total free energies (ΔG) for all stationary points for pSer-Pro and pThr-Pro peptides at the B3LYP/6-311++G(d,p)//CPCM B3LYP/6-31+G(d) level of theory in chloroform and water are presented in Tables I and II, respectively.
In chloroform, the most preferred conformations with cis and trans prolyl peptide bonds are found to be Ag+g−/cFd and Fg+g−/tBu for the pSer-Pro peptide and Ag+g−/cFd and Fg+g+/tAu for the pThr-Pro peptide, respectively, as found in the gas phase. In particular, the ΔG values of the trans conformations Fg+g−/tBu and Fg+g+/tAu for pSer-Pro and pThr-Pro peptides, respectively, are reduced by 6.60 and 5.61 kcal/mol, respectively, due to the favorable solvation. Interestingly, the relative solvation free energies for most of the local minima for both peptides are remarkably increased by about 1–13 kcal/mol using the implicit solvation CPCM method in chloroform. However, most of the preferred conformations for both peptides in the gas phase are also conserved as the preferred conformations in chloroform, which may be ascribed to the strong hydrogen bonds between the anionic oxygens of the phosphate group and the backbone NH groups, as described above. There are some exceptions for the pThr-Pro peptide; the conformation Ag+t/cDd is destabilized and the conformations Cg−g+/tBd, Fg−g+/cBd, and Cg−g+/tCd are remarkably stabilized.
In water, the conformations Fg+g−/tBu and Fg+g+/tAu with the trans prolyl peptide bond become most preferred and more stabilized by 4.28 and 6.16 kcal/mol in ΔG for pSer-Pro and pThr-Pro peptides, respectively, than the most preferred conformation Ag+g−/cFd with the cis prolyl peptide bond in the gas phase and in chloroform. The second to fifth preferred conformations are Fg+g+/tAu, Fg−g+/tBd, Fg−g+/cBd, and Cg−g+/tCd for the pSer-Pro peptide in water, whose ΔG are 0.35, 0.37, 0.40, and 0.63 kcal/mol, respectively. For the pThr-Pro peptide in water, the corresponding conformations are Cg−g+/tCd, Fg−g+/cBd, Cg−g+/tCu, and Cg−g+/tBd with ΔG = 0.54, 0.82, 1.66, and 1.99 kcal/mol, respectively.
The reason why the trans conformations Fg+g−/tBu and Fg+g+/tAu for pSer-Pro and pThr-Pro peptides, respectively, become more stable than the cis conformation Ag+g−/cFd in water, which is remarkably stable in the gas phase and in chloroform due to the strong hydrogen bonds between the anionic oxygens of the phosphate group and the backbone NH groups, can be ascribed to the larger solvation free energy of one anionic oxygen of the phosphate group for the trans conformation. As described above, the anionic oxygen Oε3 (or Oε1) of the phosphate group is nearly free for the trans conformation Fg+g−/tBu (or Fg+g+/tAu), whereas it is involved in the formation of the hydrogen bond with the NH of the C-terminal NHMe group for the cis conformation Ag+g−/cFd. The solvation free energies of H3PO4 and H2PO4− are estimated to be −13 and −81 kcal/mol, respectively, at the implicit solvation PCM HF/6-31G(d) level of theory,45 which may indicate that the free anionic oxygen of the phosphate group has approximately the value of −68 kcal/mol in ΔΔGs than the neutral OH group. Upon formation of the hydrogen bond with the NH of the C-terminal NHMe group, the solvation free energy of the anionic oxygen Oε3 (or Oε1) of the phosphate group is expected to be dramatically decreased for the cis conformation Ag+g−/cFd.
The most preferred cis conformation is Fg−g+/cBd for both the peptides, whose ΔG are 0.40 and 0.82 kcal/mol for pSer-Pro and pThr-Pro peptides, respectively (shown in Figures 2c and 3c, respectively), as described above. It should be noted that the corresponding ΔG are 16.17 and 13.39 kcal/mol in the gas phase, respectively, and 4.57 and 2.31 kcal/mol in chloroform, respectively. The larger solvation free energies of −15.77 and −12.57 kcal/mol for pSer-Pro and pThr-Pro peptides, respectively, seem to contribute to stabilize this cis conformation in water.
In particular, it should be noted that the hydrogen bonds between the NH of the pSer/pThr residue and the phosphate Oε1 or between the NH of the C-terminal NHMe group and the phosphate Oε3 appear to be responsible for stabilizing the preferred conformations for both pSer-Pro and pThr-Pro peptides in water, as described above. This is consistent with the observations by NMR16-18 and IR20 experiments, suggesting the existence of hydrogen bonds between the charged phosphoryl group and the backbone amide protons in solution.
Comparison with X-Ray Structures of Proteins
By searching the Het Group data at the PDBsum website,46 we found 280 pSer- and 242 pThr-containing entries in the Protein Data Bank (PDB).47 From these PDB entries, we identified 17 pSer-Pro and 12 pThr-Pro motifs of X-ray structures with resolutions of ≤2.3 Å, respectively, which are listed in Supporting Information Tables SIII and SIV. In particular, it should be noted that there are no entries with the cis prolyl peptide bond.
In the 17 pSer-Pro motifs of X-ray structures, there are 14 different conformations, which are Cg−g−/tFd (1F8AC), Cg−t/tAd (2GHQD), Cg−t/tFu (1T15B and 1T29B), Ctg+/tFu (3D9KZ), Eg+g+/tHu (3D9KY), Etg+/tAd (3D9PZ), Etg+/tBd (3D9KZ, 3D9LY, and 3D9NZ), Etg+/tBu (3D9LZ), Ett/tAu (1SZAZ), Fg−g+/tAd (1F8AC), Fg−t/tAu (2GHTD), Fg−t/tFu (3FQXC), Ftg+/tFu (3D9MZ), and H*tt/tBd (2GHQD), where PDB entry codes are shown in parentheses. After optimizations at the B3LYP/6-31G(d) level of theory, they are converged into Fg−g−/tCd, Fg−t/tBd, Cg−g+/tCu, Cg−g+/tCu, Eg+t/tAu, Etg+/tAd, Etg+/tAd, Ett/tAu, Ett/tAu, Fg−g+/tBd, Eg+g+/tAu, Cg−g+/tCu, Cg−g+/tCu, and Etg+/tAd, respectively. At the B3LYP/6-31+G(d) level of theory, the conformations Eg+t/tAu and Ett/tAu are converged into Fg+g+/tAu and Etg−/tAu, respectively, and the others remain the same as the conformations at the B3LYP/6-31G(d) level of theory. The relative free energies for the conformations Fg−g−/tCd, Fg−t/tBd, Cg−g+/tCu, Fg+g+/tAu, Etg−/tAu, Fg−g+/tBd, and Eg+g+/tAu are computed to be 7.87, 12.67, 1.27, 0.35, 1.30, 0.37, and 8.91 kcal/mol, respectively, at the B3LYP/6-311++G(d,p)//B3LYP/6-31+G(d) level of theory with the CPCM method in water.
We obtained 5 different conformations from the 12 pThr-Pro motifs of X-ray structures, which are Cg−g+/tFd (1Q4KD, 1Q4KE, 1Q4KF, 1UMWF, 2OJSE, and 3BZIE), Cg−g+/tFu (1UMWE), Fg−g+/tCd (3E87A and 3E87B), Fg−g+/tFd (3D0EA and 3D0EB), and Fg−g+/tFu (2ASTD). After optimizations at the B3LYP/6-31G(d) level of theory, they are converged into Cg−g+/tCd, Cg−g+/tCu, Cg−g+/tCd, Cg−g+/tCd, and Cg−g+/tCu, respectively. At the B3LYP/6-311++G(d,p)//B3LYP/6-31+G(d) level of theory with the CPCM method in water, the relative free energies of the conformations Cg−g+/tCd and Cg−g+/tCu are calculated to be 0.54 and 1.66 kcal/mol, respectively.
These conformations and their relative energies for both peptides in the gas phase and in solution are also included in Tables I and II. The differences in the backbone and side-chain conformations for the calculated preferred structures and the observed X-ray structures might be ascribed to the different end groups and the crystal packing that is governed by van der Waals contacts and intramolecular hydrogen bonds.
Population of Cis Prolyl Peptide Bond
The population of each conformation can be calculated using the normalized Boltzmann weight by the relative total free energy at the B3LYP/6-311++G(d,p)//B3LYP/6-31+G(d) level of theory at 25°C in the gas phase and using the implicit solvation CPCM method in solution.
The cis populations of the prolyl peptide bond are computed to be 100.0 and 95.7% for the pSer-Pro peptide and 100.0 and 92.6% for the pThr-Pro peptide in the gas phase and in chloroform, respectively, due to the conformation Ag+g−/cFd. In water, the cis populations are diminished to be 14.7 and 14.2% for the pSer-Pro and pThr-Pro peptides, respectively, to which the conformation Fg−g+/cBd contributes dominantly for both the peptides, as described above. Our calculated cis populations are reasonably consistent with observed values of 12–19% and 6–11% for pSer-Pro and pThr-Pro containing peptides, respectively, from NMR experiments in a buffer solution.15 The interplay of solvation and hydrogen bonds on the relative stabilities of cis conformations Ag+g−/cFd and Fg−g+/cBd for both peptides in water is discussed above.
Prolyl Cis–Trans Isomerization
We located two transition states ts1 and ts2 for the cis–trans isomerization of the pSer/pThr-Pro peptide bond in the gas phase, which are both for the clockwise rotation through ω1 ≈ +120° about the prolyl peptide bond but have the different backbone conformation and puckering, as described above. For the pSer-Pro peptide, the ts1 is feasible in the gas phase and in chloroform and the ts2 is favored in water, whereas the ts1 is preferred in the gas phase and the ts2 is favored in chloroform and water for the pThr-Pro peptide.
At the B3LYP/6-311++G(d,p)//B3LYP/6-31+G(d) level of theory in the gas phase, the rotational barriers (ΔG‡tc and ΔG‡ct) to the trans-to-cis and cis-to-trans isomerizations are estimated to be 9.28 and 17.99 kcal/mol for the pSer-Pro peptide, respectively, and 10.71 and 18.27 kcal/mol for the pThr-Pro peptide, respectively (see Table I). At the same level of theory with the implicit solvation CPCM method, the values of ΔG‡tc and ΔG‡ct are calculated to be 17.19 and 19.30 kcal/mol in chloroform, respectively, and 20.27 and 19.87 kcal/mol in water, respectively, for the pSer-Pro peptide, whereas the corresponding values are estimated to be 16.30 and 18.25 kcal/mol in chloroform, respectively, and 21.39 and 20.57 kcal/mol in water, respectively, for the pThr-Pro peptide (see Table I). These calculated results indicate that the rotational barriers ΔG‡tc and ΔG‡ct for both peptides increase as the solvent polarity increases, as seen for the Pro dipeptide,31 except for the values of ΔG‡ct of the pThr-Pro peptide in the gas phase and in chloroform, which are almost the same. Our calculated values of 19.87 and 20.57 kcal/mol for ΔG‡ct of pSer-Pro and pThr-Pro peptides in water, respectively, are consistent with the observed values of 19.6 and 20.1 kcal/mol for Ala-Ala-Xaa-Pro-Phe-NH-Np with Xaa = pSer and pThr, respectively, from NMR experiments at 25°C in a buffer solution.15 In particular, these calculated values are similar to the value of 20.55 kcal/mol for ΔG‡ct of the proline dipeptide at the same level of theory in water.48 This may indicate that the phosphorylation does not alter significantly the rotational barriers for the cis-to-trans isomerization of the prolyl peptide bond. Because of phosphorylation, there are the increases of 0.4 and 1.1 kcal/mol in ΔG‡ct for Ala-Ala-Xaa-Pro-Phe-NH-Np with Xaa = Ser and Thr, respectively, deduced from NMR experiments at 25°C.15
By analysis of the contributions to rotational barriers in the gas phase, cis–trans isomerizations for the pSer/pThr-Pro peptide bond are proven to be entirely enthalpy driven, to which the electronic energies have contributed considerably (Tables I and II), as seen for the Ala dipeptide,31 Pro dipeptide,31 and Pro derivatives.48-52 This is consistent with the experimental results on proline-containing peptides, kinetically determined as a function of temperature.53
The kinetic and spectroscopic results have been interpreted as the evidence that indicates the existence of an intramolecular hydrogen bond between the prolyl nitrogen and the following amide NH group for the transition state structure, which is capable of catalyzing the prolyl isomerization by up to 260-fold in model peptides.54 However, this intramolecular hydrogen bond does not play a role in stabilizing the preferred transition state ts2 for the pSer/pThr-Pro peptides in water because the amide hydrogen of the NHMe group is involved in a bifurcated hydrogen bond with the phosphate Oε3 and phosphoester Oγ, as described above (Figures 2d and 3d).
CONCLUSIONS
In the gas phase and in chloroform, the most preferred conformation Ag+g−/cFd has the α-helical structure for the pSer/pThr residue, the down-puckered polyproline I structure for the Pro residue, and the cis prolyl peptide bond for both pSer-Pro and pThr-Pro peptides, in which two hydrogen bonds between the phosphate oxygens with the backbone NH groups seem to play a role.
However, the trans conformations Fg+g−/tBu and Fg+g+/tAu for pSer-Pro and pThr-Pro peptides, respectively, that have a single hydrogen bond of the phosphate oxygen with either of two backbone NH groups become most preferred in water. This is because the hydration free energy of the anionic oxygen of the phosphate group is expected to dramatically decrease for the cis conformation Ag+g−/cFd upon formation of the hydrogen bond with the backbone NH groups. These calculated results are consistent with the observations by NMR and IR experiments, suggesting the existence of hydrogen bonds between the charged phosphoryl group and the backbone amide protons in solution.
From the values of >90% due to the conformation Ag+g−/cFd in the gas phase and in chloroform, the cis populations are diminished to be 14.7 and 14.2% for the pSer-Pro and pThr-Pro peptides in water, respectively, to which the conformation Fg−g+/cBd contributes dominantly for both the peptides. These calculated cis populations are reasonably consistent with observed values for pSer-Pro and pThr-Pro containing peptides, respectively, from NMR experiments.
The rotational barriers to the prolyl cis-to-trans isomerization are calculated to be 19.87 and 20.57 kcal/mol for pSer-Pro and pThr-Pro peptides in water, respectively, which are consistent with the observed values for Ala-Ala-Xaa-Pro-Phe-NH-Np with Xaa = pSer and pThr, respectively, from NMR experiments. However, the hydrogen bond between the prolyl nitrogen and the following amide NH group, which was suggested to be capable of catalyzing the prolyl isomerization, does not play a role in stabilizing the preferred transition state for the pSer/pThr-Pro peptides in water. Instead, the amide hydrogen of the NHMe group is involved in a bifurcated hydrogen bond with the anionic oxygen and phosphoester oxygen of the phosphate group.