Research highlights
An open-and-shut case
Many bacteria rely on periplasmic binding proteins (PBPs) as gatekeepers for the shepherding of nutrients and other small molecules into the cytoplasm. These proteins consist of two globular domains connected by a hinge, angled apart like a pair of open jaws. When a target binds the PBP, the jaws clamp shut and this conformational shift creates a new binding site for interaction with other membrane bound receptors.
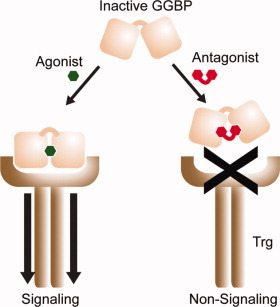
PBPs participate in numerous functions essential to bacterial pathology, including chemotaxis and quorum sensing, making them appealing targets for antibiotic design. Most such drugs work as PBP agonists, exploiting this system to gain cytoplasmic access, but a new article in ACS Chemical Biology by Borrak et al. demonstrates a promising alternative: designing antagonists that give PBPs “lockjaw” and thereby trap them in an inactive state. They investigated E. coli glucose/galactose-binding protein (GGBP), a PBP that recognizes sugars as chemoattractive stimuli. Having made the unexpected finding that a glucose variant with an alkoxy substitution, 3-OMe Glc, acts as a relatively low-affinity antagonist for GGPB, the investigators determined through structural analysis that this substrate appears to lock the PBP in the open conformation.
Encouraged by this discovery, the authors developed a strategy for generating a more effective “wedge” inhibitor, superimposing structures of open/unbound GGBP and closed/glucose-bound GGBP and designing a glucose derived molecule that makes simultaneous contact with appropriate residues in both globular domains without allowing GGBP to actually close. The resulting inhibitor, composed of two glucose molecules with a 2-methylene linker, demonstrated nearly five-fold higher affinity than 3-OMe Glc and proved an effective antagonist for blocking glucose-induced chemotaxis, suggesting that this structurebased strategy may prove generally applicable for targeting other PBPs.—Michael Eisenstein.
Borrok, M.J. et al. ACS Chem. Biol., published online 30 April 2009, doi: 10.1021/cb900021q.
Points of contact
Lipopolysaccharide (LPS) molecules released by Gram negative bacteria at the onset of infection represent an early warning for the immune system—but can also act as a lethal sneak attack against the host, triggering septic shock if the immune response stampedes out of control.
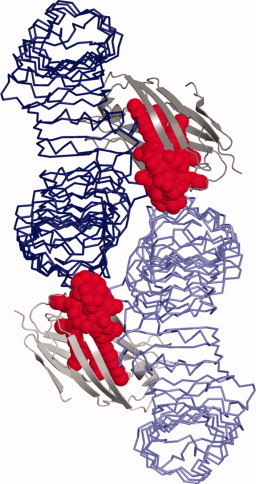
LPS stimulates immune response via the TLR4-MD-2 receptor complex, inducing dimerization and activation of a downstream signaling cascade. However, the LPS family of molecules is fairly heterogeneous—particularly in their lipid A domain, the segment primarily responsible for receptor recognition—and it remains unclear why subtle variations can markedly affect TLR4-MD-2 interaction and immunostimulatory activity.
A high-resolution crystal structure of the TLR4-MD-2-LPS complex, published recently by Park et al. in Nature, offers some new insights into this phenomenon. They observed a symmetrical structure, consisting of two of each molecule, with LPS residing primarily within a large hydrophobic pocket in MD-2 and directly mediating dimerization via both hydrophobic and hydrophilic interactions.
By identifying the fine details of these interactions, the researchers were able to understand the structural basis for previously published experimental observations regarding functional differences between LPS variants. For example, the lipid A domain may be acylated with different numbers of lipid chains: six chains confers strong inflammatory capabilities, while a four-chained-variant is negligibly active. The model presented here suggests that LPS molecules with fewer chains move deeper into the pocket of MD-2, and thereby undermine dimerization and signal transduction.
The authors conclude that future analysis of these complex interactions may help drive the development of effective LPS-derived therapeutic agents.—Michael Eisenstein
Park, B.S., et al. Nature, 2009, 458, 1191–1195.
A Fitting Approach
The convergence of the fields of molecular biology and cell biology has been increasing lately, and large impetus for this has been an increasing understanding of the structures of macromolecular assemblies. These assemblies typically consist of multiple proteins, often combined with nucleic acids, carbohydrates or lipids. Cryo-electron microscopy (cryoEM) is increasingly being used as a means to determine low-resolution structures, and an electron microscopy data bank (http://www.emdatabank.org) has been developed to collect and distribute results from this technique.
It is frequently the case that higher-resolution structures of individual proteins or other fragments of these assemblies are available from crystallography, NMR or homology modeling. In a recent paper in the Journal of Molecular Biology, Lasker and co-workers describe a method called MultiFit, which uses combinatorial optimization techniques to position atomic structures of components into their assembly density maps at resolutions as low as 25 Å. This complements, or can provide starting configurations, for other techniques that attack the same problems as some what higher resolution. These methods should lead to increased structural understanding of many important structural components of cells that have not yet been understood in structural terms.—David A. Case
Lasker, K., et al. J. Mol. Biol. 388, 180–194, 24 April 2009.




