FTIR microspectroscopic analysis of the effects of certain drugs on oxidative stress and brain protein structure
Abstract
This study reports the changes in lipids and proteins of different brain areas of nicotine, D(+)-amphetamine, and nicotine and D(+)-amphetamine treated rats by monitoring lipid peroxidation and protein β-sheet formation using infrared microspectroscopy. Compared with the untreated brain samples, the peroxide level is relatively higher in the amphetamine-treated brain sections, both in the cortex and hippocampus area. However, this peroxide increase is attenuated when administering amphetamine plus nicotine. Analogous drug-dependent trends for protein β-sheet content are observed, which suggests a connection between lipid oxidation involved in oxidative stress and β-sheet protein structure generally present in neurodegenerative diseases. The above property of nicotine is of interest, in the sense that it might reduce the production of β-amyloid proteins in Alzheimer's disease. © 2008 Wiley Periodicals, Inc. Biopolymers 89: 548–554, 2008.
This article was originally published online as an accepted preprint. The (Published Online) date corresponds to the preprint version. You can request a copy of the preprint by emailing the Biopolymers editorial office at [email protected]
INTRODUCTION
A promising strategy for the treatment of Alzheimer's disease (AD) and related disorders involving oxidative stress seems to be selective activation of brain nicotinic receptors.1, 2 Nicotine and its nontoxic derivatives have been reported to improve cognitive ability by acting directly or indirectly on the cortical neurons.3-6 However, many of the neuronal changes that nicotine induces are poorly understood. Some of the effects of this drug are positive, such as neuroprotection against neurotoxic agents, ageing, and pathological situations.6-12 Our research group has demonstrated that nicotine increases the turnover of the glycolytic pathway and Krebs cycle in neurons,13 and the NGF immunoreactivity in the frontoparietal cortex.14 The benefits of other changes, such as the modification of synaptic transmission15 and the activation of the midbrain dopamine system,16 are more debatable. Finally, some neuronal changes can be negative, such as the induction of apoptosis.8, 17 These negative results require a deeper knowledge to be gained of the neuronal and glial effects of nicotinergic treatments before their clinical use.
Infrared spectroscopy has been used in biology for studying the structure and conformation of molecules like proteins, nucleic acids, and lipids. Particularly, Fourier transform infrared (FTIR) microspectroscopy has been found to be a technique that is sensitive to molecular composition and molecular conformational changes associated either to administration of drugs or to pathological development, which is not easily detectable by morphological methods.18 The spatial resolution of this technique offers the possibility of detecting very small amounts of compounds that can be located in surface areas of the order of square micrometers. This is a clear advantage when compared with most traditional biochemical techniques requiring tissue homogenization, which can dilute locally concentrated products beyond detection limits and disrupt their spatial distribution. Since oxidation and derivative products can be highly localized in active brain lesions, data from traditional techniques would not necessarily reflect the chemical changes that occur at sites of pathology. A previous study has demonstrated the potential of this spectroscopic technique in detecting amphetamine-induced oxidative stress and concomitant protein structural changes.19 In this study, FTIR microspectroscopy has been used to investigate the molecular changes in brains from nicotine, D(+)-amphetamine, and nicotine and D(+)-amphetamine treated rats. Particularly, the use of this spectroscopic technique is focused here on protein structural changes and measurement of lipid peroxidation generated by the above drug treatments under conditions designed not to produce apoptosis. The corresponding results may help in the development of new treatments for cognitive disorders, and help to understand the mechanism of action of nicotine and nicotine–amphetamine combined drugs. In addition, the results presented in this study show some infrared bands which can be new useful biomarkers to detect the generation of lipid peroxides as precursors of free radicals.
MATERIALS AND METHODS
Experimental Animals
Four groups (A–D) of 16 3-month-old, male, Wistar albino rats, weighing 300 g, bred at the Cajal Institute, were housed in standard cages. Food and water were made available ad libitum. The temperature was controlled at 22°C and a 12 h/12 h light/dark cycle maintained. All the procedures were conducted in accordance with NIH20 and Spanish21 welfare guidelines. The experimental protocols were approved by the CSIC animal welfare committee. Drugs were administered daily via intraperitoneal injections containing 0.5 ml of the corresponding solution in 0.9% (w/v) NaCl. No behavioral or weight differences were observed between control and experimental animals during normal handling.
The treatments of the different rat groups were as follows: (A) saline (NaCl 0.9%) control solution; (B) nicotine ([−] nicotine hydrogen tartrate salt; Sigma Chem, St. Louis, MO), at a daily dose of 1 mg/kg—equivalent to 0.35 mg of (−)-nicotine free base/kg/day; (C) amphetamine (D(+)-amphetamine sulfate salt; Uquifa Lab, Barcelona, Spain), at a daily dose of 4 mg/kg—equivalent to 3.92 mg of D(+)-amphetamine free base/kg/day; and (D) nicotine and D(+)-amphetamine (both drugs administered simultaneously at the above doses). The nicotine dose administered was the same as that used in previous work showing it to be responsible for dehydrogenase activation and NGF induction, but not the induction of apoptosis.13, 14 We have previous information on the minimum amphetamine dose inducting apoptosis in the hippocampus using a similar treatment schedule as here. Here, the selected dose was 75% lower than in that study in order to prevent apoptosis.
Tissue Handling for Microspectroscopy and Immunohistochemistry for Apoptosis
Two weeks after initiating the experimental treatments (1 h after the last injection), 12 of the animals from each group were deeply anesthetized with 60 mg/kg of i.p. Equitesin (Jansen Lab, Piscataway, NJ) and then transcardially perfused via the aorta with 100 ml of 0.9% (w/v) NaCl followed by 400 ml of a fixative solution containing 4% (w/v) formaldehyde (prepared from paraformaldehyde) in 0.1M phosphate-buffered saline solution (PBS, pH 7.4). Brains were dissected out and postfixed for 3 h at 4°C in the same fixative solution, and then kept overnight at 4°C in 0.1M phospate buffer (PB, pH 7.4).
Six brains were cryoprotected in ascending sucrose solutions (15–30%), rapidly frozen with dry ice, and 12 coronal 15-μm thick serial sections, at the level of 3.60 mm anteroposterior to the bregma in the brain atlas of Paxinos and Watson22 were obtained using a Leitz sledge cryotome at −20°C (Leitz, Germany). The even cuts were placed between two ZnSe or CaF2 discs and kept at −20°C until FTIR microspectroscopical analyses in the 4000–700 cm−1 and 4000–1000 cm−1 ranges, respectively. The odd cut numbers were processed of habitual way, hematoxylin/eosin stained, to verify the anatomical structures.
The remainder six fixed brains were washed in 0.1M PBS (pH 7.4) for 1 h, dehydrated and embedded in paraffin wax. Serial coronal sections of 5-μm thick at coronal level −3.60 mm of the above atlas were obtained using a Shandon rotary microtome (Shandon, Cheshire, UK). Sections separated by 30 μm were used in the histochemical study of apoptosis. The brain atlas of Paxinos and Watson22 was used to define the anatomical areas. The areas studied were as follows: (a) the frontoparietal cortex (motor and sensory areas) and (b) the hippocampus (CA-1, CA-2, CA-3, and the gyrus dentatus).
Apoptosis
- a
The detection of terminal deoxynucleotidyl transferase by biotinylated UTP nick end-labeling using the Apoptag test (an in situ apoptosis detection kit that detects new 3′-OH DNA ends generated by fragmentation; Oncor, Gaithersburg, molecular dynamics (MD), USA). Digoxygenin-dUTP was used to label the DNA fragments, which were visualized using a peroxidase-conjugate antidigoxygenin antibody. The procedure of detection of apoptotic cells was performed according to the manufacturer's instructions. Intact cells that showed the reddish brown reaction product in the nucleus were taken as Apoptag-positive.
- b
Immunohistochemical detection of increases in caspase 3 and caspase 9 concentration using purified rabbit polyclonal caspase antiserum (1:500) (Chemicon International, Tamecula, USA), following a standard protocol used for immunodetection. Tissue sections were deparaffinated, rehydrated, and treated for 30 min with 1% (v/v) H2O2 in distilled water, rinsed twice in cold PBS (pH 7.4) for 15 min, and then boiled in 10 mM citrate buffer (pH 6.0) for 10 min. They were then cooled to room temperature and left for 20 min. After incubation for 30 min in 3% normal goat serum in PBS, to which 0.2% Triton X-100 had been added, all the sections were further incubated in a humidity chamber for 24 h at 4°C, covered by a solution containing each purified rabbit antiserum to which 0.2% Triton X-100 had been added. After previous incubation, the sections were still incubated for 2 h at room temperature in biotinylated anti-rabbit IgG secondary antibody (Vector Laboratories, Burlingame, CA) diluted 1:100 in PBS, followed by several rinses in PBS. They were then incubated again for 1 h at room temperature in avidin–biotin complex detection solution (ABC Elite kit, Vector Laboratories), and the peroxidase complex was developed at room temperature for 5 min using 0.06% 3,3′-diaminobenzidine tetrahydrochloride (DAB) as a chromogen (Sigma Chem, USA) and 0.001% (v/v) H2O2 in 0.1M Tris-HCl buffer (pH 7.4). The sections were dehydrated in a graded ethanol series, cleared with xylene, and mounted with Entellan (Merck, Darmstadt, Germany). Control procedures were performed in parallel and involved the following: (a) the use of normal rabbit serum instead of the specific antiserum and (b) the omission of the primary antiserum.
Tissue Handling for Lipid Peroxidation Analysis
The remaining four rats from each group were sacrificed by decapitation, and two anatomical regions were obtained by microdissection: (a) the frontoparietal cortex [the motor and somatosensory areas were taken together from the limits of the gyrus cingularis and retrospenial agranular cortex to the rhinal fissure (upper and lower limits), and from the 1.00 mm to −4.50 mm (anterior and posterior limits)] coronal planes according to the atlas of Paxinos and Watson22 and (b) the hippocampus (CA1, CA2, CA3, and the gyrus dentatus; from the fimbria to the above-mentioned posterior limit). Lipid peroxidation levels at the time of the assay, which are closely related to the production of free radicals, were determined using the Lipid Hydroperoxide Assay Kit manufactured by the Caiman Chemical (MI) based on the redox reactions of the hydroperoxides existing in the tissue with ferrous ions. The preparation of the homogenates, the chloroform/methanol extraction, and the measurement of ferric ions generated during the reaction were performed according to the manufacturer's instructions. The lipid peroxidation levels (mean values ± SD; n = 4) were calculated, and the results for the treatment groups were compared with those of the control group. ANOVA and the Student's t-test were used to analyze the results; significance was set at P < 0.05.
FTIR Microspectroscopic Analysis
Acquisition of spectral images and measurement of spectra were carried out on a Perkin-Elmer Spectrum Spotlight 300 infrared imaging spectrometer, as described elsewhere.19 Briefly, infrared images were acquired with a liquid nitrogen cooled mercury cadmium telluride (MCT-A) line detector composed of 16 pixel elements, which can be operated either at 6.25 or 25 μm/pixel resolution. In this work, we used the 25 μm/pixel spatial resolution. Each absorbance spectrum composing the IR images, and resulting from 10 scans at 4 cm−1 spectral resolution, was recorded for each pixel in the transmission mode. To allow meaningful comparisons, all the infrared data were uniformly pretreated. About 12 spectra in each brain area were selected and further assessed by calculating band area and curve fitting. The Grams/AI software (ThermoGalactic) was used to fit the 1600–1500 cm−1 region to a sum of Lorentzian functions by a nonlinear least-squares procedure, to determine protein β-sheet contents as described elsewhere.19
After atmospheric correction, all the spectra (in the 1000–700 cm−1 region) were converted to their second derivative using an 11-point Savitzky-Golay algorithm to minimize the influence of background artifacts. The resulting spectra in the above region were treated by principal component analysis (PCA). This analysis has been performed on the data at different locations in the spectra to find the independent sources of variation in all spectra and to reduce the number of variables describing the data set. Maps based on principal component scores have been used to find the independent sources associated with controls and drug-treated tissues.
RESULTS AND DISCUSSION
Infrared Spectra from Controls and Drug-Treated Tissues
Reactive oxygen species (ROS) are thought to be produced in living tissues as a result of oxidative stress acting over a long period of time. Accordingly, free radical reactions and the formation of lipid peroxides in tissues lead to age-related damage and eventually to various age-related pathological processes. Oxidative stress has been demonstrated to occur also in response to high doses of substituted amphetamines such as methamphetamine (METH) and 3,4-methlyene-dioxymethamphetamine (MDMA) leading to long-term neurochemical changes.19 Therefore, the spectroscopic measurement of peroxides in brain tissue samples is of interest to determine levels of the oxidative stress. Peroxide determinations can be carried out using various different ways. One of them is the use of the νOH band of hydroperoxides located in the 3500–3000 cm−1 region. Although this procedure is reliable for dried samples, this band is masked by the analogous band of water when studying fresh brain tissues (see Figure 1). Another alternative could be measurement of the CH stretching bands, because oxidation can result in disordering of the hydrocarbon lipid chains and subsequent increase of the corresponding half-band widths.19 However, the νCH bands can be influenced by water to some extent, and their spectral changes are not necessarily produced just by lipid oxidation, but also by conformational transitions of membrane proteins and other membrane-related phenomena. Therefore, in fresh tissue samples it is necessary to find other peroxide bands that are free from water interferences. Only very few studies have been reported on organic peroxides, mainly because of their unstable nature.23 Despite the fact that the OO stretch appears in a region where carbon skeletal modes may interfere, Vacque et al.23 determined it using a narrower range of frequencies for this vibration, i.e., 845–875 cm−1. This has prompted us to carry out PCA for the 900–800 cm−1 region to extract, in a multiple variable system, one, two, or more PCs that carry maximum information. The first one generally represents the maximum variance and discrimination of tissue samples. In conclusion, the spectra of brain tissue samples (see Figure 1) are dominated by bands from water, lipids, and proteins in the region above 1000 cm−1, whereas the bands below this frequency being of weak intensity. This is particularly visible in Figure 2 where an expanded scale is shown in the 1000–700 cm−1 range. Although the 873 cm−1 band appears with weak intensity in the original spectrum (Figure 2A), the height of its second-derivative peak is enhanced (Figure 2B). This band can be assigned to the νOO vibrational mode from peroxides as described earlier.23
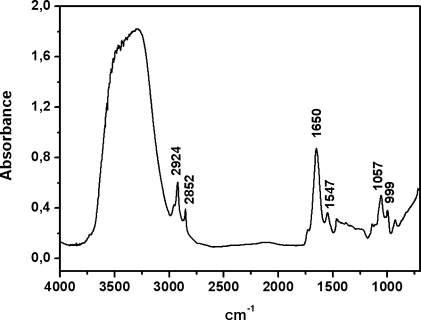
Infrared spectrum measured in the 4000–700 cm−1 region from the cortex area in the brain tissue section of a control.
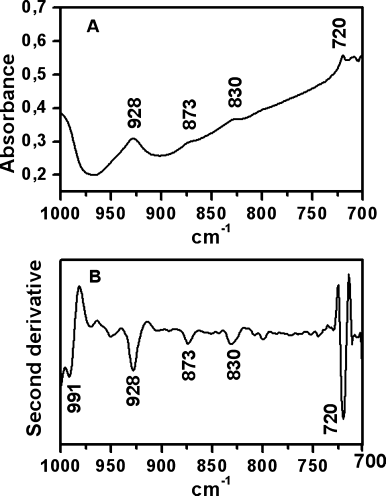
(A) Infrared spectrum measured in the 1000–700 cm−1 region from the cortex area in the brain tissue section of a control. (B) Corresponding second derivative spectrum.
The second-derivative analysis of infrared spectra permits direct quantitative analysis of peroxides through this second-derivative peak. In fact, the intrinsic shape of an infrared absorbance is approximated by a Lorentzian function.24 In the second-derivative spectrum, the peak frequency of an absorbance is practically identical with the original peak frequency, but the half-bandwidth is reduced.25, 26 The height of a second-derivative peak is proportional to the original peak height with an opposite sign, and the half-bandwidth of the second-derivative peak is proportional to the original half-bandwidth.25, 26 On this basis, the 873 cm−1 peak height (Figure 2B) relative to that of the 720 cm−1 peak (lipid chain CH2 rocking) was used here as spectroscopic quantitative evaluation of peroxide content (Table I). The use of the 873 cm−1 second-derivative peak with this aim is supported by its assignment to peroxides on the basis of group frequency considerations,23 and on the fact that the intensity of this peak increases upon amphetamine treatment, which is known to generate higher hydroperoxide levels relative to controls19 (Table I). In fact, PCA of second-derivative spectra in the 900–800 cm−1 region shows the possibility of discriminating amphetamine-treated and control tissues through the two-dimensional (2D) scatter plot displayed in Figure 3. Most part of the control samples are located on the positive part of PC1 axis, unlike amphetamine-treated brain samples that tend to fall on the PC1 negative part. The spectral significance of this negative part of the PC1 is shown in the loading plots (see Figure 4) of this principal component for the score plot of Figure 3. Thus, the most significant contribution to the negative part of PC1 axis, where amphetamine-treated samples tend to be located, stems from the predominant second-derivative peak located at 873 cm−1. This can be explained by considering higher peroxide content in the amphetamine-treated samples located on the negative part of PC1.
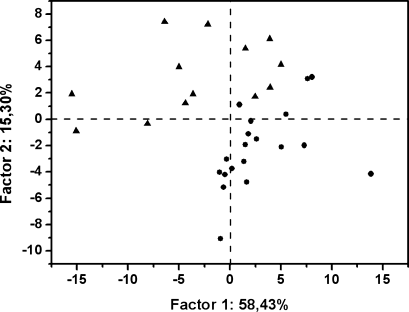
PCA of second derivative infrared spectra in the 900–800 cm−1 region from brain tissues (control (•) and amphetamine treated (▴) animal).
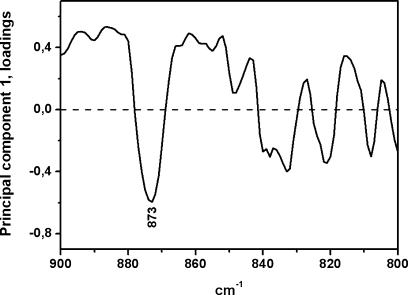
Factor loadings of PCA of control and amphetamine-treated brain tissues of infrared second derivative spectra in the 900–800 cm−1 region.
| Frontoparietal Cortex | Hippocampus | |
|---|---|---|
| Control | 0.15 ± 0.03 | 0.11 ± 0.02 |
| (36.14 ± 3.30) | (13.52 ± 2.01) | |
| 22.3 ± 1.4 | 22.1 ± 1.6 | |
| Nicotina | 0.17 ± 0.04 | 0.13 ± 0.03 |
| (38.21 ± 5.80)ns | (18.62 ± 3.18)* | |
| 22.8 ± 1.5 | 22.4 ± 1.3 | |
| D(+)-Amphetamine | 0.45 ± 0.06 | 0.23 ± 0.07 |
| (62.12 ± 4.28)*** | (32.51 ± 4.42)*** | |
| 26.7 ± 2.6 | 23.5 ± 1.4 | |
| Nicotine and D(+)-amphetamine | 0.25 ± 0.05 | 0.18 ± 0.04 |
| (42.62 ± 4.26)ns | (17.21 ± 2.80)*** | |
| 24.8 ± 2.0 | 22.6 ± 1.8 |
- These values are expressed as mean ± SD. The first value for each drug and control is the I873/I720 intensity ratio in the second derivative spectra (n = 6 animals/group). Values between parentheses correspond to lipid hydroperoxides (μM per mg of fresh tissue; n = 4 animals/group.
- ns ns ns = nonsignificant;
- * P < 0.05;
- *** P < 0.001). β-content (area percentage) in italics.
Changes in Lipid Peroxidation and Protein β-Sheet Content Induced by Nicotine and D(+)-Amphetamine
On the basis of the above assignment, we have measured the height of the 873 cm−1 second-derivative peak relative to that of the 720 cm−1 peak which is attributable to CH2 rocking motions in lipid chains. The results are included in Table I and reveal that, compared with the untreated brain samples, the 873 cm−1 intensity is relatively higher in amphetamine-treated brain sections, both in the cortex and hippocampus area. Analogous results have been found through the biochemical procedure for evaluation of peroxides. Interestingly, however, the above intensity increase is attenuated when administering amphetamine plus nicotine (see Figure 5), and the same can be said in relation to hydroperoxide content as determined through conventional biochemical methods. Particularly, in control animals, the lipid hydroperoxide level at the time of the assay in the frontoparietal cortex was double more than that in the hippocampus (Table I). In nicotine-treated rats, only the hippocampus showed a statistical significant increase (P < 0.05) in lipid hydroperoxides. However, in the D(+)-amphetamine-treated group, the two brain regions showed a very statistical significant increase (P < 0.001). When both the drugs were administered simultaneously, lipid peroxidation returned to levels with no statistical significance against the control values.
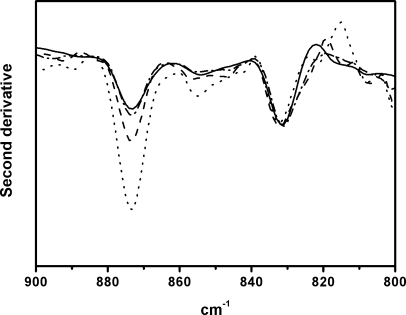
Second derivative spectra measured in the 900–800 cm−1 region from the brain cortex area of control (——), nicotine (–·–·–), nicotine and D(+)-amphetamine (– – –) and D(+)-amphetamine (······)-treated animals.
Oxidative stress manifested by lipid and protein oxidation among other indices is observed in most of the neurodegenerative and neurotoxic disorders, both as a cause and a consequence of the pathogenic pathways. In AD, ROS, the primary mediators of oxidative stress, seem to be either directly or indirectly involved in the production of amyloid β-peptides,27 a keystone in AD. Simultaneously, ROS generated during or after aggregation of these amyloid proteins are putative responsible of the cellular and molecular alterations in the cascade of pathogenic events.28, 29 In a previous article,19 it was suggested that ROS generated by amphetamine-mediated oxidative stress can induce formation of β-sheet rich proteins, which can be of amyloid β-like character. In this work, we have also found that protein β-sheet content increases upon amphetamine treatment. This increase, however, attenuates through combined amphetamine plus nicotine treatment, as occurs also with hydroperoxide levels. Thus, there seems to be a correlation between oxidative stress as reflected by peroxide levels and increasing of protein β-structure in the drug-treated brain tissues. On the other hand, the above results suggest that nicotine may play the role of inhibiting hydroperoxide and ROS formation, whereby inhibiting the appearance of protein β-sheet structure.
The ability of nicotine to regulate the oxidative stress induced by D(+)-amphetamine (and probably by other substances) could be of interest in different pathological processes such as AD, in which oxidative stress is a component of the pathological cascade leading to neurodegeneration. Nicotinergic treatment in AD may also reduce the production of β-amyloid peptides, a putative source of oxidative stress.30 The results obtained with the nicotine and D(+)-amphetamine-treated animals are of great interest. According to unifying theories on the role of psychostimulant drugs,31 this treatment should have theoretically increased the response seen with each treatment on its own. However, this was not the case, as we found, by Western blot analysis, that 50–100% increase in the dose of nicotine or D(+)-amphetamine leads to a more intense induction of cyclooxygenase 2 (protein related to neurodegeneration) than that promoted by the double drug treatment. This suggests the existence of different response mechanisms to these two psychostimulants which are not complementary. One important reason for these unexpected results might lie in the observation that nicotine reduced lipid peroxidation (free radical production or neutralization).
CONCLUSIONS
The control and drug-treated brain sections were examined by infrared microspectroscopy in order to find spectroscopic biomarkers for the detection of peroxides, and their relationships with other molecular parameters that may be indicative of pathological processes. The fact that the trend in drug- dependent peroxide levels as measured by infrared microspectroscopy is analogous to the trend in hydroperoxide changes found by biochemical methods supports the use of the second-derivative infrared spectra of brain sections for peroxide evaluation. This reliable method first used here is advantageous, in the sense of being directly and rapidly applied to fresh tissue samples, using a spectroscopic region which is not influenced by water vibrations.
It might be also concluded that the administration of nicotine agonists, at controlled doses, which have no side effects on the brain and cardiovascular system, might improve the cognitive symptoms that characterize several brain diseases. These agonists might work via several mechanisms including the lowering of oxidative stress.




