Prediction of radiotherapy response in cervix cancer by Raman spectroscopy: A pilot study
Abstract
Radiotherapy is the choice of treatment for locally advanced stages of the cervical cancers, one of the leading female cancers. Because of intrinsic factors, tumors of same clinical stage and histological type often exhibit differential radioresponse. Radiotherapy regimen, from first fraction of treatment to clinical evaluation of response, spans more than 4 months. Clinical assessment by degree of tumor shrinkage is the only routinely practiced method to evaluate the tumor response. Hence, a need is created for development new methodologies that can predict the tumor response to radiotherapy at an early stage of the treatment which can lead to tailor-made protocols. To explore the feasibility of prediction of tumor radioresponse, Raman spectra of cervix cancer tissues that were collected before (malignant) and 24 h after patient was treated with 2nd fraction of radiotherapy (RT) were recorded. Data were analyzed by Principal Components Analysis (PCA) and results were correlated with clinical evaluation of radioresponse. Mean Raman spectra of RT tissues corresponding to different levels of tumor response, complete, partial, and no response, showed minute but significant variations. The unsupervised PCA of malignant tissues failed to provide any classification whereas RT spectra gave clear classification between responding (complete and partial response) and nonresponding conditions as well as a tendency of separation among responding conditions. These results were corroborated by supervised classification, by means of discrimination parameters: Mahalanobis distance and spectral residuals. Thus, findings of the study suggest the feasibility of Raman spectroscopic prediction of tumor radioresponse in cervical cancers. © 2008 Wiley Periodicals, Inc. Biopolymers 89: 530–537, 2008.
This article was originally published online as an accepted preprint. The “Published Online” date corresponds to the preprint version. You can request a copy of the preprint by emailing the Biopolymers editorial office at [email protected]
INTRODUCTION
Cervical cancer is one of the leading causes of cancer death in the world and accounts for 23% mortality among Indian women. Around 370,000 new cases are diagnosed every year. One fourth of the global newcases are reported in India. It is estimated that 90% of deaths can be prevented on early diagnosis which emphasizes the need of effective screening methods. Pap smear and colposcopy are the two widely used screening methods. Cervical malignancy progresses through stages from CIN I to CIN III, CIS (carcinoma in situ), and finally to invasive cancers.1 Radiotherapy in combination with chemotherapy is the choice of treatment for locally advanced stages of cervical malignancy—stage II B and above.2
Radiation treatment regimen, as will be discussed later in the Materials and Methods section, from first of fraction of treatment to clinical evaluation of tumor response, extends more than 4 months. Radiation resistance is one of the major hurdles in radiotherapy of cancers. Intrinsic factors such as DNA aneuploidy, S-phase fraction and proliferation kinetics, tumor vascularity and hypoxia and glutathione content are believed to influence radiation resistance of a tumor.3-5 Clinical assessment, details will be discussed in Materials and Methods section, is the only routinely practiced procedure to evaluate the tumor response to the treatment. The degree of tumor shrinkage is commonly used index of radioresponsiveness.6, 7 For example, in complete response there is 100% disappearance in tumor volume, decrease in tumor volume of 65% and above is seen in partial response, and below 65% decrease is considered as no response condition.8 Development of predictive assays for tumor response based on samples that are collected at early stages of treatment could be very significant for cervix cancer management. These methods can lead to individualized treatment regimen. There are several studies reported in the literature on prediction of tumor response using radiobiological and magnetic resonance spectroscopy methods. Radiobiological studies indicate that prediction of tumor response to radiation could be assessed by estimation of glutathione (GSH) levels.3 In other studies, expression of proteins: c-erbB-2, Ki-67, and the mitotic index of proliferating cell population are also demonstrated as predictor of tumor response.9, 10 Magnetic resonance imaging is also shown to distinguish tumors of low grade and high grade responsiveness to radiation therapy in carcinoma of uterine cervix. Further, monitoring the radiation response by both magnetic resonance imaging and NMR spectroscopy is also reported in literature.8, 11 Although these studies demonstrate the feasibility of prediction of tumor radioresponse, so far there are no prescribed methods in clinical practice.
Applications of optical spectroscopic methods in medical diagnosis and screening have been active areas of research in recent times.12-21 These methods are shown to be rapid as there is very little or no sample preparation requirements, more objective, and have the advantage of in vivo/in situ applicability, thus, a painful biopsy can be avoided. Multi Drug Resistance (MDR) characterization, a serious hurdle in chemotherapy, of cell lines22 and discrimination of a cell type in a mixed cell population by vibrational spectroscopy is also reported.23 Prospectively, spectroscopy techniques can be useful in early detection, monitoring, and follow-up without a biopsy.
Cervical cancer has been one of the well-studied forms of malignancy by optical spectroscopic methods such as Raman12, 13 FTIR,14-17 fluorescence,18-21 and combination of reflectance and fluorescence spectroscopy.18 In a previous study Raman spectroscopic methods for discrimination of normal and malignant tissues of cervix have been developed.13 A more recent Raman microspectroscopy study had demonstrated the feasibility of classifying formalin-fixed malignant cervix tissues that were collected before commencement of radiation treatment and 24 h after patient was exposed to 2nd fraction of radiotherapy.24 However, this study could not correlate spectral variations in terms of tumor radioresponse as all the subjects recruited in the study are found to be of same class, namely, complete response. Raman spectroscopy is also used to study the radiation induced damages to understand the characterization of ionizing radiation and proton radiation.25, 26
In present study, we have explored the feasibility of Raman spectroscopic prediction of tumor response to radiation in carcinoma of uterine cervix. To achieve this, Raman spectroscopic studies of cervix tissues that were collected, from the same subjects, before the commencement of treatment and 24 h after patient was exposed to 2nd fraction of radiotherapy, were carried out. The Raman spectroscopic findings were correlated with clinically evaluated tumor response. The tissues which were collected before treatment will be henceforth referred to as malignant tissues and the samples that were collected 24 h after 2nd fraction of radiotherapy as RT tissues. Principal Component analysis (PCA) was employed, both in supervised and unsupervised modes, for spectral data analysis. Discriminating parameters scores of factors, Mahalanobis distance, and spectral residuals were explored for the best discrimination of tissues based on tumor response to radiation treatment. The results obtained in this study are presented and discussed in the article.
MATERIALS AND METHODS
Tissue Samples
Cervix biopsies, in saline, from malignant subjects of stage IIIA and IIIB at the time of admission (before commencement of radiation treatment) and 24 h after the patient was administered 2nd fraction of radiotherapy (RT tissues) were collected from Department of Radiation Oncology, Shirdi Sai Baba Cancer Hospital. Normal samples were harvested from regular hysterectomies with no indications of malignancy and were used as controls. These samples were collected from Department of Obstetrics and Gynecology, MAHE, Manipal. Depending on the size of the sample, three or more spectra were recorded on each sample at different locations. Each spectral point is considered as separate sample. The rationale behind this approach has been explained in our earlier papers.27 A total of 88 spectra from 25 malignant tissues and 106 spectra from 25 RT tissues were analyzed. We have also employed 68 spectra from 16 normal tissues as controls.
After 2nd Fraction of Radiotherapy (RT) Samples
Radiation Treatment Regimen
Following is the radiation treatment regimen employed in our cancer center: Stage IIIB patients were subjected to External Beam Radiotherapy (EBRT) of 45 Gy in 20 fractions over a period of 4 weeks by using linear accelerator. Patient was allowed to rest for 2 weeks for parametrial regression. This was followed by two doses of remote after loaded High Dose Rate intracavitary brachytherapy (HDR) of 8.5 Gy to point A, once a week. Then, the patient was clinically assessed to evaluate tumor response after 4 weeks of rest.
Clinical Assessment of Tumor Response to Radiation Therapy.
The immediate response to radiation therapy was assessed by per vaginal, per rectal examination at the end of 4 weeks after exposure to the second dose of High Dose Rate intracavitary brachytherapy. The tumor response to radiation therapy was graded based on degree of shrinkage volume of the tumor. In the case of complete response conditions, shrinkage volume of tumor would be 100%, shrinkage volume of 65% and above is considered as partial response where as shrinkage volume less than 65% is considered as no response condition.6, 7
In the present study, RT tissues were collected 24 h after the patient was exposed to 2nd fraction-of-External Beam Radiotherapy (EBRT), i.e., after exposure to 4.5 Gy of EBRT.
Laser Raman Spectroscopy
Raman spectra of tissue samples, moist in saline, were recorded using conventional Raman setup assembled by us.18 In this set up, 785 nm emission from diode laser was used for excitation and HR 320 spectrograph coupled to Spectrum One liquid nitrogen cooled CCD was employed for detection of Raman signals. Rayleigh scattering was rejected by a notch filter (Kaiser Optics). Theoretical laser spot size is approximately 17 microns25 and this could be larger for tissue due to their diffusing nature. Other experimental conditions were as follows: Laser power 100 mW, integration time 30 s, accumulations 20, and these settings were kept constant through out the study. During spectral data acquisition, tissues were oriented such that of probing laser beam was focused on to the epithelial side. In such arrangement, spectral data are expected to be emanating mostly from the upper layers due to loss of signals that emerge from lower layers because of multiple scattering.
In this study spectra were recorded in pixel space. In order to convert spectra to Raman shift space, a known standard, Tylenol (4 – acetamidophenol), spectra was recorded before each measurement. Then the recorded tissue spectrum was calibrated with a cubic order fit to known frequencies of Tylenol using algorithms implemented in GRAMS. Cubic Order fit is one of the four calibration curve types, which requires minimum of four calibration levels to produce a valid calibration curve. Cubic curve type uses central part of each section to extract the load value at constant displacement increments, thus smooth curve can be drawn through the given data points. This approach has been shown to produce highly reproducible spectra.28, 29
Data Analysis
Baseline of Raman spectra was corrected by fitting and subtracting a third order polynomial function. The baseline corrected spectra were then vector normalized to δCH2 band using Grams 32 (Galactic Industries Corporation, USA). Calibrated and corrected spectra were then analyzed by PCA. PCA was carried out under different conditions: entire spectrum and derivative spectrum, full region (800–1700 cm−1) and selected regions (1250–1500 cm−1, 1500–1700 cm−1). Total percentage variance, Eigen values and factor profiles were employed for standardizing the conditions. First derivative spectra in 1250–1500 cm−1 region with seven factors gave best results in our analysis. Further analysis was carried out under same conditions. The standard sets corresponding to different levels of tumor radioresponse were developed and analysis was carried out in supervised mode. In this case, Mahalanobis distance and spectral residuals were discriminating parameters. Mean spectra of clusters corresponding to different tissue types were computed and used in understanding the biochemical differences among them.
RESULTS AND DISCUSSION
The present study is aimed at exploration of Raman spectroscopic prediction of tumor response to radiation treatment. It is well known that there is no standard protocol to assess the tumor response to radiation therapy. As described earlier, radiotherapy is the choice of treatment for locally advanced stages of cervical cancers (stage II B and above). It is shown that tumor response toward radiation therapy varies despite same clinical stage and histological type. Intrinsic factors such as antioxidants levels are shown to be potential predictive markers of tumor response. As it is well-known radiation induces oxidative stress, which in turn triggers antioxidants in order to repair radiation-induced-deleterious effects.
In the present study, as described earlier, 25 each of malignant and RT samples were analyzed. Out of twenty-five patients recruited in the study, eighteen subjects showed complete response, three patients showed partial response, and remaining four patients showed no response on clinical examination.
Typical mean spectra of normal, malignant, complete response, partial response, and no response tissues were shown in Figures 1a–1e, respectively. Mean normal spectrum was characterized by spectral features (broader amide I, and peaks at 1278, 948, and 864 cm−1) of structural proteins like collagen (Figure 1a). Mean malignant spectrum was characterized by sharper amide I, minor blue shift in δCH2 band and sharper features in amide III region which can be assigned to noncollagenous-proteins, lipids, and DNA.30, 31 These features corroborate earlier findings.12, 13 Mean spectra of complete response, partial response and no response tissues show very minute but significant variations with respect to malignant spectra (Figures 1b–1e). In order to appraise intrasample variability of Raman signals among complete response, partial response, and no response conditions, mean and standard deviation were computed as shown in Figure 2. Solid line indicates the mean spectra and grey line indicates the standard deviations in the spectral profiles. From the figure, variations in the spectral profiles of complete response and partial response conditions were less pronounced (Figures 2a and 2b) compared with that was observed for the case of no response conditions (Figure 2c). As will be explained later, this aspect was also reflected in scattered plots of RT tissue wherein cluster corresponding to no response condition exhibit larger spread compared with other two conditions. Reasons for this behavior cannot be attributed to clinical conditions. It is pertinent to note that, spectra of malignant and RT tissues are expected to exhibit very minor variations. This could be explained as tissues were of same malignant type and latter specimens were collected after two fractions of radiation, which could be too early to observe therapeutic effects. Moreover, tissues were collected 24 h after radiation treatment which provides ample time for the systems to repair most of the radiation-induced-effects. As mentioned earlier, radiation induces oxidative stress, which in turn activates the production of antioxidants. Thus only spectral variations, which can be expected between malignant and RT tissues could be due to differences in the antioxidant levels.
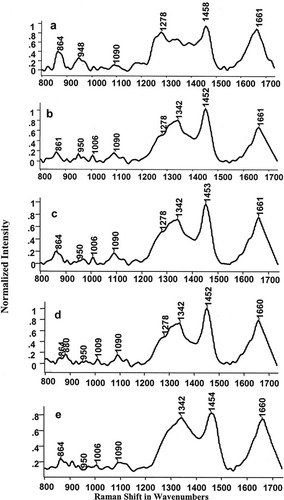
Mean spectra of cervix tissues: (a) normal; (b) malignant; (c) complete response; (d) partial response; (e) no response.
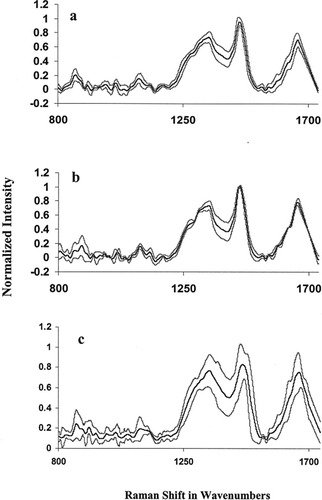
Mean standard spectra of cervix tissues. (a) Complete response; (b) partial response; (c) no response (dark line-mean spectra, grey line-mean ± standard deviation).
Optical spectroscopy methods facilitate objective discrimination which is due to fact that spectral data are amenable to multivariate statistical tools, both supervised and unsupervised algorithms. In the present study, PCA was employed to classify tissues based on tumor response to radiotherapy. PCA is a well-known data compression method where large spectral data are reduced into small number of independent variations known as factors or principal components and contributions of these factors are called scores. PCA was carried out in two different approaches: unsupervised classification using scores of factor as discriminating parameter and multiparametric supervised classification. The first approach, PCA by unsupervised classification was carried out in a three-step process. In our analysis, as mentioned earlier, PCA was carried out under different conditions and the analysis of first derivative spectra in 1250–1500 cm−1 region gave best discrimination. In the first step, spectra from all tissue types, namely, normal, malignant, and RT tissues were pooled and analyzed. This resulted into two clusters corresponding to normal and malignant + RT tissues (Figure 3a). Mean and standard deviation values of score of factor 1 for normal, malignant, and RT spectra are −0.076 ± 0.020, 0.023 ± 0.053, and 0.017 ± 0.055, respectively. Even a second-step PCA of malignant and RT spectra could not classify the tissue types. Mean and standard deviation values of score of factor 1 for malignant and after RT are 0.005 ± 0.069 and −0.004 ± 0.068, respectively (Figure 3b). To explore the feasibility of classification based on tumor radioresponse, a third, exclusive PCA of malignant tissues was carried out (Figure 4a). In this analysis, nonresponding cases exhibited some tendency of separation, but this is outweighed by the overlap with complete response spectra, Figure 4a. Loadings of factor 1 and 2 used in the analysis were shown in Figures 4b and 4c. The percentage variances of the respective factors are indicated in parenthesis. Same approach, i.e., exclusive PCA, was considered for RT tissues. In this case, score of factor 1 provided good classification between responding (complete and partial response) and nonresponding conditions, Figure 5a. As can be seen from the figure, contribution of factor 1 is positive for both complete response and partial response spectra. And score of factor 1 is largely negative for no response spectra. Mean and standard deviation values of score of factor 1 for complete response, partial response, and no response spectra are 0.043 ± 0.028, 0.059 ± 0.017, and −0.17 ± 0.105, respectively. Based on score of factor 2, partial response spectra come out as a separate group from complete response spectra (Figure 5a). Mean and standard deviation values of score of factor 2 for complete response, partial response, and no response are −0.044 ± 0.067, 0.041 ± 0.042, and −0.170 ± 0.168, respectively. Loadings of factor 1 and 2 contributed for the classification were shown in Figures 5b and 5c, respectively, and their percentage variances were presented in parenthesis. A broad and negative peak in 1450–1500 cm−1 region seems to be major feature in the profile (factor loadings) of factor 1 based on which classification among responding and nonresponding conditions could be achieved. Loadings of factor 1 also exhibits minor positive peaks in 1250–1300 cm−1 and 1400–1450 cm−1 regions and a negative peak around 1350 cm−1. A strong and broad positive band at 1450 cm−1 and negative peak in 1450–1500 cm−1 region are the major features of the loadings of factor 2 which has major contribution in observed tendency of separation between responding conditions. Minor negative peaks in 1250–1300 cm−1 and 1350–1400 cm−1 region are also seen in the loadings of factor 2. Since, as mentioned earlier, we had carried out PCA of first derivative spectra in selected region to bring out classification of tissues, the profiles factor loadings may not be very useful in understanding or the correlation at the biochemistry level. However, as a whole this analysis did not yield very clear separation between complete response and partial response conditions though there is a clear separation between responding and nonresponding conditions.
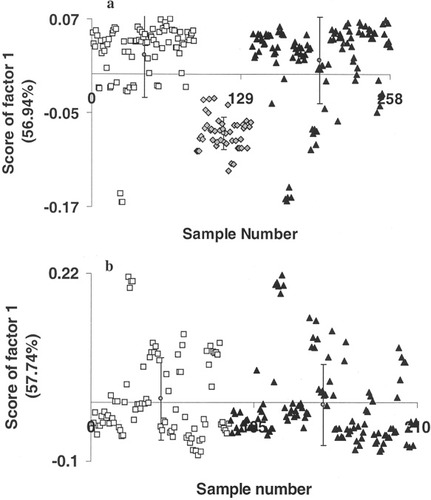
Unsupervised PCA of first derivative cervix tissue spectra in 1250–1500 cm−1 region. The variances of factors are indicted in parentheses. (a) Cluster analysis of normal (♦), malignant (□), and RT tissues (▴). (b) Classification of malignant (□) and RT tissues (▴).
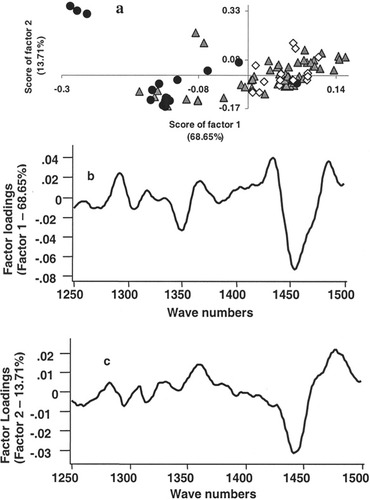
PCA of spectra of malignant cervix tissues. (a) Unsupervised classification of first derivative spectra of RT tissues in 1250–1500 cm−1 region. (▴ Complete response, • no response, and ⋄ partial response). (b) Loadings of factor 1. (c) Loadings of factor 2. The variance of the factors is indicted in parenthesis.
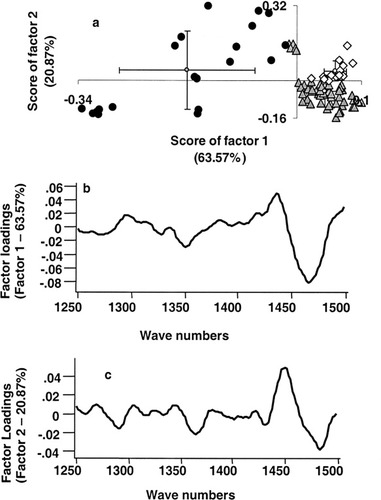
PCA of spectra of RT cervix tissues. (a) Unsupervised classification of first derivative spectra of RT tissues in 1250–1500 cm−1 region. (▴ Complete response, • no response, and ⋄ partial response). (b) Loadings of factor 1. (c) Loadings of factor 2. The variance of the factors is indicted in parenthesis.
PCA, once standard sets are developed, provides several discriminating parameters besides scores of factors, which include Mahalanobis distance (a measure of proximity of two spectra) and spectral residuals (squared error sum of difference between recorded and simulated spectrum). In our analysis, Mahalanobis distance is measured in terms of standard deviation. If Mahalanobis distance value is three or more means possibility of that particular spectrum belonging to the particular standard set is 0.5% or less.32 Theory, method of calculation and advantages of above parameters for discrimination purpose are discussed elsewhere.13, 18, 27, 33, 34 This approach of analysis has several advantages over unsupervised PCA. In this approach, once standard sets are developed for each class, pretreated spectra can be compared against all the available standard sets to compute scores of factor, Mahalanobis distance and spectral residuals, unlike unsupervised approach where PCA of entire data is required to compute scores of factor and in turn for diagnosis of each case.
In the present study, 106 spectra from 25 RT tissues: 72 spectra of 18 complete response tissues; 20 spectra of four no response tissues, and 16 spectra of three partial response tissues, were employed. Standard sets for all the three conditions were developed and used for analysis. However, since we have larger data for complete response condition, we had emphasized on the results obtained against this standard set. Randomly selected 27 spectra were used to develop the complete response standard set. This standard set was verified by rotating out spectra of the standard set and evaluated using rest of the spectra. Results obtained in this analysis are shown in Figure 6a. It is expected that when test spectra and standard set belong to same class, analysis yield lower values for Mahalanobis distance and spectral residuals and vice-versa. Mean Mahalanobis distance values for complete response, partial response and no response spectra are 1.168 ± 0.868, 0.306 ± 0.156, and 15.522 ± 9.87, respectively, and mean spectral residual values for complete response, partial response and no response spectra are 0.001 ± 0.0009, 0.0005 ± 0.0002, and 0.012 ± 0.007, respectively (Figure 6b). Thus, a clear classification among the responding (complete response and partial response) and nonresponding conditions can be obtained.
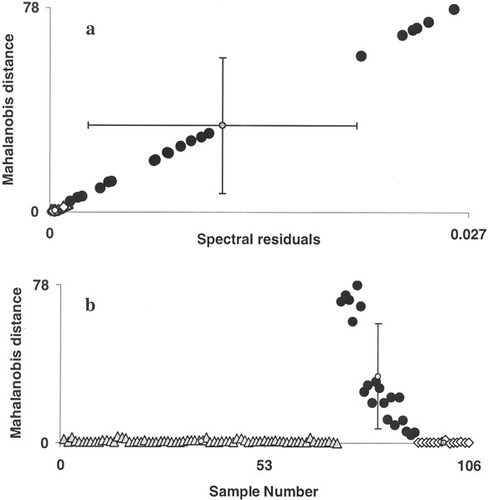
Supervised classification of first derivative spectra (in 1250–1500 cm−1 region) of RT tissues. Discriminating parameters Mahalanobis distance and spectral residual were computed against complete response standard set. (a) Plot of Mahalanobis distance vs. spectral residuals. (b) Plot of Mahalanobis distance vs. sample number. (▴ Complete response, • no response, and ⋄ partial response).
CONCLUSIONS
Spectral profiles of malignant tissues show very significant differences from normal spectra. Tissues collected after 2nd-fraction-of-radiotherapy was categorized based on clinical evaluation into three groups namely, complete response, partial response and no response. Mean spectra corresponding to these different therapeutic response conditions show very minor but significant variations. Spectra corresponding to complete response group show much less variations from malignant spectra, compared with partial response and no response. PCA, employed to classify the tissues based on tumor response, was carried out in a three-step process. PCA of malignant spectra did not yield any clear classification on the lines tissue response to radiation. In the case of RT tissues 3rd and exclusive PCA produced clear classification between responding (complete response and partial response) and nonresponding conditions, and a tendency of classification between complete response and partial response is also noticed. The supervised classification also produced same results, i.e., classification of responding and nonresponding conditions. Thus, the findings of the study demonstrate the feasibility of predicting tumor response to radiotherapy of cervix cancers by Raman spectroscopy. These results could be significant from the point view of radiotherapy of cervix cancers. Once tumor response of subjects could be predicted sufficiently early, as in this study after 2nd fraction of treatment, suitably modified treatment modalities can be implemented for nonresponding subjects.
Acknowledgements
The work was carried out under the Department of Atomic Energy; Board of Research in Nuclear Sciences, Govt. of India project entitled “Laser spectroscopy as predictor of tumor response to radiotherapy in cervical cancer,” No. 2003/34/17/BRNS/1903. Authors acknowledge technical support of Ms. Keerti and Mr. Chetan Anand. Dr. C. Santosh, Incharge, CLS is acknowledged. One of the authors (M.K.) is grateful to DAE-BRNS for providing the research fellowship to carry out this work.




