Dendrimers destabilize proteins in a generation-dependent manner involving electrostatic interactions
Abstract
Dendrimers are well-defined chemical polymers with a characteristic branching pattern that gives rise to attractive features such as antibacterial and antitumor activities as well as drug delivery properties. In addition, dendrimers can solubilize prion protein aggregates at very low concentrations, but their mode of action is unclear. We show that poly(propylene imine) dendrimers based on di-aminobutane (DAB) and modified with guanidinium surface groups reduce insulin thermostability and solubility considerably at microgram per microliter concentrations, while urea-modified groups have hardly any effect. Destabilization is markedly generation-dependent and is most pronounced for generation 3, which is also the most efficient at precipitating insulin. This suggests that proteins can interact with both dendrimer surface and interior. The pH-dependence reveals that interactions are mainly mediated by electrostatics, confirmed by studies on four other proteins. Ability to precipitate and destabilize are positively correlated, in contrast to conventional small-molecule denaturants and stabilizers, indicating that surface immobilization of denaturing groups profoundly affects its interactions with proteins. © 2008 Wiley Periodicals, Inc. Biopolymers 89: 522–529, 2008.
This article was originally published online as an accepted preprint. The “Published Online” date corresponds to the preprint version. You can request a copy of the preprint by emailing the Biopolymers editorial office at [email protected]
INTRODUCTION
Dendrimers are monodisperse hyperbranched polymers containing specific branching and ending points.1-3 The typical building block is polypropylene imine (PPI, usually with a diaminobutyrate core) or polyamino amine (PAMAM, with an ammonia or diaminoethanoate core). Regular branching leads to a globular structure where each new branching point (generation) provide an additional shell on the surface. Because of this exponential growth, dendrimer molecules can become quite large, with fifth generation structures showing sizes in the same range as medium sized proteins (ca. 5 nm in diameter).4 The dendrimer surface can be derivatized in a controlled manner with a variety of groups, while the interior tends to be shielded off from the solvent and can be used for sequestering small molecules. The density of the surface groups can be varied according to the choice of building blocks and cores. These properties have led to the use of dendrimers for drug delivery5 and as enzyme mimics.6 As confirmed by numerous molecular dynamics studies, increased shielding of the interior in higher generation dendrimers means that the surface will dominate dendrimer properties.7 PPI dendrimers with polyamine surfaces are usually positively charged at neutral pH, because of the surface amines' pKa around 9.5–10,8 while the interior amines will be more acidic because of low solvatisation,9 with pKa-values down to around eight for the most deeply buried tertiary amines.8 Consequently, they tend to be more expanded below pH 9 or at low ionic strength because of interbranch repulsion but fold back on themselves at higher pH and higher ionic strength. Their positive charge also makes them prone to interact with negatively charged membranes and proteins.4 In contrast, noncharged dendrimers show much weaker interactions with other biological components.
Remarkably, dendrimers dissolve prion protein aggregates, which are otherwise only soluble in solvents containing both detergents and high denaturant concentrations (typically 6M guanidinium chloride).10, 11 Cationic dendrimers, particularly generation 4 and higher, are effective at concentrations of 7 μg/ml or less, whereas dendrimers with surface hydroxyl groups and linear polymers only have minor effects. PPI- and PAMAM-based dendrimers also inhibit fibrillation of both prion peptides and the Aβ peptides,12-14 with inhibition increasing with dendrimer generation. The effect appears to require protonated amino groups and should therefore be stronger at lower pH,14 although this will be modulated by the effect of pH on protein structure and the reduction of electrostatic attractions as the negative side chains protonate.
Strikingly, cationic dendrimers affect proteins at concentrations four orders of magnitude (weight/weight) below that of conventional denaturants, despite their similarity in chemical structure. A few reports have already appeared on the details of these interactions. Thus, a very recent phosphorescence study reported structural changed in flexible regions of the polypeptide.15 Increasing concentrations of PAMAM dendrimers destabilize bovine serum albumin16 but slightly increase the thermal stability of human serum albumin.17 To provide more insights into these apparently contradictory observations, we here describe interactions between insulin and different modifications of a PPI-type dendrimer based on di-aminobutane (DAB). As illustrated in Figure 1, the amine end points in the DAB dendrimer (which at neutral pH contain a positive charge localized on the nitrogen atom) have been modified to either guanidinium groups (where the positive charge is delocalized among several atoms) or urea (making the end group neutral but polar). We observe strong effects of the positively charged dendrimers on the thermal stability (i.e., the melting temperature) and solubility of human insulin. More specifically, there is an unusual and generation-dependent correlation between ability to precipitate and destabilize proteins. Using data from proteins with other pI values, we conclude that dendrimer-mediated protein destabilization is driven by electrostatic interactions.
Structures of the three classes of dendrimers used in this study based on a diaminobutyrate (DAB) core, terminating either in an unmodified DAB group, a guanidinium group (G) or a urea group (U). The structures represent second-generation dendrimers. Reproduced with permission from Cordes H, et al. Biomacromolecules 2007, 8, 3578–3583, © American Chemical Society.
RESULTS
Insulin Stability and Solubility Depend on Dendrimer Type and Generation
To evaluate insulin stability in a simple and rapid manner, we chose thermal denaturation followed by far-UV circular dichroism at 220 nm. Under our conditions (pH 8, no zinc), insulin is mainly dimeric at room temperature; thermal denaturation is proposed to involve a simultaneous dissociation to monomer and unfolding.19 Insulin denatures with a midpoint denaturation temperature tm around 75°C (Figure 2A). tm decreases in the presence of different dendrimers, showing characteristic trends (Figure 2B–2D). The urea-dendrimers (U) reduced tm to a much smaller extent than the guanidinium-dendrimers (G) and the unmodified PPI dendrimer class DAB. G and DAB dendrimers were comparable in efficiency. The reduction in tm was strongest for generation 3 for both G and DAB, and tm decreased in this case approximately linearly with dendrimer concentration. For the other generations, tm tended to stabilize at a lower plateau at higher dendrimer concentrations. There was no significant change in the associated enthalpy of unfolding with dendrimer concentration (data not shown). In view of the many different interactions and coupled equilibria that could give rise to nonlinear variations in tm with dendrimer concentration, we have not undertaken a more systematic analysis of the dependence of tm on dendrimer concentration. Instead, we will use the measured or (in the case of G3 dendrimers) extrapolated reduction in tm at 50 μg/ml dendrimer as a measure of the destabilization effect of the different dendrimers.
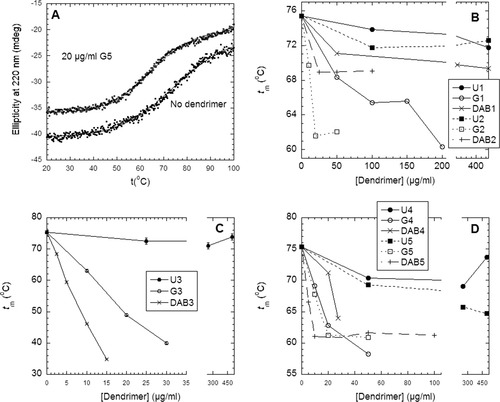
A: Thermal scans of the unfolding of insulin in the absence and presence of dendrimers, monitored by the change in ellipticity at 220 nm. Data are fitted as described.20 B–D: Insulin's denaturation temperature tm as a function of dendrimer concentration for generation 1–5 at pH 8. Errors < 1°C.
At concentrations of G and DAB (but not U) higher than those in Figure 2, incubation with insulin led to sample turbidity. We therefore systematically measured insulin solubility in G, using tyrosine fluorescence to measure the concentration of soluble insulin (Figure 3A). This led to the precipitant ranking G3 > G2 > G4 ≈ G5 > G1 (G3 most efficient), which correlated well with the dendrimers' destabilizing effect (Figure 3B). A similar correlation between solubility and stability was seen for DAB dendrimers (Figure 3B). No precipitation was seen in the presence of U (data not shown).
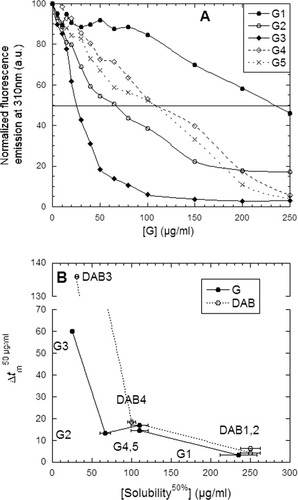
A: Solubility of insulin as a function of G concentration at pH 8. The drawn line at 50% solubility is used to estimate the midpoint concentration of precipitation [Solubility]50% where 50% of insulin remains soluble. The error in determining solubility is ∼10%. B: Correlation between insulin's [Solubility]50% and the degree of destabilization caused by 50 μg/ml G and DAB. The different generations are indicated in the plot.
pH-Studies Indicate That Dendrimer-Insulin Interactions are Linked to Electrostatics
The previous experiments had been executed at pH 8, where insulin has a net charge of −2. At pH 6, this reduces to −1, while it increases to −5 at pH 10, though it remains predominantly dimeric.21 G remains protonated over the whole pH-interval (the Arg guanidinium group has a pKa around 12) while DAB's surface amines become neutral around pH 10; the less basic interior amines are also uncharged at this pH.8 Electrostatics are likely to play a significant role in insulin-dendrimer interactions which should therefore be sensitive to changes in pH. The modest change in negative charge between pH 8 and 6 is reflected in insulin solubility in G dendrimers, which is not affected by the change in pH from 8 to 6 (Figure 4A), except for a slight decrease for G4 and G5. This must reflect a slightly stronger binding to higher-generation dendrimers despite the slight decrease in negative charge. An increase in charge at pH 10 does not affect solubility in G5. Insulin stability is most reduced by G dendrimers at pH 8, slightly less at pH 10, and to an even smaller extent at pH 6 (Figure 4B). This reflects a broad overall trend toward increased destabilization with increased negative charge on insulin. However, this is opposite to the effect of the free denaturant, guanidinium chloride (GdmCl), which decreases insulin stability to a greater extent at higher pH than at lower pH. The midpoint of denaturation of insulin in GdmCl increases from 3.72 ± 0.10 at pH 6 to 4.10 ± 0.08M at pH 8 and 4.27 ± 0.11M at pH 10 (data not shown). Insulin thermal stability in the absence of dendrimers or denaturant is higher at pH 6 and 10 (tm 82.2°C at both pH-values) than at pH 8 (tm 75.4°C). Thus, the immobilization of guanidinium groups clearly affects how it interacts with insulin.
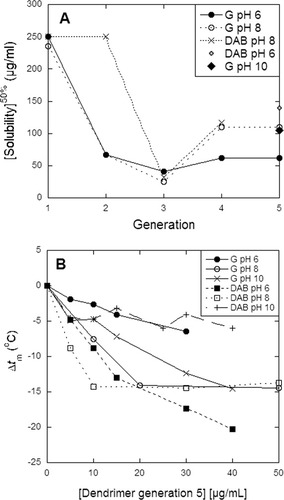
A: Insulin's [Solubility]50% as a function of dendrimer generation for G at pH 6 and 8 and for DAB at pH 8. Values for G5 at pH 10 and DAB5 at pH 6 are also indicated. DAB could not precipitate insulin at pH 10, and no denaturation by U is seen at any pH value. B: Change in insulin's denaturation temperature tm for G5 and DAB5 at pH 6–10. tm of insulin in the absence of G5 is 82.2°C, 75.4°C, and 82.2°C at pH 6, 8, and 10, respectively.
For DAB5, the situation is different. Insulin solubility in DAB5 increases only slightly from pH 8 to 6 and the effect on stability is rather insignificant (Figure 4B). At pH 10, DAB5 is unable to precipitate insulin to any significant extent (data not shown), in good agreement with the loss of positive charge on the dendrimer surface. Correspondingly, DAB5 only gives rise to a very modest decline in tm at pH 10 (Figure 4B). Thus insulin-DAB interactions show a strong correlation between solubility and stability, just as we observe with U dendrimers. The difference between G and DAB's destabilizing effects between pH 6 and 10 must be ascribed to differences in dendrimer properties.
Dendrimers Only Affect Insulin Aggregation to a Small Degree
Inspired by PAMAM dendrimer's reported ability to inhibit fibrillation of prion proteins,10 we investigated the effect of our dendrimer compounds on insulin fibrillation. Although insulin most readily aggregates to form fibrils at low pH and elevated temperatures,22 it is also possible to induce organized aggregates under more physiological conditions (pH 8 at 37°C) by prolonged shaking of the sample in a plate reader.23 By following the formation of fibrils using the fluorescent dye Thioflavin T, we obtain a characteristic sigmoidal time profile with a lag time of around 10 h before a very large increase in ThT fluorescence sets in (insert to Figure 5). FTIR spectroscopy confirms the formation of highly β-sheet rich structures typical of amyloid (data not shown). The different dendrimers (Figure 5) increase lag times by 10 (DAB) to 15 (G,U) h, while the rate of elongation (the steepness of the growth curve) and the final level of fibrillation is not affected. This is in good agreement with a previous report that dendrimers mainly affect the Aβ peptide's lag-time while only having minor effects on the kinetics of elongation and final concentration of fibrils.24 There is generally a reasonable correlation with the dendrimer generations' physical effects on solubility and stability; U dendrimers show the same effects at all generations, while DAB and G show a drop in retardation around generation 3. According to AFM scans, we observe large aggregates for insulin that contain fibril-like segments, and this morphology is not significantly affected by the presence of dendrimers (Figure 6).
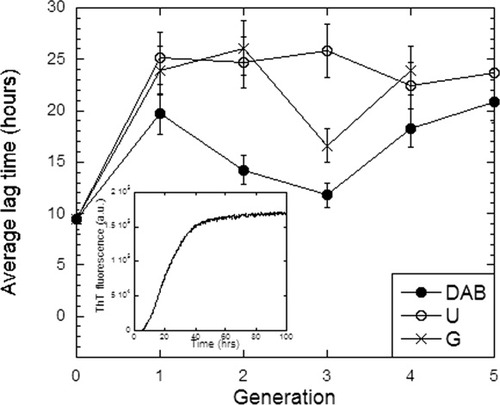
Average lag-time for fibrillation of insulin as a function of dendrimer generation. Insert: Fibrillation time profile for insulin in the absence of dendrimers.
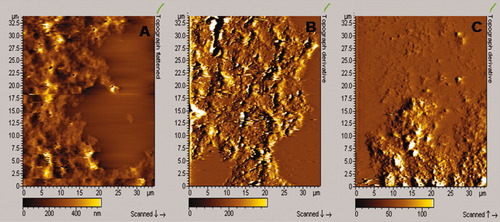
A: AFM images of 2 mg/ml insulin in 20 mM phosphate pH 7 and 100 mM NaCl after 100 h of incubation in the presence of (A) no dendrimer (B) 50 μg/ml DAB3, and (C) 10 μg/ml G3.
Destabilizing and Stabilizing Effects of Dendrimers on Other Proteins with Different pI Values
To test whether the destabilization of insulin by dendrimers is a general phenomenon, we also studied several other proteins using DAB dendrimers. Based on our insulin data, we reasoned that protein–dendrimer interactions would be heavily influenced by protein charge, and accordingly studied proteins spanning a wide range of pI values.
S6
The ribosomal α/β protein S6 from T. thermophilus25, 26 has a pI value of 6.55,27 but a very shallow titration curve around neutral pH gives it a net negative charge of ca. −1 at pH 8. It is too thermostable to be analyzed by conventional thermal scans,28 but equilibrium denaturation in GdmCl in the presence of DAB3 or DAB4 reveals that DAB3 significantly destabilizes S6; the midpoint of denaturation decreases from 2.75 ± 0.045 to 2.39 ± 0.03M (data not shown), corresponding to a destabilization by 0.93 ± 0.08 kcal/mol.26 In contrast, the midpoint is unaltered in 20 μg/ml DAB4. This confirms our observations on the specific aggressivity of third generation dendrimers.
Cutinase
The lipolytic enzyme cutinase from F. solani pisi has a pI of 7.8,20 making it effectively neutral at pH 8. Cutinase is stabilized by DAB dendrimers generation 4–5 to a small but reproducible extent, while lower-generation dendrimers have insignificant effect (Figure 7).

Cutinase's denaturation temperature tm as a function of [DAB1–5] at pH 8.
Tnfn3
The all-β protein Tnfn329 has a calculated pI around 8.1, making it electrically neutral at pH 8. Its stability is not affected by dendrimers. The denaturation temperature of (56.2 ± 0.1)°C is unaffected by the presence of 25 μg/ml DAB (data not shown).
Hen egg white lysozyme
Hen egg white lysozyme has a pI around 10.8 and retains a positive charge of +8 at pH 8. Lysozyme undergoes thermal a denaturation around 73.3°C, and this is unaffected by up to 100 μg/ml DAB3 or DAB5 (data not shown).
DISCUSSION
Dendrimers Affect Protein Stability Mainly by Electrostatic Interactions
The purpose of this study was to shed more light on the effect of the widely used PPI-type dendrimers on protein stability. Different end-point functionalizations were expected to lead to different types of protein-dendrimer interactions. Unmodified DAB is expected to exert its effects mainly through ionic interactions via the protonated surface amino groups (pKa around 9–10) and thus be most effective below pH 9. The guanidinium group's significantly higher pKa makes it protonated throughout the pH interval analyzed here (6–10), though the delocalized charge on the guanidinium group might alter the strength of interactions. In contrast, the polar but noncharged urea class should mainly work through hydrogen-bonding. Free urea and guanidinium groups have a strong chaotropic effect which is believed to reflect weak binding of these groups to the protein backbone through preferential interactions.30 This might be hampered by its immobilization on the dendrimer surface.
Our results with insulin clearly indicate that protein–dendrimer interactions are strongly modulated by electrostatics. G dendrimers are by and large equally destabilizing and precipitating toward insulin between pH 6 and 10, while DAB only destabilizes and precipitates at pH 6 and 8, but much less at pH 10 where the positive charge has been lost or reduced. Noncharged U dendrimers do not show any effect at all. Thus, the chaotropic effect of urea and gGuanidinium-groups immobilized on dendrimer surfaces seems to be very limited and the ability of dendrimers to present ligands in a multivalent fashion confers no advantage. This indicates that chaotropes need full mobility for protein destabilization rather than working in a more indirect fashion (e.g., by altering water structure and destabilizing hydrophobic interactions) and do not exert their effects by cooperative binding on the protein surface.
It is noteworthy that DAB and G have a comparable effect at pH 8 but DAB destabilizes to a greater extent than G at pH 6 (Figure 4B). This may be rationalized as follows: at pH 6, DAB is fully protonated and the localized charge on DAB's amine nitrogen leads to a stronger electrostatic interaction with the negatively charged insulin than the more delocalized positive charge on the gGuanidinium group. At pH 8, DAB is only partially protonated, and this leads to a reduced electrostatic attraction comparable with that shown by the delocalized G charge.
These conclusions are reinforced by experiments in which we use proteins with different pI values to vary the protein charge while keeping pH (and dendrimer charge) constant. We see a large variability in the response to dendrimers. Although S6 was destabilized by DAB-3, neither Tnfn3 nor lysozyme were affected and cutinase was in fact slightly stabilized in the order DAB-5 > DAB-4 > DAB-3 > DAB-1. The clear pattern is that two proteins with low pI values (S6 and insulin with pI values of 6.55 and 5.2, respectively) are destabilized by dendrimers while lysozyme (pI 10.8) is not. Consistent with this, insulin does not react with negatively charged sialic acid dendrimers G3.31 Proteins with no charge around pH 8 are either unresponsive (Tnfn3) or only affected to a small degree (cutinase). The small but reproducible stabilization of cutinase may relate to specific interactions due to cutinase's affinity for hydrophobic substrates.
Obviously, other effects can contribute to protein–dendrimer interactions. Protein–dendrimer interactions involve a mixed bag of specific and general interactions, including electrostatics, binding to clustered anionic patches on the surface and presumably also nestling of partially unfolded structures inside the dendrimer interior.3 Specific interactions can lead to low-μM affinities and 1:1 binding stoichiometries.15, 32 This will make it difficult in general to predict whether a given protein will be destabilized or unaffected by dendrimers. However, our data show that overall charge can provide useful starting information.
An optimal Dendrimer Generation for Interactions Suggests That Both Dendrimer Surface and Interior Contact Proteins
In addition to these general electrostatic effects, it is striking that the 3rd generation dendrimer is the most effective destabilizer and precipitator of insulin. A similar specificity, based on more limited data, is observed for the entirely unrelated protein S6. What could be the basis for the specific strength of the 3rd generation? Increasing generations leads to a greater density of end points at the surface, providing stronger electrostatic binding to proteins. The flip side is that the surface becomes more effectively covered by the endpoints, leaving the interior sequestered from the solvent and the protein. In addition, the outer segments of higher generation dendrimers can fold back and nestle in the interior.33 If the protein is also able to interact with interior groups by sequestering or partial unfolding, the 3rd generation may represent the optimal compromise between surface density and interior accessibility. A similar 3rd generation optimum has been seen for dendrimer binding to RNA and inhibition of Tat protein binding.34 The balance of surface and interior may vary in other systems because of individual variations in protein conformations and flexibility.
Insulin exists in a complex equilibrium between monomer, dimmer, and hexamer, controlled by external ligands (Zn2+, Na+, phenol, etc.), solvent conditions (pH, ionic strength, temperature), and protein concentration.21 However, under our conditions (50 mM Tris, pH 8.0, and no zinc present), zinc is mainly a dimer at room temperature.19 Monomerization and unfolding appear to occur simultaneously during thermal unfolding of insulin, leading to an unfolded monomer via a partially unfolded dimeric intermediate.19 Thus, preferential binding of dendrimers to denatured monomeric insulin would shift the equilibrium toward denaturation and lower the tm. While insulin fibrillation at both neutral and low pH requires the accumulation of an expanded insulin monomer,23 the weak effects of dendrimers on insulin aggregation (Figure 5) suggest that this is not a primary interaction partner. We speculate that the dendrimers stabilize a more denatured monomeric state of insulin which can bind more extensively to the dendrimer surface and interior. This binding may at the same time allow effective neutralization of negative charges, particularly if several dendrimer molecules are involved. In this way, a charge-neutralization network may arise, leading to the observed precipitation. Similar combinations of binding-unfolding-neutralization-precipitation effects may be observed when protein is incubated together with for example anionic lipids and detergents.35, 36 In these cases, binding is also accompanied by structural changes which presumably extend protein accessibility and may allow the build-up of an insoluble network. The key step in this process is the formation of a conformationally altered complex between protein and ligand, and this is likely to vary from protein to protein.
MATERIALS AND METHODS
Materials
Zinc-free human insulin was generously provided by Novo Nordisk A/S. The S6 mutant Ala35Gly from Thermus thermophilus and cutinase from Fusarium solani pisi were purified as described.25, 37 Guanidinium-, urea-, and DAB dendrimers were produced as previously reported.12, 18 All experiments were performed in 50 mM Tris pH 8, except experiments at pH 6 and pH 10 using 50 mM NaOAc and 50 mM Gly, respectively. Insulin was suspended in water to a concentration of 10 mg/ml, after which HCl was added to ∼10 mM to dissolve the protein. The required buffer was subsequently added to 50 mM. Equilibrium unfolding of S6 in guanidinium chloride in the presence of 20 μg/ml DAB-generation 3 or DAB-generation 4 was performed and analyzed as described.25
Spectroscopy
Far-UV CD spectra were recorded in a 1 mm quartz cuvette on a JASCO J-810 spectropolarimeter with a Jasco PTC-423S temperature control unit. Thermal scans were carried out in 0.5 mg/ml insulin and different concentrations of dendrimers by following the ellipticity at 220 nm with a scan speed of 2°C (steps of 0.2°C) with a 4 s integration time. Thermal scans were analyzed as described20 to yield the midpoint denaturation temperature tm.
Solubility studies were carried out by incubating 0.5 mg/ml insulin with different concentrations of dendrimer for at least 30 min, after which the solution was centrifuged for 15 min at 14.000 rpm and the tyrosine fluorescence of the supernatant was measured using excitation at 290 nm and emission at 310 nm. Fluorescence measurements gave much less scatter than absorption measurements at 280 nm. The dendrimer contribution to fluorescence was insignificant.
Thermal scans of 4 μM cutinase were performed in 10 mm quartz cuvettes on a Cary-Varian Eclipse fluorimeter with a scan rate of 90°C/h between 15 and 75°C using excitation and emission wavelengths of 295 nm and 350 nm, respectively, 1 s acquisition times and 10 nm slits widths.
Plate Reader Measurements
Insulin aggregation kinetics was followed by incubating 2 mg/ml insulin in a 20 mM phosphate buffer pH 7 100 mM NaCl with 20 μM Thioflavin T (ThT) and 1–250 μg/ml U, G, and DAB dendrimers of generation 1–5 in triplicates. One hundred microliter sample was transferred to a black polystyrene 384-well Nunc microtiter plates with transparent bottom. The plates were covered with Hampton Research Crystal-clear sealing tape and placed in a Spectramax Gemini XS fluorescence plate reader at 37°C which read fluorescence emission from the plate bottom at 485nm (excitation 450 nm) every 10 min preceded by 5 min of auto mixing. The aggregation lag time is operationally defined as the time needed to reach 2.5% of the maximum ThT-emission.
Atomic Force Microscopy (AFM)
The morphology of the ThT-positive insulin aggregates was visualized on a PicoSPMI apparatus (Molecular Imaging, Tempe, AZ) using silicon nitride cantilevers (BudgetSensors, Sofia, Bulgaria) with a force constant of 0.27 N/m. Ten microliter of aggregated insulin was loaded to fresh cleaved mica followed by incubation at room temperature for 10–15 min. The air-dried sample was washed 10 times with 200 μl sterile filtered water and the sample was dried under a gentle stream of air for a few minutes at room temperature. The image was recorded in contact mode using a cantilever (Sini AFM probe, NanoAndMore GMBH) using a scanning speed of 105,329 nm/s. Images were visualized by the software Picoscan 5.5.3 from Molecular Imaging Corporation.
Acknowledgements
We thank Lotte Langkjær for useful discussions about insulin and Novo Nordisk A/S for providing insulin samples.




