Membrane interactions of designed cationic antimicrobial peptides: The two thresholds†
This paper is dedicated to the memory of Professor Elkan Blout. C.M.D. had the privilege of being a post-doctoral associate in Elkan's lab at Harvard Medical School. It was the gift of Elkan that under his mentorship, one not only learned science, but also learned how to be a scientist. Elkan had the knack to recognize the important problems of the day, and see the big picture solutions. And equally important, he set the tone that encouraged each of us to work independently while at the same time enjoying the interchange of the group's ideas. I will always value Elkan's support and development of the young me, and the wonderful interactions I had with him, his wife Gail, and with labmates, over many subsequent years.
Abstract
Novel cationic antimicrobial peptides (CAPs) designed in our lab—typified by sequences such as KKKKKKAAX-AAXAAXAA-NH2, where X = Phe/Trp—display high antibacterial activity but exhibit little or no hemolytic activity towards human red blood cells even at high doses. To clarify the mechanism of their selectivity for bacterial versus mammalian membranes and to increase our understanding of the relationships between primary sequence and bioactivity, a library of derivatives was prepared by increasing segmental hydrophobicity, in which systematic substitutions of Ala for two, three, or four Leu residues were made. Conformationally constrained dimeric and cyclic derivatives were also synthesized. The peptides were examined for activity against pathogenic bacteria (Pseudomonas aeruginosa), hemolytic activity on human red blood cells, and insertion into models of natural bacterial membranes (containing anionic lipids) and mammalian membranes (containing zwitterionic lipids + cholesterol). Results were compared with corresponding properties of the natural CAPs magainin and cecropin. Using circular dichroism and fluorescence spectroscopy, we found that peptide conformation and membrane insertion were sequence dependent, both upon the number of Leu residues, and upon their positions along the hydrophobic core. Membrane disruption was likely enhanced by the fact that the peptides contain potent dimerization-promoting sequence motifs, as assessed by SDS-PAGE gel analysis. The overall results led us to identify distinctions in the mechanism of actions of these CAPs for disruption of bacterial versus mammalian membranes, the latter dependent on surpassing a “second hydrophobicity threshold” for insertion into zwitterionic membranes. © 2008 Wiley Periodicals, Inc. Biopolymers 89: 360–371, 2008.
This article was originally published online as an accepted preprint. The “Published Online” date corresponds to the preprint version. You can request a copy of the preprint by emailing the Biopolymers editorial office at [email protected]
INTRODUCTION
The innate immune system provides broad-spectrum recognition and rapid elimination of host-invading microorganisms. It is distinct from the adaptive or clonal immune repertoire wherein a host response is initiated only following antigen stimulation of specific lymphocyte subpopulations.1 Antimicrobial peptides successfully serve as the key innate immunity agents for a wide range of species ranging from plants and insects to mammals and limit colonization and infection by bacteria, fungi, parasites and enveloped viruses and demonstrate synergy with conventional antibiotics. Some antimicrobial peptides are also effective against tumor cells2-5 and have been proposed to play a role in the regulation of inflammatory responses.6
Antimicrobial peptides share a limited number of characteristics, including relatively short length (a few tens of residues), net positive charge,7 and solubility in water or saline.6 Highly positively-charged antimicrobial peptides, termed cationic antimicrobial peptides (CAPs), exist predominantly as random coil structures in solution, but adopt folded secondary structures in hydrophobic environments, typically with amphipathic helical character wherein charged residues are grouped on one face of the helix, while hydrophobic residues are grouped on the opposite face.8 In general, CAPs are active in the low-medium micromolar range and show little target or L- vs. D-residue specificity (enantiomers usually exhibit similar or two-fold higher activity when compared with their L-counterparts), indicating that they interact with achiral components of the cell membrane9, 10 through a mechanism of physical disruption. CAPs likely attach in an asymmetrical manner from the outer leaflet of the biological membrane, and penetrate it, physically disrupting the bacterial cellular bilayer, leading to lysis and eventually cell death.11 The activity of CAPs on each particular cell membrane is differentiated by variations in their lipid composition.3 Thus, for mammalian cells, the primary plasma membrane features are an essentially neutral (zwitterionic) outer leaflet, the presence of cholesterol (up to 25 mol%), and predominance of phosphatidylcholine lipids.12 In contrast, outer membranes of bacterial cells contain 20–25% of anionic lipids,13 giving them an overall negative charge. As well, bacterial membranes lack sterols in general with few exceptions, and their most common zwitterionic lipid is phosphatidylethanolamine. Currently, ∼900 sequences for gene-encoded eukaryotic antimicrobial peptides and proteins have been reported, with about 300 of them characterized as linear α-helical peptides rich in lysine and arginine residues and containing C-terminal amidation, as typified by the widely studied CAPs magainin and cecropin (http://bbcm1.univ.trieste.it/∼tossi/pag5.htm). Several thousand additional antimicrobial peptides have been designed de novo and produced synthetically.11
The increasing prevalence of antibiotic resistance necessitates the development of new and efficient ways to combat bacterial infection. CAPs are potential candidates and offer a viable alternative to conventional antibiotics because of their broad activity spectrum (antibacterial, antiviral and antifungal), rapid onset of killing, and potentially low levels of induced resistance. Accordingly, an understanding of CAPs mechanisms of action is essential for rational design of their successful, potent drug-lead compounds as alternatives to current antibiotic treatments.14 Biophysical studies have demonstrated that there are some general nonreceptor-mediated mechanisms responsible for peptide antimicrobial activity, which appear to involve permeabilization of phospholipid bilayer membranes via “barrel-stave,” “toroidal pore,” or “carpet detergent-like” arrangements.3, 8, 11, 15 Since the majority of antimicrobial peptides are positively charged at physiological pH, while outer leaflets of mammalian and bacterial plasma membranes are totally neutral and anionic, respectively, the mechanisms of their antimicrobial and hemolytic activities might differ in detail.
While some antimicrobial peptides are already in clinical and commercial use, future design of novel antimicrobial peptides will necessitate the optimization of multiple parameters, including toxicity against host mammalian cells, the development of allergies, susceptibility to proteolytic degradation, salt sensitivity, and high manufacturing cost. The first limitation of the clinical development of CAPs is the requirement for a high degree of selectivity between microbial and human cells, such that host cells are not damaged. Hence, the continuing goal is to engineer peptides with an improved therapeutic index, i.e., high efficacy and target specificity.
In this context, our laboratory has developed a novel series of synthetic, short membrane-active peptides with separated positively charged “Lys tags” adjacent to a nonamphipathic core of eleven residues.16 The core is built largely from residues that tend to be abundant among CAPs—Ala, Phe, and Leu. This series of peptides, typified by structures such as KmAAXAAXAAXAAKn-NH2, where m, n = 0, 4, 6, 10; X = Phe/Trp, was based on a model series of 25-residue prototypic peptides of sequence KKAAAXAAAAAXAAWAAXAAAKKKK-NH2, where X = each of the 20 commonly-occurring amino acids,17, 18 which were originally designed as transmembrane mimetic α-helical segments of integral membrane proteins. The presence in both series of peptides of a nonamphipathic hydrophobic core segment distinguishes them from essentially all known gene-encoded linear CAPs that typically form amphipathic helices upon interaction with a hydrophobic membrane environment. The designed peptides from both series display excellent antimicrobial activity against free-swimming (planktonic) gram-positive and gram-negative bacteria16 and pathogenic yeast.19 In addition, while the majority of peptides from the “shorter” series showed absence of toxicity to mammalian cells, substitution of four core Ala residues to more hydrophobic residues caused appearance of significant hemolytic activity in erythrocytes, suggesting that hydrophobic character became elevated to a level where partial insertion into zwitterionic bilayers that mimic the outer leaflet of mammalian plasma membrane composition becomes possible.20 Peptide 6K-F17 (KKKKKKAAFAAWAAFAA-NH2) from the “shorter” series with six Lys residues at the N-terminus showed the best characteristics among the family—the highest antimicrobial activity along with negligible hemolytic activity.16, 19
Since these totally synthetic nonamphipathic core peptides were found to be active in the low-medium micromolar range, and show little target or L- vs. D-residue specificity16—similarly to the majority of gene-encoded CAPs—their microbial and mammalian cell membrane disruption can likely be ascribed, at least in large measure, to one of the characterized nonstereospecific mechanisms described above. A further aspect of their mechanism of action, that contrasts to the naturally-occurring magainin II amide GIGKFLHAAKKFAKAFVA-EIMNS-NH221 and the mammalian cecropin P1 SWLSKTAK-KLENSAKKRISEGAIAIQGGPR-NH2,22 is that the designed peptides were subsequently found to have a significant tendency to dimerize in membrane environments even at low concentrations below their minimum inhibitory concentration (MIC) values,20 a possible indicator of oligomerization and hence membrane disruptive power. From a biophysical viewpoint, the efficacy of the α-helical CAPs will depend on net charge, overall hydrophobicity, peptide chain length, and the degree of ordered structure both in aqueous and membrane environments. However, all of these factors are ultimately dictated by the choice of peptide primary sequence. In the present study, we therefore sought to unify the various hypotheses concerning CAP activity, through step-by-step systematic characterization of their properties as a direct function of peptide sequence and hydropathy.
MATERIALS AND METHODS
Materials
Reagents for peptide synthesis, cleavage, and purification included Fmoc-protected amino acids (Novabiochem); dansyl- and dabcyl-chlorides (Molecular probes, Eugene, OR); Fmoc-PAL-PEG-PS-resin, piperidine (Applied Biosystems, Foster City, CA); DMF, methanol, diethyl ether, acetonitrile, (Caledon Laboratories, Georgetown, Ontario, Canada); DIEA, triisopropylsilane (Aldrich); HATU (GL Biochem, Shanghai, China); ultrapure buffer-saturated phenol (Invitrogen). Lipids—POPC, POPE, POPG, and cholesterol (Avanti Polar Lipids, Alabaster, AL). Reagent kits for micro BCA protein assays were from Pierce (Rockford, IL); Mueller Hinton Broth (MHB) and Bacto-Agar were from Difco laboratories. All chemicals were used without further purification. Buffers were prepared in double-distilled water (DDW) with pH adjustment when necessary.
Peptide Synthesis and Characterization
Peptides were synthesized on a PS3 Protein Technologies Inc. peptide synthesizer by standard solid-phase protocols similar to those previously described.17, 18 For cyclization of peptide (K7-D17)7K-F18-1D17 a low load (>0.15 mmol/g) PAL-PEG-PS resin was used to incorporate an amide function at the peptide C-terminus upon peptide cleavage. DIEA (1.0M in DMF) was used with a four-fold excess of protected amino acids and HATU coupling reagent. N-terminal Fmoc groups were removed at the last step of the synthesis.
The lactam ring cyclization (Scheme 1) was accomplished manually, while N-terminal Fmoc and all side-chain protecting groups were still attached to the peptidyl-resin. Side chain protecting groups—Mtt and -O-2-PhiPr on residues Lys7 and Asp17, respectively—were removed selectively (1% trifluoracetic acid (TFA), 2% triisopropylsilane, 97% dichloromethane) on ice for 30 min.23 The resin was then washed with 5% DIEA (vol/vol) in dichloromethane and the intermolecular amide bond was created through application of five-fold excess of HATU in DMF at room temperature for 24 h.
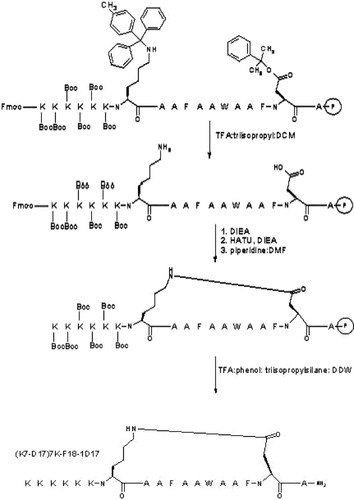
Synthesis of a cyclic peptide analog of 6K-F17.
Following a standard cleavage procedure (89% TFA, 4.5% (vol/vol) phenol, 4.5% DDW, 2% triisopropylsilane), crude deprotected peptides were precipitated in cold ethyl ether, dissolved in water and purified on a reverse-phase C18 high performance liquid chromatography column (Vydac, 250 × 21.5 mm) using a linear gradient of 20–50% B (Buffer A: 0.1% TFA, 95% DDW, 5% acetonitrile; Buffer B: 0.1% TFA, 95% acetonitrile, 5% DDW) for the first 35 min, then 50–100% B for another 10 min at 10 mL/min flow rate. Absorbance was monitored at 215 nm. Major peak fractions containing product were collected, pooled and lyophilized. Molecular masses were confirmed by MALDI mass spectrometry. Peptide concentrations were determined in triplicate by standard micro BCA assay and their purities (above 95%) and retention time (Rt) values were assessed by RP-HPLC at room temperature on a Vydac C4 column (250 × 4.6 mm) with linear gradients of Buffers A and B at a flow rate of 1 mL/min, starting with 15% B for the first 5 min, then a linear gradient of 15–70% B for another 55 min. Stock peptide solutions were stored at −20°C.
Hydrophobicity
Core segment hydrophobicity (CSH) values were calculated using the Liu-Deber hydrophobicity scale.18
Hemolytic Activity
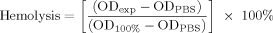 ()
()The hemolytic activity was determined as minimal hemolytic concentration (MHC)—the maximal peptide concentration that caused no hemolysis of RBCs after 1 h. Since RBCs were in isotonic medium, no detectible release of hemoglobin (<1%) was observed from negative controls.
Antibacterial Activity
The antimicrobial activity of each peptide was tested under aerobic conditions in sterile 96-well microtiter plates (Costar) in a final volume of 100 μL by following standard microtiter dilution protocols25 in MHB. Pseudomonas aeruginosa nonclinical strain wild-type mPAO1 was maintained as a glycerol stock at −80°C. Bacterial cells were grown in MHB at 37°C for overnight and were diluted in the same medium to a final concentration of 2 × 104 to 2 × 105 colony forming units (CFU/mL) as determined by optical density at 600 nm. Ten-microliter aliquots of serial two-fold dilutions in buffer (0.2% BSA, 0.01% acetic acid) of the lyophilyzed peptides were added to microtiter plates followed by 90 μL of bacterial suspension. Peptide antibacterial activity, expressed as the MIC—the lowest peptide concentration that resulted in 100% prevention of microbial growth as evidenced by absence of turbidity after 19 h of incubation at 37°C. Turbidity was measured as OD at 600 nm using a Genesys 5 microplate autoreader Spectrophotometer (Rochester, NY). Positive controls contained no peptide and demonstrated visible turbidity after 19 h of incubation at 37°C. All assays were carried out in triplicate.
Gel Electrophoresis
SDS-PAGE electrophoretic separation was performed at 125 mV with peptide samples dissolved in sample buffer (NuPAGE, Novex, San Diego, CA) to 35 μM, heated at 85°C for 10 min and separated on precast 12% Bis-Tris NuPage gels (1.0 mm × 10 well) in NuPAGE MES SDS buffer. In hetero-oligomerization experiments SPGS-34 was mixed in equal amounts with other peptides before loading to keep the total peptide concentration identical to that used in homo-oligomerization experiments. Coomassie Blue staining was used on all gels to visualize peptides. The MWexp/MWtheor values were calculated by densitometry using See blue and Mark12 markers and the NIH 1.62 Image Program (software available at http://www-cellbio.med.unc.edu/henson_mrm/pages/NIH.html).26
Circular Dichroism
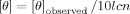 ()
()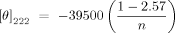 ()
()Preparation of Large Unilamellar Vesicles
LUVs were prepared as described previously.28 The desired mixtures of lipids were dried in glass tubes first under nitrogen and then lyophilized overnight to obtain lipid films. The films were suspended for 1 h on a water-bath at 40–50°C in Tris-HCl buffer, pH = 7.0 (10 mM Tris, 10 mM NaCl), sealed with parafilm under nitrogen, and then freeze-thawed five times under nitrogen to produce large multilamellar vesicles. Each suspension was extruded nine times through polycarbonate membranes with 0.1-mm diameter pores (Nuclepore, Pleasanton, CA) on an Avanti mini-extruder apparatus. Vesicles were used the same day and appropriate aliquots of the desired peptides were added.
Fluorescence Measurements
Fluorescence emission spectra of peptide Trp residues were measured on a Hitachi F-400 Photon Technology International C-60 fluorescence spectrometer at excitation wavelength of 280 nm with a 2nm bandpass, and integrated between 305 and 390 nm with a 6-nm bandpass. Interactions of CAPs with zwitterionic or negatively-charged vesicles were characterized by measuring changes in emission intensity maxima wavelength λmax (blue shifts) of the peptide intrinsic Trp relative to normal salt aqueous conditions. Samples of peptides (4 μM) in 1 mM of detergent were used in a 0.5 mL semimicro quartz cuvette (10-mm excitation path length and 4-mm emission path length) (Hellma, Concord, ON). All spectra were integrated, corrected for light scattering effects by subtraction of background, and by the correction function of FELIX software provided by the manufacturer.
RESULTS
Peptide Design
To obtain additional details about the mechanism of bioactivity of synthetic CAPs, as well as to improve their antimicrobial activity, while preserving selectivity, we designed a library of 12 peptides based on the 6K-F17 prototype, and comprehensively investigated their antibacterial and cytotoxic activities, secondary structures and folding in various environments, and interactions with model membranes (Table I). The peptide net charge was maintained as +7 in all analogs (including the N-terminal -NH3+ moiety), while minor changes in charge distribution and length were made. In the present study we used 6K-F17 (KKKKKKAAFAAWAAFAA-NH2) as a framework to alter core hydrophobicity by systematically replacing Ala residues with Leu to increase average core hydrophobicity. The resulting new designs represent mono, double, triple, and quadruple Ala-to-Leu substituted 6K-F17 derivatives (Table I). This designed “Leu-series” of further analogs has two key variables: the number of Leu residues and the relative position(s) of the Leu in hydrophobic core. The full library contains one isomer each with single and quadruple substitutions, five isomers of two, and two isomers of three Leu residues. To distinguish among the isomeric peptides, we specify Leu positions along the sequence by subscript numbers at the end of each peptide designation.
| Peptide | Positions Along Hydrophobic Corea | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | CSHb | |
| 7K-F18-1D17 | A | A | F | A | A | W | A | A | F | D | A | 1.23 |
| 6K-F17 | A | A | F | A | A | W | A | A | F | A | A | 1.48 |
| 6k-f17 | a | a | f | a | a | w | a | a | f | a | a | 1.48 |
| 6K-F17-1L11 | A | A | F | A | L | W | A | A | F | A | A | 1.89 |
| 6K-F17-2L11,13 | A | A | F | A | L | W | L | A | F | A | A | 2.31 |
| 6K-F17-2L10,14 | A | A | F | L | A | W | A | L | F | A | A | 2.31 |
| 6k-f17-2l16,17 | a | a | f | a | a | w | a | a | f | l | l | 2.31 |
| 6K-F17-2L8,16 | A | L | F | A | A | W | A | A | F | L | A | 2.31 |
| 6K-F17-2L7,8 | L | L | F | A | A | W | A | A | F | A | A | 2.31 |
| 6K-F17-3L8,11,13 | A | L | F | A | L | W | L | A | F | A | A | 2.73 |
| 6K-F17-3L11,13,16 | A | A | F | A | L | W | L | A | F | L | A | 2.73 |
| 6K-F17-4L8,11,13,16 | A | L | F | A | L | W | L | A | F | L | A | 3.14 |
- a Core sequences are shown; each peptide labeled 6K has Lys residues at positions 1–6. Peptide 7K-F18-1D17 = KKKKKKKAAFAAAAFDA-NH2. In the Table, positions in core of 7K-F18-1D17 are shifted left by one number for alignment. Amino acids in lowercase are D-enantiomers; Leu and Asp residues are italicized.
- b Values correspond to the hydrophobicity of the core segment (CSH) determined by the Liu-Deber scale.18, 29 Lys residues are not included in the calculation. CSH values greater than +0.4 are above the “first threshold” for membrane insertion (see text).
CAPs with inter-molecular disulfide bonds form a major class of innate immune system agents,3, 4 and peptide cyclization is a known tool for metabolic stability improvement through global conformational constraint on otherwise more flexible structure(s).30, 31 This tactic has been applied successfully on magainin II32 and mellitin33 sequences, also demonstrating that linearity of the peptides is not essential for the disruption of the target phospholipid membrane. Thus, we designed two mono Ala-to-Asp substituted peptides—one is an intra-molecular side chain-to-side chain cyclic peptide, termed (K7-D17)7K-F18-1D17 (see Materials and Methods section and Scheme 1 for details of synthesis), along with its linear analog 7K-F18-1D17 (Table I). The functional groups for lactam ring closure are located 12 residues apart (i, i + 12) from one another to preserve their α-helical secondary structure. To mimic the presumed membrane-bound dimeric oligomerization state of the designed 17-residue CAPs,20 a helix-loop-helix hairpin “covalent anti-parallel dimer” model peptide KKKKAAFAAWAAFAAKSPGSKAAFAAWAAFAAKKKK-NH2 (termed SPGS-34), was synthesized. It consists of two Lys-tagged nonamphipathic cores of 11 residues each connected by the short hydrophilic β-turn mimic loop of Ser-Pro-Gly-Ser.34
Antimicrobial Activity
P. aeruginosa infections are the major cause of morbidity in adult patients with cystic fibrosis, and also represent a serious problem in patients hospitalized with cancer and burns.35 The MIC values for the present library of peptides against the gram-negative P. aeruginosa mPAO1 strain were measured (Table II). The D-enantiomer 6k-f17 was the most active peptide (MIC = 2 μM) of the series, while the SPGS-34 hairpin was the least active (MIC = 128 μM). Magainin II amide showed intermediate activity (MIC = 16 μM). The remainder of the peptides displayed good-to-moderate antimicrobial effects; their activity in general decreased as increasing Leu residues were incorporated into the sequence. The isomers with the same numbers of Leu residues showed different MICs, as previously noted in other CAPs,36, 37 although the full range was limited (8–64 μM). Cyclic and linear derivatives of 7K-F17-1D17 showed similarly favorable MIC values (MIC = 8 μM), and based on previous results, at least two-fold lower values could be expected for the D-enantiomers of these peptides.
| Peptide | Hemolytic Activity (%) [error (%)] at Different Peptide Concentrationsa | MHCb (μM) | MICc (μM) | Therapeutic indexd | |||
|---|---|---|---|---|---|---|---|
| 650 μM | 325 μM | 162 μM | 81 μM | ||||
| 7K-F18-1D17 | 0.3 (0.0) | −0.2 (0.0) | 0.3 (0.0) | −0.4 (0.0) | 650 | 8 | >81 |
| 6k-f17 | 2.3 (0.0) | 0.3 (0.0) | 0.7 (0.0) | 0.3 (0.0) | 325 | 2 | 162 |
| 6K-F17-1L11 | 3.8 (0.0) | 3 (0.0) | 1.5 (0.0) | 0 (0.0) | 81 | 16 | 5 |
| 6K-F17-2L11,13 | 2.4 (0.1) | 2.2 (0.0) | 2.3 (0.0) | 2.2 (0.0) | <81 | 16 | <5 |
| 6K-F17-2L10,14 | 23.7 (1.2) | 16 (0.0) | 10 (0.4) | 11.4 (0.1) | <81 | 8 | ≪10 |
| 6k-f17-2l116,17 | 17 (2) | 4.4 (0.0) | 7.1 (0.1) | 5.8 (0.0) | <81 | 8 | <10 |
| 6K-F17-2L8,16 | 19 (2) | 4.1 (0.1) | 15.1 (0.3) | 6.5 (0.1) | <81 | 64 | <1 |
| 6K-F17-2L7,8 | 20.4 (0.9) | 13.2 (0.1) | 9.5 (0.0) | 5 (0.0) | <81 | 8 | <10 |
| 6K-F17-3L8,11,13 | 23.8 (0.6) | 16.8 (0.4) | 18.9 (0.5) | 16.4 (ND) | ≪81 | 32 | ≪2.5 |
| 6K-F17-3L11,13,16 | 20.1 (0.0) | 20.0 (0.6) | 11 (ND) | 21.8 (0.2) | ≪81 | 16 | ≪5 |
| 6K-F17-4L8,11,13,16 | 34.8 (0.9) | 50.6 (5.6) | 30.3 (1.7) | 34.2 (2.8) | ≪81 | 64 | ≪1.3 |
| (K7-D17)7K-F18-1D17 | ND | ND | ND | ND | ND | 8 | ND |
| Magainin II amide | 101 (2) | 91.2 (0.7) | 82.5 (7.5) | 81.1 (0.8) | ⋘81 | 16 | ⋘5 |
| SPGS-34 | ND | ND | ND | 1.2 (0.0) | <81 | 128 | >0.6 |
| Cecropin P1 | 1.3 (0.0) | ND | ND | −0.2 (0.0) | 81 | ND | ND |
- ND, not determined.
- a Percent of hemolysis in human RBC ± (error) at various peptide concentrations, relative to 100% positive control of Triton X. Values are representative of two experiments.
- b MHC is the highest nonhemolytic concentration of a given peptide.
- c MIC values are the lowest antimicrobial concentration for the mPAO1 strain of P. aeruginosa. Values are representative of results from three experiments. MICs may be converted to micrograms per milliliter by dividing molecular mass (in Daltons) by 1000 and then multiplying by the value in micromolar units.
- d Therapeutic index is given as the MHC to MIC ratio.
Hemolytic Activity
The toxicity of drug leads to mammalian cells is frequently expressed as hemolytic activity. The hemolytic activities of our synthetic CAPs, as well as of magainin II amide against human RBCs, were determined at four peptide concentrations (650, 352,162, and 81 μM) (Table II), and MHC values were estimated in this range. The total absence of hemolysis at the highest tested concentration (MHC = 650 μM) was observed only for the peptide with the lowest CSH18 of 1.2—7K-F18-1D17 (Table I). Peptides with CSH values between 1.5 through 1.9, as well as cecropin P1, showed slight hemolytic activity at concentrations of 162 μM and above. In contrast, those peptides with CSH of 2.3 and above (with two Leu residues and higher), along with SPGS-34 and magainin II, showed modest or significant hemolytic activities at every concentration tested (MHC values below 81 μM). Overall, the most hydrophobic peptide among the Ala-substituted series of 6K-F17, 6K-F17-4L8,11,13,16, displayed the highest hemolytic profile (up to 50%), while magainin II amide showed almost complete hemolysis at all concentrations tested. Isomers with the same number of Ala-to-Leu mutations showed similar but not identical activities; for example, 6K-F17-2L11,13 showed the lowest hemolytic activity among double-substituted isomers.
Therapeutic Index and Selectivity
The therapeutic index is a widely employed parameter to quantify the specificity of antimicrobial reagents. It is expressed as ratio between MHC and MIC values (Table II); thus, larger therapeutic index values indicate greater clinical potential. Calculated therapeutic indexes for each peptide studied in the present work indicate an obvious decrease of selectivity with increasing Ala-to-Leu substitutions (Table II) from 162 times to <1. Among all studied peptides, 6k-f17 and 7K-F18-1D17 showed the largest therapeutic indexes of 162, and >81, respectively, while the remainder of the 6K-F17 derivatives have therapeutic indexes below 10. The naturally occurring magainin II CAP showed a therapeutic index below 5 in our hands, while for cecropin A, indices in the range of 6–17 have been reported.38 Our best designs therefore showed therapeutic indices considerably superior to both of the latter natural CAPs.
Hydrophobicity
RP-HPLC retention behavior is an excellent tool to estimate relative peptide apparent hydrophobicities.39 The peptide retention times (Rt values) are shown in Table III. As expected, the tetra-substituted Ala-to-Leu peptide 6K-F17-4L8,11,13,16 was retained the longest on the HPLC column among the Ala-substituted series, followed by triple, double, hairpin (SPGS-34), mono-Leu, cyclic, and nonsubstituted peptides, followed finally the least hydrophobic Ala-to-Asp substituted—7K-F18-1D17. Magainin II amide showed the longest Rt value, reading out as the most hydrophobic peptide among those tested.
| Peptide | RP-HPLC, Rt (min)a | SDS-PAGE, MWexp/MWtheorb (±0.15) |
|---|---|---|
| 7K-F18-1D17 | 24.6 | 2.5 |
| 6k-f17 | 25.6 | 2.3 |
| (K7-D17)7K-F18-1D17 | 27 | 2.1 |
| 6K-F17-1L11 | 29.6 | 2.4 |
| SPGS-34 | 30.6 | 1.3 |
| 6K-F17-2L7,8 | 31.3 | 2.3 |
| 6K-F17-2L11,13 | 32.9 | 2.2 |
| 6K-F17-2L10,14 | 33.2 | 2.4 |
| 6K-F17-2L8,16 | 33.9 | 2.3 |
| 6k-f17-2l16,17 | 34.2 | 2.2 |
| 6K-F17-3L11,13,16 | 35.6 | 2.1 |
| 6K-F17-3L8,11,13 | 36.7 | 2.2 |
| 6K-F17-4L8,11,13,16 | 40.2 | 2.1 |
| Magainin II amide | 42.1 | 1.4 |
| Cecropin P1 | ND | 1.2 |
- ND, not determined.
- a Rt values are the retention times of the peptides in analytical RP-HPLC experiments at pH = 2. See Methods section.
- b MWexp/MWtheor are the ratios between experimental (estimated by Nu-PAGE gel) and theoretical molecular weights of the peptides; values close to two = dimer.
Importantly, the subset of five isomeric double-Leu peptides displayed a range of Rt values, despite the fact that all five have identical core residue hydrophobicities. For example, among double Ala-to-Leu substituted isomers, the peptide with both Leu residues located at the C-terminal, 6k-f17-2l16,17, showed the longest Rt (highest apparent hydrophobicity; Rt = 34.2 min; Table III), while 6K-F17-2L7,8 with both Leu residues at the N-terminal side of the core immediately following the Lys tags had the shortest retention time (31.3 min.). To verify the effect of different interactions of the isomeric peptides with the HPLC column, a mixture of all five double-substituted isomers in equal amounts was injected using the same conditions and all peaks were resolved (data not shown). This result confirms that peptide isomers have different physical properties and potentially different antimicrobial and biophysical properties as well.36
Helicity
TM Finder data analysis29 predicts that nonamphipathic segments of these charge-segregated peptides will insert into cell membranes and adopt an α-helical secondary structure. CD data performed in different media are consistent with these predictions. The studied CAPs are found largely in extended “random coil” conformations in aqueous buffer of low ionic strength (Fig. 1a), except SPGS-34, which is partially helical under these conditions (Fig. 1d). In SDS micelles, we observed typical CAPs transitions to helical-form patterns, indicating membrane insertion of the peptides, including the cyclic derivative (K6-D17)7K-F18-1D17 (Figs. 1b–1d). Along the Leu-substitution series, the mean residue ellipticity at 222 nm ([θn]222) generally increases with the number of Leu substitutions and indicates involvement of between 5 and 11 residues in helix formation.
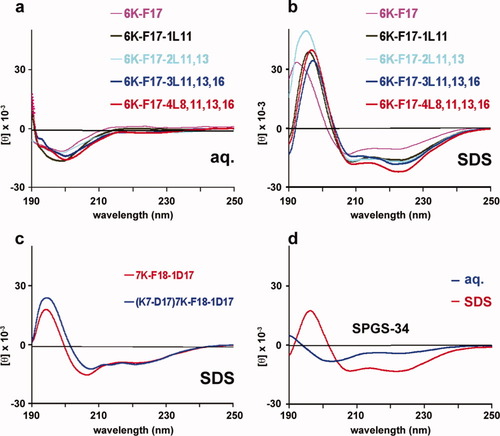
Circular dichroism spectra of selected CAPs (30 μM) recorded at 25° in 10 mM Tris-HCl, pH 7.0, and 10 mM NaCl. (a) Spectra in aqueous buffer only; (b,c) Spectra in the same buffer in the presence of 25 mM SDS. (d) SPGS-34 in buffer and in the presence of 25 mM SDS. Spectra of peptides are as indicated in the diagrams. The estimated uncertainty of peptide concentrations is ±5%. See text for further discussion.
Electrophoresis
SDS-PAGE electrophoresis has proven to be an efficient qualitative method for determining association states of many TM proteins, and indeed, many small membrane proteins maintain their oligomeric structure in the presence of SDS.31, 34 Interestingly, all 6K-F17 derivatives including cyclic (K7-D17)7K-F18-1D17 appeared as discrete SDS-resistant dimers on SDS-PAGE based on observed MWexp/MWtheor values of 2.1–2.5 (Table III). The monomeric behavior of hairpin SPGS-34 was the lone exception and likely represents formation of a tightly packed hairpin. Hetero-oligomerization was not observed when mixtures of SPGS-34 with either 6K-F17-2L11,13 or 6K-F17-2L7,8 were analyzed by SDS-PAGE (not shown), indicating only a single potential interface for peptide-peptide interactions along the hydrophobic core. In contrast, magainin II amide and cecropin P1 ran as monomers on SDS-PAGE20 (Table III).
Fluorescence
Measurement of fluorescence emission shifts in the λmax of the single Trp residue present in the nonamphipathic cores of all Leu-substituted analogues of 6K-F17 is a particularly useful tool for analysis of their membrane insertion/conformation in various media. As well, peptide Trp fluorescence emission positions upon interaction with model cell membranes has been observed to be closely correlated with their hemolytic interactions.40 In the present work, additional evidence of CAP selectivity for bacterial vs. mammalian cell membranes was obtained by exposure of the Trp-containing 6K-F17 derivatives to freshly prepared anionic phospholipid vesicles, corresponding to a typical bacterial membrane (LUV-POPC:POPG = 75:25) vs. zwitterionic vesicles corresponding to a typical mammalian membrane (LUV-POPC, and LUV-POPE, in the presence or absence of cholesterol) (see Fig. 2). When introduced to anionic membrane models, all peptides displayed significant blue shifts in Trp λmax values, ranging from 14 to 25 nm. These results provide evidence of peptide insertion into the bilayer41 and effective removal of the peptides from the bulk aqueous environment even at concentrations below their MIC values. In contrast, the same series of peptides displays variable behavior in the presence of zwitterionic vesicles, which generally correlates with their Rt values. Thus, minor blue shifts (<5 nm) were seen for the less hydrophobic peptides (6k-f17, 6K-F17-1L11 and most of the Ala-to-Leu double-substituted isomers) indicating a minimal level of attachment of the peptides to the bilayer surface. Under the same conditions, 6K-F17-2L8,16 and 6k-f17-2l16,17, along with both triple-substituted isomers and 6K-F17-4L8,11,13,16, showed an indication of partial/shallow Trp insertion into the zwitterionic bilayer moiety with larger blue shifts of 5–15 nm. In correlation with Rt values, double-substituted peptides with Leu residues close to the C-terminus showed higher insertion potential relative to their other isomers. Though some minor differences in fluorescence spectra were noted for PE and PC phospholipids (the former caused smaller blue shift values), the presence of cholesterol in the bilayer core did cause a discernible decrease in blue shifts in λmax values, by a few nm in general (see Fig. 3). Even though the total lipid compositions of LUVs employed in fluorescence studies do not completely resemble those of human RBCs or bacteria, nor maintain the innate asymmetry of outer versus inner RBC bilayer leaflets, our fluorescence-based LUV insertion data were nevertheless consistent with the corresponding observation of hemolytic effects as Leu-content increased, legitimizing comparison of the biophysical and biological approaches.
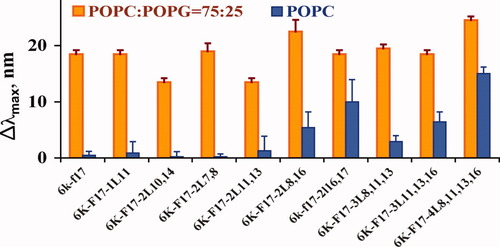
Blue shifts (nm) in the wavelength maximum emission of Trp fluorescence upon exposure of selected CAPs (4 μM) to freshly prepared anionic and zwitterionic vesicles (1 mM of lipids). Blue shifts are given as Δλmax = λmax(aqueous) − λmax(LUV). Orange bars: LUV-anionic [25% anionic lipids—POPG, 75% zwitterionic lipids—POPC; similar to the composition of bacterial cell membranes]. Blue bars: LUV zwitterionic [0% anionic lipids, 100% zwitterionic lipids—POPC, 0% cholesterol—similar to the outer leaflet of erythrocyte cell membranes]. Error bars represent the standard deviation of three experiments.
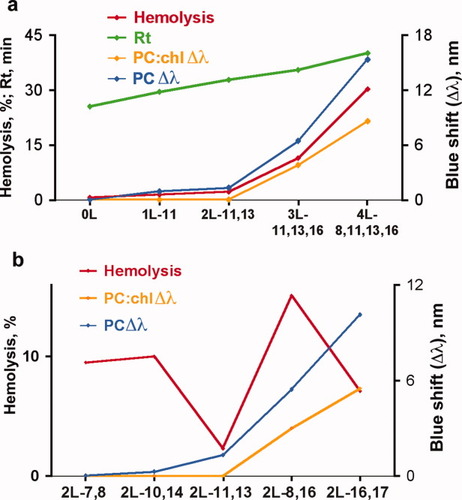
(a) Hemolysis, HPLC retention times, and blue shifts in Trp fluorescence spectra in zwitterionic membranes for CAPs with increasing Leu residue content. Left vertical axis: Units are read in % for hemolysis values (red line), or in minutes for HPLC retention times (green line). Hemolysis experiments are performed in human RBCs at peptide concentration = 160 μM. Right vertical axis: Blue shifts in the wavelength maximum emission of Trp fluorescence (Δλmax) upon exposure to freshly prepared zwitterionic LUVs [75% POPC, 25% cholesterol] at 1 mM total lipid concentration. PC is freshly prepared unilamellar 100% POPC vesicles. Fluorescence measurements are performed at peptide concentrations = 4 μM; see Methods for further experimental details. Curves are plotted from left to right for peptides with increasing Leu-residue content. Sequences are as given in Table I; 2L11,13 refers to peptide 6K-F17-2L11,13, etc. (b) Experiments similar to those described in (a) for the sub-library of double-Leu substituted isomers. PC:chl = 75% POPC: 25% cholesterol vesicles. Peptide HPLC retention times are as given in Table III.
DISCUSSION
Hydrophobicity Thresholds for Membrane Insertion of CAPs
The gradual increase in CSH values along the series of nonamphipathic CAPs from mono- to tetra-substituted Ala-to-Leu peptides causes a gradual increase of their RP-HPLC retention times and roughly speaking, in their MIC values (i.e., a decrease in antimicrobial activity). However, the onset of hemolytic effects on RBCs, paralleled by peptide insertion into mammalian model membranes, appears only once CSH reaches prescribed levels. In previous studies from our laboratory, a “hydrophobicity threshold” (termed here the “first threshold”) was defined as the minimal average segmental hydrophobicity required for peptide insertion from water into micelles or anionic bilayer membranes42, and therefore for antimicrobial activity.16 This threshold was shown to correspond approximately to just above that of a poly-Ala segment. A similar pattern was noted earlier for Lys-tagged 25-residue peptides with 19-residue core hydrophobic segments.17 However, when this paradigm was applied to zwitterionic mammalian-like membranes, we found for similar peptides containing highly charged Lys tags attached to a core hydrophobic sequence,20 that peptides ultimately cannot leave the bulk water for attachment/insertion into erythrocyte-like bilayers until their CSH begins to approach sufficiently high levels, as in the case where the sequence contains at least two Ala-to-Leu substitutions (see Fig. 2). One can thus define a “second hydrophobicity threshold” for peptide insertion into zwitterionic (mammalian) membranes—and as observed in the overall studies reported here—generally correspondingly for hemolytic activity. In the present work, we are able to localize this “second threshold” to the gap between CSH values of 1.9–2.3 among the sets of 6K-F17 analogues (Table I).
The highest therapeutic index (MHC/MIC = 162) of peptide 6K-F17, can also be explained by this “two threshold” paradigm. Since hemolysis of mammalian cells results in principle from insertion of the peptide into the hydrophobic core of the erythrocyte membrane, it seems reasonable to assume that relatively low core hydrophobicity would prohibit direct peptide attachment to the membrane via hydrophobic interactions. On the other hand, because the mechanism of action against bacterial cells does allow the attachment of multiple Lys side chains to the anionic phospholipid head groups in the interface region, insertion into the hydrophobic core of the membrane by peptides with average CSH above the “first threshold” will be facilitated by the initial incorporation of the peptide into the membrane surface region.
Role of Positional Hydrophobicity
The set of peptides with systematic variation of Leu residue location in the sequence allows us to assess a possible key role of positional hydrophobicity on CAP bioactivity and hemolytic strength, in a series where peptide composition, charge, and length are otherwise identical. In our experiments, lipid:peptide (L:P) ratios were maintained high enough (250:1) at low peptide concentration (4 μM, well below the majority of MIC values and below the hemolytic threshold for the conditions tested) to mimic the initial steps of peptide-mediated membrane disruption. Among five isomers of double-Ala-to-Leu substituted peptides (6k-f17-2l16,17 is studied as the D-enantiomer), CSH values are correspondingly equal in each case, but Rt values range from 31.3 to 34.2 min, and hemolytic activity ranges from 2.2 to 11.4% at 81 mM and 2.4 to 23.7% at 650 mM peptide. Yet the antimicrobial activities of these peptides are broadly similar: allowing for a doubling of the MIC for the L-version of the Leu16,17 isomer, four of the five isomers have MICs = 8– 16 μM, and only the Leu8,16 isomer is higher at MIC = 64 μM. The 3-Leu and 4-Leu isomers are discernibly higher with MICs of 32–64 μM. The trend toward decreasing antimicrobial activity with increasing Leu content may reflect the circumstances that (i) the “active” interface needed for insertion becomes involved instead in peptide-peptide interactions, such that at least a portion of the peptide population becomes unable to penetrate the microbial cell membrane despite electrostatic attachment; and/or (ii) the increased segmental hydrophobicity imparts an increased potential to peptide self-association/oligomerization in the aqueous phase, thus limiting the concentration of peptide actually impacting on the bacterial cell surface. This latter situation would have little effect on the access of the peptides to zwitterionic mammalian membranes, thus uncoupling, at least in part, any direct correlation between hemolysis levels and antimicrobial activity.
Designed CAPs Form Dimers in Hydrophobic Environments
Essentially all Lys-tagged 11-residue core peptides we have designed have a strong tendency toward dimerization under membrane-mimetic conditions, as confirmed by SDS-PAGE gels (Table I) and FRET spectroscopy.20 In the present work, all 2-, 3-, and Leu isomeric peptides migrate on SDS-PAGE as dimers. Such dimerization must relate to the presence of highly potent helix-helix interaction motifs in their sequence. Dimerization in this peptide series may arise largely from the fact that their nonamphipathic cores contain at least one AxxxA in their sequences. Whether or not other oligomerization promoting motifs may be present, two “small” residues separated by three residues, such as AxxxA43-45 represent likely candidates for driving dimerization. In the context of antimicrobial and hemolytic activities, such strong dimer-forming ability in hydrophobic environments might serve as a key determinant, i.e., the promotion of even higher oligomerization states within the membrane surface region, which in turn may be expected to create substantially larger disturbances in the bilayer once insertion occurs. It may be further noted that in CAPs that act through barrel-stave and toroidal pore mechanisms, peptides are believed to oligomerize before or during bilayer insertion,10, 46 so that their ability to oligomerize enhances the effectiveness of membrane disruption. Although we did not undertake to measure the affinity constants of this series of dimers, which may span a considerable range given the variations in core hydrophobicity, the SDS-resistant behavior of these dimers suggests high affinity for all of them. It is therefore unlikely that this affinity correlates with antimicrobial activity, although the more hydrophobic peptides may associate to higher oligomers as a function of Leu residue placement, particularly in aqueous media.
Novel Conformationally Constrained CAPs Derived from 6K-F17
Neither of our attempts to significantly improve the antimicrobial activity by either intermolecular cyclization or hairpin dimerization succeeded. Cyclic (K7-D17)7K-F18-1D17 showed values equal to those of its linear precursor 7K-F18-1D17, and MIC values slightly higher than 6k-f17 with P. aeruginosa.
Although induced dimers (through formation of intermolecular disulfide bridges) of many antimicrobial peptides have shown increased activity,47 the SPGS-34 hairpin—that resembles a parallel dimer of 6K-F17 cross-linked via the C-terminals with a short loop—has the least antimicrobial activity of any “monomeric” peptide in the present study. We suspect that the monomeric state of the helix-loop-helix SPGS-34 hairpin itself, and the lack of its hetero-oligomerization in mixtures with other single “TM” CAPs on SDS-PAGE (not shown) is explicable by the presence of just a single potential SDS-resistant oligomerization face upon the nonamphipathic core helix.
Insertion of CAPs into Anionic and Zwitterionic Bilayer Membranes
As proposed earlier in a “grip and dip” model for antimicrobial action,16 the Lys residues anchor the peptides into the anionic bacterial membrane surface, and the uninterrupted core of hydrophobic residues (with amidated C-terminus) can then substantially penetrate the membrane. Given the strong dimerization properties of this category of CAPs, one can envisage an antiparallel dimer (with Lys residues maximally separated) as a powerful perturbant, disrupting the packing of lipid chains as it diffuses laterally—largely parallel to the bilayer surface—through the bacterial membrane in “submarine” fashion.
In contrast, zwitterionic membranes offer no direct route to penetration by these CAPs until CSH exceeds the “second threshold”; this is manifested by the generally increasing blue shifts of Trp fluorescence spectra as a function of increasing Leu content from one to four residues (see Fig. 2), which in turn are also broadly correlated with HPLC retention times and hemolytic activity (Fig. 3a). The data acquired from the double-Leu-substituted subset of our peptide library provide some further mechanistic insights into this process. In the latter series, evidence was also obtained of Trp insertion into the hydrophobic phase of zwitterionic lipid bilayers, but principally when one or both Leu residues are located close to the uncharged C-terminus of the sequence (Table I, Fig. 3b); hemolytic activity displayed no correlation with this process. These latter results suggest initially that in the absence of electrostatic forces (i.e., the membranes are zwitterionic, the C-terminal positions are most remote from Lys sites), reorientation of the peptide perpendicularly to the bilayer surface, with accompanying “corkscrew-like” insertion, becomes possible, while the Lys-tag of six consecutive positively charged residues remains above the surface in the aqueous phase. Insertion of peptides in this manner would likely orient them similarly to the transmembrane segments of native membrane proteins, and as such would be expected to cause less disruption to the bilayers than the parallel insertion experienced by the bacterial membrane. This latter situation probably allows many hydrophobic isomers of the present CAP series to induce minimal hemolysis even at relatively high concentrations. Peptides that have amphipathic sequences, including most natural CAPs like magainin, may bind zwitterionic membrane phosphate groups weakly through distributed cationic residues, producing a situation geometrically more similar to their parallel orientation in bacterial membranes, and hence increasing their hemolytic propensity. Interestingly, no specific correlations were observed in the present work between Leu-residue positions and hemolytic activity.
CONCLUSION
Analysis of the characteristics of a novel family of synthetic CAPs family, highlighted by a Lys-tagged N-terminus and a core sequence of hydrophobic (largely Ala and Leu residues) led to the estimation of a second hydrophobicity threshold for peptide insertion into zwitterionic membranes, and the delineation of potential pathways for the mechanisms underlying their ability to disrupt bacterial and mammalian cell membranes.
Although unequivocal correlations between positional hydrophobicity and antimicrobial activity did not emerge, the discernible differences we observed among peptide isomers provided some important insights into the manner in which primary sequence can be designed to optimize CAP antimicrobial activity.
Acknowledgements
We are grateful to Dr. Arianna Rath for helpful discussions throughout the course of this work. L.L.B. is the recipient of a CIHR New Investigator Award. E.G. held a post-doctoral award from the CIHR Strategic Training Program in Structural Biology of Membrane Proteins Linked to Disease.




