Investigation of molecular structure of bombesin and its modified analogues nonadsorbed and adsorbed on electrochemically roughened silver surface
Abstract
This work describes the molecular structure of bombesin (BN) and its analogs on the basis of the absorption infrared and Raman results described below. In these analogues is replaced one ([D-Phe12]BN, [Tyr4]BN, and [Lys3]BN) or two ([Tyr4,D-Phe12]BN, [D-Phe12,Leu14]BN, and [Leu13-®-Leu14]BN) amino acid residues within the peptide chain with a synthetic amino acid, creating antagonists to bombesin, which are useful in the treatment of cancer. It is also used surface enhanced Raman scattering (SERS) to study the differences and changes in the vibrational spectra of BN and its analogs, which were attached to an electrochemically roughened silver surface as these peptides interacted with target proteins. This work explores the use of SERS for molecules anchored to a macroscopic silver surface to interrogate the interaction of these peptides with protein receptors. The results presented here show that all peptides coordinate to the macroscopic silver surface through an indole ring and the methylene group of Trp8, the CO fragment, and an amide bond; however, the orientation of these fragments on the electrochemically roughened silver surface and the strength of the interactions with this surface is slightly different for each peptide. For example, the interaction of CH2 of [D-Phe12]BN, [Tyr4,D-Phe12]BN, [D-Phe12,Leu14]BN, [Leu13-®-Leu14]BN, and [Lys3]BN with the silver surface perturbed the vertical orientation of the Trp8 indole ring on this surface. Hence, the indole ring adopted a close to perpendicular orientation on the silver surface for BN and [Tyr4]BN, only. © 2007 Wiley Periodicals, Inc. Biopolymers 89: 506–521, 2008.
This article was originally published online as an accepted preprint. The “Published Online” date corresponds to the preprint version. You can request a copy of the preprint by emailing the Biopolymers editorial office at [email protected]
INTRODUCTION
One of the important developments during the period from 1955 to1973 was the emergence of systematic studies of the actions of peptides on the central nervous system, including psychotropic and behavioral effects. During this period, the studies of Anastasi et al. were concerned with bombesin (BN).1 This tetradecapeptide [pGlu-Gln-Arg-Leu-Gly-Asn-Gln-Trp-Ala-Val-Gly-His-Leu-Met-NH2, where pGlu is 5-oxo-proline (pyrologlutamic acid)], was originally isolated from the skins of toads, Bombina bombina and B. variegata. It is an endogenous neurotransmitter in many animals, including mammals, structurally and pharmacologically related to ranatensin and the mammalian gastrin-releasing peptides (GRP).2
BN exhibits both direct and indirect potent pharmacological effects on the central nervous system3 and the gastrointestinal tract.4 It elicits the release of other peptide hormones including insulin, glucagon, gastrin, cholecystokinin, prolactin, and growth hormone.5
Bombesin and bombesin BN-like peptides show a wide spectrum of biological activities. These include inhibition of feeding, thermoregulation, a contractive effect on the uterus, colon, or ileum,6 stimulation of gastrin release and exocrine and endocrine secretions,7 induction of gastrin cell hyperplasia, hypertensive action, and hyperglycaemic effects.8 It has also been proven that BN-like peptides are present in joint fluid in arthritis and are increased in rheumatoid arthritis9; they might also be involved in the pathogenesis of neuropsychiatric disorders, such as schizophrenia.10 Apart from the classical role of BN, it also acts as a growth factor and therefore, has cytokine activity.11 The growth of human mammary carcinoma cells is also enhanced by bombesin, which induces the synthesis of endothelins that act on stromal cells of the mammary gland in a paracrine manner.12 In serum-free medium, BN stimulates pancreatic DNA content in vivo in rats13 and proliferation of murine fibroblasts (3T3 cells) in the absence of other growth factors.14 The biological activity of bombesin as a mitogen is coupled to the activation of Ca2+-mobilizing G-proteins.15 Moreover, Kresch and others have reported that BN inhibits cell death by apoptosis in developing fatal rat lung,16 although, Salido et al. have demonstrated that BN inhibits apoptosis in several prostate cancer cell lines.17
Several reports demonstrate the presence of high concentrations of BN in pulmonary tumors.18 For example, analogues of BN have been shown to inhibit the in vitro and in vivo growth of a small-cell lung carcinoma (SCLC)19, 20 and human pancreatic tumor tissue21 xenografted to nude mice. Thus, BN has been found to be a growth factor for a number of human cancer cell lines, which has made this neurotransmitter an attractive goal for many research groups. A number of these cancers are known to secrete peptide hormones related to GRP or bombesin. Consequently, antagonists to BN have been proposed as agents for the treatment of these cancers.22
After bombesin cell receptors were established on SCLC cells, receptors were also found to be present on human breast and prostate cells.23 Relie et al. showed that the PC-3 and DU-145 human prostate cancer cell lines possess specific high-affinity receptors for BN and are suitable models for the evaluation of anti-neoplastic activity of new bombesin antagonists in the treatment of androgen-dependent prostate cancer.24 Bombesin also increases the penetration of the two human prostatic carcinoma cell lines, the relatively indolent LNCaP cells and the aggressively growing and invasive PC-3 cells, in an in vitro invasion of reconstituted basement membrane (Matrigel).25 High-affinity binding sites for GRP were found on human colorectal cancer tissue,25 suggesting that BN-like peptides may have a role in the pathogenesis of colorectal cancer, and bombesin receptor antagonists may be of value in the treatment of receptor-positive tumors. Inhibitory effects of bombesin antagonist RC-3095 and somatostatin analogue RC-160 were also seen on the growth of HT-29 human colon cancer xenografts in nude mice.26
The design of bioactive peptide derivatives has been one of the widely used approaches for the development of peptide-based therapeutic agents. The locations of the modifications that give rise to antagonists are determined by the location of the active site in the naturally occurring peptide. For example, cleavage of a peptide bond in the active site of naturally occurring linear bombesin is unnecessary for its in vivo biological activity. Introduction of a nonpeptide bond between the carboxyl-terminal and adjacent amino acid residues, or the replacement of the natural C-terminal and adjacent amino acid residues with a synthetic, β-, or γ- amino acid residue, incorporation of D-amino acids, nonpeptide bonds, CH2NH, and ester modifications, or the deletion (“des”) of the C-terminal amino acid residue or amide bond in an analogue are useful in creating or enhancing its antagonist activity.
These alterations give rise to certain peptides having improved characteristics. For example, Rivier reported work directed toward restricting the conformational freedom of the bioactive C-terminal decapeptide of bombesin by incorporating intramolecular disulfide bridges.27 Heinz-Erian et al. replaced L-histidine at position 12 (His12) in BN with D-phenylalanine (D-Phe) ([D-Phe12]BN) and observed bombesin antagonist activity in dispersed acini from guinea pig pancreas.28 Alteration of the His12 in BN, i.e. [D-Phe12]BN, [D-Phe12,Leu14]BN, and [Tyr4,D-Phe12]BN did not stimulate amylase release from guinea pig pancreatic acini when present alone, but each analogue inhibited bombesin-stimulated secretion. These results demonstrate that [D-Phe12] analogues of BN function as BN receptor antagonists and are the only bombesin receptor antagonists that interact only with the bombesin receptor. Because of their specificity, these analogues may prove useful for defining the role of BN in various physiological or pathological processes. Second, the L-norleucine in position 14 (Nle14) of this ligand is unlikely to be responsible for its high affinity, because [D-Phe1,Nle9]litorin, which has a D-Phe and Nle in equivalent positions to the hBRS-3 ligand, has low affinity. However, the presence of a penultimate phenylalanine could play an important role in combination with alterations in the other locations, because, in general, the peptides with this substitution had a higher affinity for the hBRS-3 than those with L-leucine in this position (Leu14) ([Leu14]BN).29 On the other hand, L-lysine at the three position of BN ([Lys3]BN) has the same C-terminal heptapeptide sequence as human and porcine GRP, which is very suitable for a comparison study of BN and GRP.30 Whereas, the L-methionine at 14 position (Met14) could be replaced with its D isomer with a retention of 10% biological activity, any other alteration of the C-terminus (deletions or free acid with the exception of the N-methylamide) drastically reduced the biological potency of those peptides.31
The most potent analogues of bombesin are those in which positions one to five (not included) are altered, i.e. [Tyr4]BN, [Lys3]BN, and [Tyr4,D-Phe12]BN, indicating again that the decapeptide C-terminal is sufficient for full potency.31
The NMR studies on BN do not offer evidence of its preferred structure in solution. On the other hand, the relaxation data indicate that a different mobility along its peptide chain exists. In fact, the NOE effects in the 6–13 fragment of BN demonstrate that this section is not as mobile as the N-terminus.32 This finding may reflect a tendency to a possible structuring of this part of the peptide chain, indicating that the hepta- to nonapeptide segment of the C-terminus of bombesin contains the pharmacologic message for triggering the biological responses (Trp8 and His12 are essential for biologic activity)33 and is believed to be responsible for receptor recognition.26, 34
The biological importance of the above-described neurotransmitter has motivated us to perform a number of spectroscopic studies on it. Among many molecular spectroscopy methods vibrational spectroscopy, i.e. absorption infrared and Raman, is a very valuable method for qualitative and quantitative measurements of different structural components and determination of the secondary structure of peptides and proteins.35-39 While, one of the Raman techniques, so-called surface enhanced Raman scattering (SERS), is a simple and rapid method for studying adsorption phenomena at the peptides/protein level.35, 36 This interaction is believed to be of great significance for the understanding of a mechanism of a substrate binding to its receptor. That is due to the facts1: in the presence of a solid surface, the process of protein adsorption is often energetically favorable, (2) the adsorption of proteins does not affect their binding capabilities, implying that their structures are not strongly perturbed on the surface, and (3) based on the specific interactions of molecule functional groups with respect to the surface, the geometry of adsorbed species can be deduced.36, 40-43
As is evident from the above paragraps, the biological activity of bombesin and its modified analogeues has been widely investigated. However, there are only a few reports44, 45 about they structure and no one about adsorbed structure. So, the main goals of this study is to describe in detail structure of bombesin and its modified analogues, to determine they adsorption mechanism onto the electrochemically roughened silver surface as well as to propose, based on the obtained SERS information, the reliable mechanism of bombesin binding to its receptor. It is also an attempt to correlate the observed changes in the adsorption mechanism of modified analogues with their biological activity.
Thus, the present study encompasses BN and its analogs, having the following modification: a replacement of one ([D-Phe12]BN, [Tyr4]BN, and [Lys3]BN) or two ([Tyr4,D-Phe12]BN, [D-Phe12,Leu14]BN, and [Leu13-®-Leu14]BN) (where: D-Phe12, D-phenylalanine at 12 position, Tyr4, L-tyrosine at 4 position, Lys3, L-lysine at 3 position, Leu13, L-leucine at 13 position, and Leu14, L-leucine at 14 position of the peptide chain and ®—reduced peptide bond) amino acid residues within the peptide chain with a synthetic amino acid, creating antagonists to bombesin that are useful in the treatment of cancer. These spectroscopic investigations particularly relate to adsorbed structures of the above-mentioned peptides deposited onto the electrochemically roughened silver substrates, their likely adsorption mechanisms on this surface, and changes in this process due to natural amino acid replacement. To acquire this information, the SERS technique was used, while Raman and infrared spectroscopies were used for examination of the nonadsorbed structure of these peptides. So, the infrared, Raman, and SERS spectra of BN and its six analogues are reported in this work.
EXPERIMENTAL
Neurotransmitters
BN and its modified analogues: [D-Phe12]BN, [Tyr4]BN, [Tyr4,D-Phe12]BN, [D-Phe12,Leu14]BN, [Leu13-®-Leu14]BN, and [Lys3]BN were purchased at Bachem, Switzerland. Their purity and chemical structures were proven by means of the 1H and 13C NMR spectra (Bruker Avance DRX 300 MHz spectrometer) and electrospray mass spectrometry (Finnigan Mat TSQ 700).
FT-Raman Measurements
FT-Raman measurements were performed for samples placed in a glass capillary tube. Raman spectra were recorded on a Bio-Rad step-scan spectrometer (model FTS 6000) combined with a Bio-Rad Raman Accessory (model FTS 40) and liquid-nitrogen-cooled germanium detector. Typically, 1000 scans were collected with the resolution of 4 cm−1. Excitation at 1064 nm was used from a Spectra-Physics continuum-wave Nd+3: YAG laser (model Topaz T10-106c).
FT-IR Measurements
Thin palettes containing 1 mg of each peptide dispersed in 200 mg of KBr were used for the infrared measurement. The spectra were recorded at room temperature as an average of 30 scans using a Brucker infrared spectrometer (model EQUINOX 55) equipped with a Nernst rod as the excitation source and a DT-GS detector in the 400–4000 cm−1 range with the spectral resolution of 4 cm−1.
Spectral Analysis
Second-derivatives of absorption infrared and Raman spectra, in the range of the amide I and II bands of BN and its modified analogues: [D-Phe12]BN, [Tyr4]BN, [Tyr4,D-Phe12]BN, [D-Phe12,Leu14]BN, [Leu13-®-Leu14]BN, and [Lys3]BN were calculated with the derivative function (Sav-Golay) of the Grams/AI(7.0) program from Galactic (Galactic Industries, Salem, NH).
SERS Measurements
Before adsorption of peptides, silver substrates were roughened electrochemically by three successive oxidation-reduction cycles in a 0.1M KCl aqueous solution from −0.3 to 0.3 to −0.3 V at a sweep rate of 5 mV s−1. The cycling was finished at −0.3 V, then the applied potential was changed to −0.4 V and the silver electrode was kept for 5 min at this potential; after that, the working electrode was removed at an open circuit potential and very carefully rinsed with water. The roughening was carried out in a conventional three-electrode cell with a large platinum sheet as the counter-electrode and a 0.1M KCl Ag electrode as the reference (all potentials are referred to the potential of this electrode).
SERS spectra at the macroscopic silver substrates were recorded with an ISA T64000 (Jobin Yvon) Raman spectrometer equipped with Kaiser SuperNotch-Plus holographic filters, 600 gooves/mm holographic grating, an Olympus BX40 microscope with a 50× long distance objective, and a 1024 × 256 pixel nitrogen-cooled CCD detector. A Laser-Tech model LJ-800 mixed argon/krypton laser provided excitation radiation of 514.5 nm.
RESULTS AND DISCUSSION
Infrared and Raman Studies
Figure 1 shows the infrared spectra of BN and its modified analogues [D-Phe12]BN, [Tyr4]BN, [Tyr4,D-Phe12]BN, [D-Phe12,Leu14]BN, [Leu13-®-Leu14]BN, and [Lys3]BN, while Figure 2 compares the Raman spectra of these compounds in solid state. Unfortunately, due to the very low amount of above-mentioned neurotransmitters, it was not able to measure the Raman spectra of these molecules in solution. Therefore, this study refers to the Raman spectra of BN in solution reported previously by Carmona et al.44 The solid state Raman spectra are shown in this work, since it is established and accepted that in aqueous solution many proteins, including bombesin, do not change their secondary structures,44-46 except in extreme pH conditions. As can be seen, these Raman spectra are fruitful in band numbers compared to the infrared spectra of these peptides. Additionally, it should be mentioned that the vibrational patterns of each of the seven investigated peptides are similar, although in terms of wavenumbers, shapes, band intensities, and spectral features due mainly to Phe and Tyr, there are some differences.
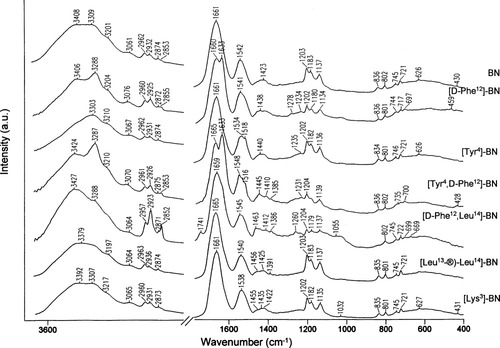
FT-IR spectra of BN, [D-Phe12]BN, [Tyr4]BN, [Tyr4,D-Phe12]BN, [D-Phe12, Leu14]BN, [Leu13-®-Leu14]BN, and [Lys3]BN in the spectral range of 3650–400 cm−1.
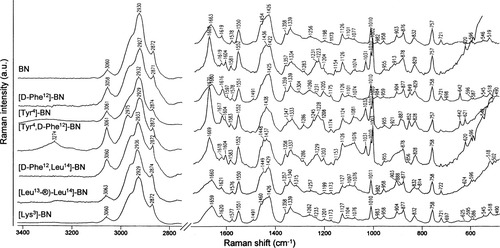
FT-Raman spectra of solid BN, [D-Phe12]BN, [Tyr4]BN, [Tyr4,D-Phe12]BN, [D-Phe12,Leu14]BN, [Leu13-®-Leu14]BN, and [Lys3]BN in the spectral range of 3400–480 cm−1. Measurement conditions: excitation wavelength, 514.5 nm; power at laser output, 100 mW.
The literature reports on vibrational spectroscopic studies of bombesin are limited.44, 47 Thus, Table I gathers detailed band assignments of the infrared and Raman spectra of the investigated peptides, mostly based on these previous Raman studies of BN43 and small proteins.35, 36 This allows all readers to clearly understand the assignment of the SERS bands discussed further in this work.
| Assignment | Wavenumbers (cm−1) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BN | [D-Phe12]BN | [Tyr4]BN | [Tyr4,D-Phe12] BN | [D-Phe12,Leu14] BN | [Leu13-®-Leu14] BN | [Lys3]BN | ||||||||||||||||
| FT-RS | FT-IR | SERS | FT-RS | FT-IR | SERS | FT-RS | FT-IR | SERS | FT-RS | FT-IR | SERS | FT-RS | FT-IR | SERS | FT-RS | FT-IR | SERS | FT-RS | FT-IR | SERS | ||
| νas(NH)bonded amines | 3408 | 3406 | 3424 | 3427 | 3379 | 3392 | ||||||||||||||||
| νs(NH)bonded amines or ν(NH)amides | 3309 | 3288 | 3303 | 3287 | 3288 | 3307 | ||||||||||||||||
| νs(NH)bonded amides | 3201 | 3204 | 3210 | 3210 | 3197 | 3217 | ||||||||||||||||
| Phe (ν2) | 3060 | 3061 | 3058 | 3076 | 3061 | 3067 | 3061 | 3070 | 3060 | 3064 | 3063 | 3064 | 3060 | 3065 | ||||||||
| νas(CH3) | 2962 | 2960 | 2962 | 2975 | 2961 | 2957 | 2963 | 2960 | ||||||||||||||
| νas(CH2) | 2930 | 2932 | 2927 | 2925 | 2932 | 2931 | 2929 | 2926 | 2933 | 2923 | 2936 | 2936 | 2929 | 2931 | ||||||||
| νs(CH3) | 2872 | 2874 | 2871 | 2872 | 2874 | 2874 | 2874 | 2875 | 2872 | 2871 | 2874 | 2874 | 2872 | 2873 | ||||||||
| νs(CH2) | 2853 | 2855 | 2853 | 2852 | ||||||||||||||||||
| AI | Random-coil | 1663 | 1661 | 1669 | 1660 | 1660 | 1661 | 1648 | 1670 | 1665 | 1641 | 1669 | 1659 | 1660 | 1665 | 1643 | 1659 | 1661 | 1647 | |||
| Antiparallel β-sheet | 1633 | 1633 | ||||||||||||||||||||
| νas(CO) in pGlu, Asn, and/or Gln, and/or δas(NH2) | 1626 | 1626 | ||||||||||||||||||||
| W1 (benzene+pyrrole ν(N1C8)) and/or Tyr | 1619 | 1619 | 1604 | 1616, 1597 | 1617 | 1604 | 1618 | 1602 | 1621 | 1620 | ||||||||||||
| Phe (ν8a) | 1604 | 1604 | 1604 | |||||||||||||||||||
| W2 and/or Phe (ν8b) | 1578 | 1592 | 1581 | 1578 | 1593 | 1583 | 1583 | 1576 | 1577 | 1595 | ||||||||||||
| W3 (pyrrole ν(C2C3)) and/or νas(CO)in pGlu, Asn, and/or Gln | 1550 | 1542 | 1562 | 1552 | 1541 | 1565 | 1551 | 1534 | 1552 | 1548 | 1563 | 1552 | 1545 | 1550 | 1540 | 1551 | 1538 | |||||
| AII and/or W4 (ν(CC)) | 1508 | 1506 | 1507 | 1508 | 1520 | 1503 | 1506 | |||||||||||||||
| di-substituted aromatic ring | 1491 | 1491 | 1518 | 1516 | 1491 | |||||||||||||||||
| W5 and ρs(CH2) | 1454 | 1454 | 1452 | 1455 | 1453 | 1448 | 1463 | 1463 | 1449 | 1456 | 1460 | 1455 | 1453 | |||||||||
| δ(CH2) | 1436 | 1438 | 1440 | 1438 | 1445 | 1435 | 1437 | 1429 | 1425 | 1442 | 1426 | 1435 | 1437 | |||||||||
| W6+(pyrrole [νs(N1C2C3) + δ(N1H)] + benzene δ(CH)), δas(CH3), and/or δ(CH2) | 1426 | 1423 | 1435 | 1422 | 1435 | 1425 | 1441 | 1410 | 1412 | 1422 | ||||||||||||
| νs(CO) in pGlu, Asn, and/or Gln | 1398 | 1399 | 1400 | 1385 | 1397 | 1386 | 1409 | 1391 | 1400 | 1400 | ||||||||||||
| W7 (indole ν(N1C8); Fermi resonance) and/or ρw(CH2) | 1358 | 1355 | 1357 | 1354 | 1359 | 1357 | 1347 | 1365 | 1358 | 1364 | 1357 | 1371 | 1367 | 1358 | 1356 | |||||||
| W7 and/or ρw(CH2) | 1339 | 1339 | 1334 | 1339 | 1333 | 1337 | 1340 | 1339 | ||||||||||||||
| δi.p.(CH) | 1315 | 1310 | ||||||||||||||||||||
| ρt(CH2) | 1304 | 1290 | 1292 | 1304 | 1291 | 1296 | 1287 | 1315 | 1294 | 1292 | ||||||||||||
| Phe (ν3) and/or Tyr | 1283 | 1278 | 1286 | 1286 | ||||||||||||||||||
| AIII, W10, and/or δ(CCαH) | Random-coil | 1256 | 1274 | 1274 | 1260 | 1276 | 1245 | 1276 | 1260 | 1257 | 1257 | 1275 | 1262 | 1275 | ||||||||
| B-sheet | 1231 | 1234 | 1231 | 1235 | 1228 | 1231 | 1229 | 1233 | ||||||||||||||
| W10 | 1245 | 1240 | 1246 | 1252 | 1243 | |||||||||||||||||
| ρtCH2) | 1225 | 1224 | 1224 | 1226 | 1223 | 1225 | ||||||||||||||||
| Tyr, His, and Phe (ν7a) | 1198 | 1203 | 1204 | 1202 | 1205 | 1202 | 1208 | 1204 | 1203 | 1204 | 1199 | 1202 | 1201 | 1202 | ||||||||
| Tyr and Phe (ν9a) | 1183 | 1180 | 1175 | 1182 | 1174 | 1179 | 1173 | 1182 | 1173 | 1182 | ||||||||||||
| δ(N1H) and/orρt(NH2) in Asn and/or Gln | 1150 | 1148 | 1150 | 1150 | 1161 | 1148 | 1149 | |||||||||||||||
| ν(CC)T alkyl chain and/or W13 | 1154 | 1153 | ||||||||||||||||||||
| 1126 | 1137 | 1136 | 1126 | 1134 | 1134 | 1126 | 1136 | 1124 | 1139 | 1126 | 1137 | 1127 | 1135 | 1127 | 1135 | |||||||
| ν(CC)T alkyl chain and/or ρt(CH2) | 1101, 1077 | 1077 | 1074 | 1072 | 1101,1074 | 1078 | 1081 | 1076 | 1084 | 1097,1076 | 1104,1076 | 1082 | ||||||||||
| Phe (ν18a) | 1031 | 1032 | 1031 | |||||||||||||||||||
| W16 (benzene andpyrrole ring breathing out-of-phase) | 1010 | 1007 | 1010 | 1005 | 1010 | 1008 | 1010 | 1010 | 1010 | 1020 | 1011 | 1004 | 1010 | 1006 | ||||||||
| Phe (ν12) | 1002 | 1003 | 1003 | |||||||||||||||||||
| ν(CC) | 955 | 963 | 963 | 963 | ||||||||||||||||||
| ν(CCO) | 922 | |||||||||||||||||||||
| ν(CC) | 982, 958, 903 | 955,913 | 984,959,904 | 955,921 | 955 | 982,958,903 | 983,958,904 | |||||||||||||||
| W17 (δ(N1H) and Fermi resonance between ring breathing and o.o.p ring bend overtone ρr(CH2) | 876 | 894 | 878 | 888 | 877 | 891 | 887 | 878 | 888,877 | 877 | 887 | |||||||||||
| ν(CC) and/or νs(CNC) secondary amide | 856 | 849 | 858 | 852 | 856 | |||||||||||||||||
| ν(CC) | 832 | 836,802 | 829 | 836,801 | 829 | 834,801 | 828 | 836,802 | 828 | 802 | 832,814 | 835,801 | 832 | 835,801 | ||||||||
| W18 and/or ν(CO) in pGlu, Asn, and/or Gln | 757 | 745 | 757 | 744 | 758 | 746 | 757 | 758 | 745 | 758 | 745 | 758 | 745 | |||||||||
| W19 | 721 | 721 | 717 | 721 | 721 | 735 | 722 | 722 | 721 | 721 | 721 | |||||||||||
| ν(CS) PC-T | 697 | 698 | 700 | 699 | 697 | |||||||||||||||||
| Tyr | 642 | 642 | ||||||||||||||||||||
| ν(CS) PH-G, His, and/or Phe (ν6b) | 626 | 620 | 626 | 621 | 620 | 624 | 625 | 627 | ||||||||||||||
| ω(COO−) and/or AVI | 596 | 596 | 595 | |||||||||||||||||||
| W | 587 | 586 | ||||||||||||||||||||
| W | 545 | 548 | 545 | |||||||||||||||||||
| τ(CO) + δ(CO) in pGlu, Asn, and/or Gln | 519 | 518 | 518 | 518 | ||||||||||||||||||
The structural information is prominently derived from the analysis of the conformation-sensitive amide bands, particularly the amide I, II, and III bands. Among them, the amide I and III bands have relatively strong Raman intensity, while amide II is IR-active. The frequencies of these bands are sensitive to the molecular geometry and hydrogen bonds formed by the peptide backbone that determine its specific arrangement, such as α-helices, β-sheets, turns, and disordered arrangements.37, 38 Usually, the nonperiodic structure (random-coil, disordered) is characterized by the amide I band in the 1660–1665 cm−1 region, the broad, less characteristic amide III band near 1243–1253 cm−1, and the amide II band occurring around 1535 cm−1. The presence of the antiparallel β-sheet conformation in a protein shows the infrared amide I band between 1615 and 1640 cm−1, while the β-plated sheet conformation of a protein is generally signaled by the amide I at the frequency of 1670–1680 cm−1, the strong band of the amide II around 1530 cm−1, and the sharp amide III feature around 1230–1240 cm−1.
BN is known to exist as a random coil structure in pure water and to adopt a helix conformation between L-Asn6 and L-Gly11 at high concentrations of 2,2,2-trifluoroethanol (TFE)48, 49 and in phospholipid bilayer.50 The disordered peptide arrangement is also adopted by BN in the solid state, as reflected in Figures 1 and 2. The strong IR (Figure 1, top trace) and broad, medium intensity Raman (Figure 2, top trace) bands at 1661 and 1663 cm−1, respectively, fall in the range that is typical of the random coil secondary structure.51 Consequently, the 1542 cm−1 infrared and the 1256 cm−1 Raman bands are indicative of the formation of this structure.
As is evident, the width of the contributing component bands within the amide regions of the BN infrared and Raman spectra is greater than the separation between the maxima of adjacent bands. As a consequence, the individual component bands cannot be resolved in these experimental spectra. A deconvolution procedure usually allows increasing separation of the overlapping components present within the broad band envelope.38, 39, 51 So, the calculated second-derivative Raman spectrum of BN shows several components at 1619, 1658, 1669, and 1688 cm−1 (Table II). The highest frequency band component at 1688 cm−1 may have the β-pleated sheet character, while the 1658 cm−1 band is due to the disordered conformation. The structural assignment of the 1669 cm−1 band is not clear, since this band falls in an overlapped frequency interval for the disordered and β-sheet structures. In fact, some of random coiled copolymers of L-and D-lysine give rise to an amide I band at 1671 cm−1, while poly(L-Ala) in the β-sheet conformation shows this vibrational mode at 1669 cm−1.52 Finally, the prominent band at 1619 cm−1 in the second-derivative BN Raman spectrum corresponds to the β-strands. The infrared second derivative spectra show the features that are consistent with the above structural details attributed to BN in the solid state.
| Assignment | Wavenumbers (cm−1) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BN | [D-Phe12]BN | [Tyr4]BN | [Tyr4,D-Phe12]BN | [D-Phe12,Leu14]BN | [Leu13-®-Leu14]BN | [Lys3]BN | ||||||||
| FT-RS | FT-IR | FT-RS | FT-IR | FT-RS | FT-IR | FT-RS | FT-IR | FT-RS | FT-IR | FT-RS | FT-IR | FT-RS | FT-IR | |
| AI | ||||||||||||||
| Turn | 1688 | 1692 | 1691 | 1697 | 1691 | 1693 | 1691 | 1688 | ||||||
| β-sheet | 1669 | 1678 | 1670 | 1670 | 1677 | 1670 | 1676 | 1670 | 1677 | 1678 | 1677 | |||
| Random-coil | 1658 | 1660 | 1656 | 1657 | 1659 | 1662 | 1658 | 1659 | 1660 | 1657 | 1658 | |||
| Random-coil | 1643 | 1644 | 1646 | 1643 | ||||||||||
| B-sheet | 1629 | 1629 | 1630 | |||||||||||
| W1 (Trp) | 1619 | 1620 | 1620 | 1616 | 1614 | 1618 | 1614 | 1620 | 1619 | 1619 | 1619 | 1618 | ||
| AII | ||||||||||||||
| Random-coil | 1548 | 1543 | 1549 | 1550 | 1548 | 1549 | 1548 | |||||||
| β-sheet | 1533 | 1523 | 1533 | 1532 | 1531 | 1531 | 1531 | |||||||
The amide I infrared absorption band of [Tyr4]BN, [Leu13-®-Leu14]BN, and [Lys3]BN centered at 1661, 1665, and 1661 cm−1, respectively, is characteristic of predominantly random coil structure and, in terms of wavenumber and band shape, is similar to the amide I band of BN (Figure 1, top trace). Also, the pattern of the amide I region in the Raman spectra of these three analogues is like that of BN. In this region, one broad, medium intensity band around 1660 cm−1 is enhanced (see Figure 2) (see Table I for detailed band frequencies). For [D-Phe12]BN and [Tyr4,D-Phe12]BN, two absorption bands at 1660 and 1633 cm−1 and 1665 and 1633 cm−1 (see Figure 1) are observed, respectively. The former spectral feature (∼1660 cm−1), as in the case of BN, is assigned to the nonperiodic structure, while the second one corresponds to the antiparallel β-sheet conformation. However, in the Raman spectra of these two analogues, only one sharp, strong intensity band at ∼1670 cm−1 (β-sheet) is enhanced (see Figure 2). This observation suggests that the [D-Phe12]BN and [Tyr4,D-Phe12]BN structures are dominated by the β-sheet conformation. In the case of the last analogue investigated in this work, [D-Phe12-Leu14]BN, the 1669 cm−1 Raman band, which shows a shape between that of BN and [Tyr4,D-Phe12]BN (see Figure 2), and the 1659 cm−1 asymmetric infrared band with a small shoulder on the lower-wavenumber side are due to the random-coil arrangement of the backbone of this peptide. The calculated second-derivative spectra of all bombesin analogues reveal several overlapping bands in the amide I range that support the above discussion. Their frequencies, together with their assignments to the proper conformation, are summarized in Table I. Briefly, the amide I bands span between 1645 and 1630, 1656 and 1662, 1669 and 1678, and 1688 and 1693 cm−1 are due to the antiparallel β-sheet, random-coil, β-plated sheet, and β-turn conformations of the peptide backbone, respectively (see Table II for detailed bands wavenumbers). While, the calculated lowest frequency band component (∼1614 cm−1) is assigned to the aromatic ring vibrations of the Trp residue, W1.
The prediction of the secondary structure for all peptides investigated in this work, on the basis of the amide II and III bands, is in agreement with the results obtained from the analysis of the amide I region. Therefore, a band at around 1548–1534 cm−1 in the all infrared spectra presented here is due to the random-coil conformation (see Table I for detailed wavenumbers). However, in this regionof the second-derivative infrared spectra, three band components are calculated at around 1550–1543, 1533–1523, and 1508–1515 cm−1. The former two bands are assigned to the random-coil and β-sheet arrangement of the peptide backbone, respectively, whereas, the third one is assigned to vibrations of the di-substituted aromatic ring of L-Tyr or L-His (a semicircle stretch).53 In addition to these, both the random-coil and β-sheet structures give rise to the amide III bands in the Raman spectra. For BN, [Tyr4]BN, [Tyr4,D-Phe12]BN, [Leu13-®-Leu14]BN, and [Lys3]BN (see Figure 2), the random-coil conformation is observed between 1262 and 1245 cm−1, whereas for [D-Phe12]BN, [Tyr4]BN, [Tyr4,D-Phe12]BN, [D-Phe12, Leu14]BN, and [Lys3]BN the ∼1231 cm−1, the spectral feature due to the β-sheet structure is enhanced.
Trp8 and His12 are the native aromatic amino acids of BN, [Leu13-®-Leu14]BN, and [Lys3]BN (see Table I for amino acid sequence). In [D-Phe12]BN, [D-Phe12,Leu14]BN, and [Tyr4,D-Phe12]BN the His12 residue is replaced by D-Phe. Additionally, in [Tyr4,D-Phe12]BN Leu4 is replaced by Tyr, similar as in [Tyr4]BN. Considering both the aromatic amino acid content of the investigated neurotransmitters and the fact that the vibrational spectra of peptides are usually dominated by spectral features due to aromatic ring vibrations, it is expected that the main differences between spectra presented in Figures 1 and 2 arise from the absence of either the Tyr, Phe, or His modes.
All peptides investigated in this work contain Trp8, which gives rise to several well-defined Raman bands listed in Table I.54, 55 With no doubt, the medium-strong intensity bands due to the indole ring system of Trp8 (W18, W17, W16, W7, W6, and W3) are observed at around 744–758, 876–888, 1010, 1339/1358, 1422–1429, and 1551 cm−1 in the Raman spectra of all peptides (see Table II for detailed bands wavenumbers), while only one of the above-mentioned modes is clearly observed, at ∼745 cm−1, in the infrared spectra of all molecules. Among the Trp Raman-active bands, one at 1339 cm−1 (W7) is sensitive to the indole ring microenvironment.56 Thus, a strong, sharp band at this frequency is usually taken as a diagnostic band for an exposed Trp residue, since its intensity exhibits significant solvent and/or pH dependence.56 Actually, this band is the lower-frequency component of a Trp doublet at 1358/1339 cm−1. All the Raman spectra presented in Figure 2 show this doublet with the higher intensity of the low-wavenumber component over the intensity of the second band from this doublet (I1358/I1339 > 1). This intensity ratio of these doublets suggests that the indole ring of the Trp8 residues is exposed to the environment. The other three characteristic Trp bands, i.e. W19 (∼721 cm−1), W2 (1576–1583 cm−1), and W1 (∼1619 cm−1), are slightly enhanced in the all Raman spectra. From these, only the former band (699–721 cm−1) is observed in the all infrared spectra presented here (see Figure 1).
The Phe Raman bands at 1583 (ν8b) and ∼1604 cm−1 (ν8a) due to the phenyl ring vibrations of [D-Phe12]BN, [D-Phe12,Leu14]BN, and [Tyr4,D-Phe12]BN overlap with the W2 and W1 vibrations of the Trp8 ring. Different Phe Raman bands are observed at 620 (ν6b), 1003 (ν12), 1031 (ν18a), 1173–1183 (ν9a), and 1198–1204 cm−1 (ν7a). Detailed assignment of these bands is given in Table I. Three of these bands (ν6b, ν9a, and ν7a) overlap with those due to the p-hydroxyphenyl ring of Tyr4.57
Other spectral feature in the spectra of [Tyr4,D-Phe12]BN and [Tyr4]BN are due to the Tyr4 residue vibrations. They are observed at 829/850, 1173–1183, 1198–1208, ∼1426, and 1619 cm−1. These bands are due to a Fermi resonance between the ring-breathing vibrations and the overtone of an out-of-plane ring bending of the p-substituted benzene ring of Tyr, in-plane CH deformation, ring-C stretching, CH ring bending, and CC ring stretching (ν8a) vibrations, respectively.58
Additionally, in the Raman spectra of BN, [Leu13-®-Leu14]BN, and [Lys3]BN, some minor bands could be observed at around 631, 1031, and 1094 cm−1, due to the imidazole ring system of the His12 residue.59 These bands are obscured by the bands of the Tyr4 residue in [Tyr4]BN as well as the stretching vibrations of the CN bonds (see Table I). In the discussed vibrational spectra, the other two His bands at around 1198–1208 and 1278–1286 cm−1 could be observed. The former one may be additionally assigned to the NH deformation of the imidozole ring (δ(NH2)).
The contribution of the aliphatic side-chain vibrations in the region between 1400 and 1800 cm−1 has been thoroughly investigated by Venyaminov and Kalnin.60 Among the proteinogenous aliphatic amino acids, only Asn, Gln, and Arg show a significant intensity in the region discussed above. The strong, broad Raman band in the wavenumber range of 1422–1460 cm−1 in the all spectra discussed here is due to the CH2 (δ(CH2)) and CH3 (δ(CH3)) group deformations. Two other bands of medium intensity at ∼1304 and ∼1339 cm−1 are present and could be due to the twisting and wagging vibrations of the CH2 group (ρt(CH2) and ρw(CH2)), respectively. They overlap with the bands of the Trp8 residue. On the other hand, bands due to the methane and methylene vibrations hardly adsorb in the infrared spectra (see Figure 1).
In the CC stretching region (800–1150 cm−1), the vibrational spectra of all peptides presented here show several bands of weak intensity (see Table I) that could be attributed to the CC as well as CN stretching vibrations. For example, a 518 cm−1 band could be due to τ(CO) + δ(CO), while a band at ∼596 cm−1 in the Raman spectra could be assigned to the COO− wagging mode (ω(COO−)) and/or amide VI mode. Likewise, a weak spectral feature around 1385 cm−1 in the infrared spectra could be due to the CO stretch (ν(CO)).
Surface-Enhanced Raman Scattering Studies
Figure 3 compares the SERS spectra of BN and its modified analogues: [D-Phe12]BN, [Tyr4]BN, [Tyr4,D-Phe12]BN, [D-Phe12,Leu14]BN, [Leu13-®-Leu14]BN, and [Lys3]BN deposited onto the electrochemically roughened silver surface. Table I lists the observed frequencies of the most important SERS bands of these spectra. Assignment to the normal coordinates of these bands is also given. This corresponds to both Raman studies of several indole compounds,61 L-tryptophane,54, 55 gramicidin A in phospholipids bilayeres,62 filamentous virus fd,63 small proteins35, 36 and SERS investigation of tryptamine immobilized on a gold electrode,64 an indole ring-terminated self-assembled monolayer on the silver electrode,65 L-tryptophan,66 L-tryptophan-containing peptides,41, 42 and proteins35, 36, 43 adsorbed on different silver surfaces.
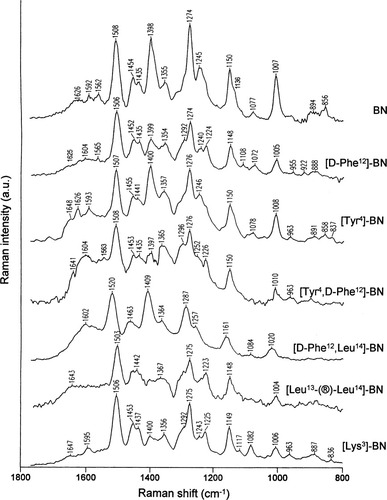
SERS spectra of BN, [D-Phe12]BN, [Tyr4]BN, [Tyr4,D-Phe12]BN, [D-Phe12,Leu14]BN, [Leu13-®-Leu14]BN, and [Lys3]BN adsorbed on electrochemically roughened silver substrate in the spectral range of 1800–800 cm−1. Measurement conditions: ∼10−4 M; excitation wavelength: 514.5 nm; power at sample ∼4 mW.
Considering the fact that the SERS spectra of peptides containing the aromatic residues usually show bands that are exclusively or predominantly due to the vibrations of aromatic amino acids,35, 36, 41-43 it is expected that in all the SERS spectra presented in this work, spectral features due to aromatic amino acid vibrations are enhanced. As mentioned earlier, BN, [Leu13-®-Leu14]BN, and [Lys3]BN contain the Trp8 and His12 residues in their backbones, whereas [D-Phe12]BN and [D-Phe12,Leu14]BN consist of Trp8 and D-Phe12. The remaining two analogues, [Tyr4]BN and [Tyr4, D-Phe12]BN, include Tyr4, Trp8, and His12 and Tyr4, Trp8, and D-Phe12, respectively. Thus, bands due to proper residue vibrations may determine the pattern of the SERS spectrum of the proper analogue, under the requirement that a given residue interacts with the silver surface. However, as is evident from Figure 3, the SERS patterns of all the spectra show many similarities in regard to the observed bands and their relative intensities. This may suggest that only the Trp8 residue, the single common aromatic residue of all peptides investigated in this work, influences the SERS spectra of these peptides immobilized onto the electrochemically roughened silver surface. Again, this points out that Trp8 of all the peptides interacts with the macroscopic silver surface. In the adsorption of these Trp-containing peptides, one must consider the possibility of the indole ring-metal interaction. The indole ring has two potential adsorption sites, the ring π-system and the electron lone pair of nitrogen in the ring. The evidence for or against the indole ring-metal surface interaction may be found from the indole ring mode pattern.
The absence or very weak intensity of bands due to the Tyr4, His12, or D-Phe12 vibrations indicates that these side-chain groups are neither enhanced or weakly enhanced by the distance effect67 nor by the aromatic ring orientation on the silver surface68 (the orientation for the vibrations of the aromatic rings ofTyr4, His12, or D-Phe12 would have weak (close to zero or zero) components of atomic movement normal to the surface, so their stretches would receive very little amplification).
In analysis of the presented SERS spectra, particularly important are the Raman bands at 1010 and 1550 cm−1 due to the out-of-phase benzene and pyrrole ring breathing (W16) and the pyrrole ring stretching vibration ν(C2C3) localized primarily at the C2C3 bond (W3), respectively. These two bands appear at 1007 and 1562 cm−1 in the BN SERS spectrum (Figure 3, top trace). The first-mentioned spectral feature decreases in intensity and slightly shifts (Δ = −3 cm−1) from its position in the ordinary Raman spectrum, while the second one shifts by 12 cm−1 to higher wavenumbers. In addition, the band width (full width at half maximum, FWHM) of this band increases from 17 to 22 cm−1. Hence, it can be concluded that the indole ring of Trp8 strongly binds through its pyrrole ring to the macroscopic silver surface. It is worth pointing out that the orientation of the indole ring relative to the peptide backbone may be determined based on the frequency of W3. The frequency of W3 varies as a function of the absolute value of the CαCβ-C3C2 torsional angle (χ2,1), which produces limitations on the possible ring orientations (the 60–120° range).69 According to the relationship and in comparison with other Trp containing-peptides, the average (χ2,1(angle for the tryptophan side chain of BN in the solid state seems to be 99° and (120° for BN adsorbed on the electrochemically roughened silver surface.
The SERS spectra of only two of the investigated analogues, [D-Phe12]BN and [Tyr4,D-Phe12]BN, show the W3 band at 1565 and 1563 cm−1, respectively. Similar to BN, the relative intensity substantially decreases and the position shifts up (Δ = 13 and 11 cm−1, respectively) in these SERS spectra (see Figure 3) in comparison to the corresponding Raman spectra (see Figure 2). Hence, it can be concluded that the Trp8 ring of [D-Phe12]BN and [Tyr4,D-Phe12]BN is slightly removed from the silver surface. Because W3 for the bombesin analogues is either hidden under the bands envelope or is hardly enhanced, it is impossible to precisely measure its FWHM and determine the indole ring orientation relative to the amide backbone for the proper peptide.
On the other hand, the W16 mode in the SERS spectra of all analogues immobilized onto the electrochemically roughened silver surface is enhanced (see Table I for detailed band positions). No substantial shift of this band (Δ = 0–4 cm−1) is noticed for [Tyr4,D-Phe12]BN, [Tyr4]BN, [Lys3]BN, and [D-Phe12]BN, while its 7–10 cm−1 position movement is observed for [Leu13-®-Leu14]BN and [D-Phe12,Leu14]BN. This could suggest that some changes in the interaction of Trp8 with the silver surface occur when the Met14 residue is replaced by Leu14. These changes probably cause preferential binding of Trp8 to the silver surface for [Leu13-®-Leu14]BN and [D-Phe12, Leu14]BN. The band broadening of this mode observed in the SERS spectra of all peptides investigated in this work, in comparison to its FWHM in the corresponding Raman spectra, confirms the above statement for BN, that the π-electron system of Trp8 directly interacts with the electrochemically roughened silver substrate. However, the strength of this interaction is different for each peptide. Comparison of the relative intensity of the W16 band between the SERS spectra presented in Figure 3 shows that its intensity decreases in the direction: BN > [Tyr4]BN > [D-Phe12]BN ≈ [Tyr4,D-Phe12]BN ≈ [D-Phe12,Leu14]BN ≈ [Leu13-®-Leu14]BN ≈ [Lys3]BN. Additionally, the SERS relative intensity for BN seems to be comparable with that exhibited in ordinary Raman spectra of bombesin and its analogues. These facts indicate a slightly different orientation of the Trp8 ring on the macroscopic silver surface for these peptides. Hence, it can be concluded that the indole ring of BN adopts an almost perpendicular orientation on the electrochemically roughened silver surface, whereas for [Tyr4]BN, it leans slightly out from this position. In turn, the indole rings of [D-Phe12]BN, [Tyr4,D-Phe12]BN, [D-Phe12,Leu14]BN, [Leu13-®-Leu14]BN, and [Lys3]BN are somewhat more tilted with respect to the silver surface than that of [Tyr4]BN.
Most of the other Trp8 ring modes, such as those at 1126 (W13), 1256 (W10), 1358 (W7), and 1578 cm−1 (W2) in the ordinary Raman spectrum of BN (Figure 2, top trace) show shifts upon adsorption on the macroscopic silver surface, appearing at 1136 (shoulder), 1245, 1355, and 1592 cm−1, respectively, in the BN SERS spectrum (Figure 3, top trace). Among these, the W2 (benzene vibrations in the indole ring) band is hardly enhanced, while W13, W10, and W7 are of medium intensity. This supports the previous statement that the indole ring of BN interacts with the silver surface mainly through its pyrrole ring. At similar frequencies, these modes are enhanced in the SERS spectra of the remaining peptides immobilized on the macroscopic silver surface (see Table I). Also, they show comparable relative intensities to those exhibited in the BN SERS spectrum; however, small differences can be noted.
The enhancement of the higher-wavenumber component of W7 and a lack of its lower counterpart in the SERS spectra of bombesin and its six analogues (see Figure 3) indicates that the Trp8 ring is in contact with the aliphatic side-chain when the peptides interact with the electrochemically roughened silver surface. This means upon adsorption the indole ring changes arrangement from exposed to buried in the globin.
Now, four ring modes, which contain substantial contributions from the indole ring NH motion will be considered. These correspond to the bands at 876 (W17), 1173 (δ(N1H)), 1426 (W6), and 1619 cm−1 (W1) in the ordinary Raman spectrum of BN (Figure 2, top trace). These bands are shifted to ∼894, 1150, 1435, and ∼1600 cm−1, respectively, in the BN SERS spectrum (Figure 3, top trace), indicating a direct interaction between the indole ring nitrogen lone pair and the macroscopic silver surface. For [D-Phe12]BN, similar bands appear at 888, 1435, and 1604 cm−1 in the SERS spectrum. In the SERS spectra of [Tyr4]BN, [Lys3]BN, and [Tyr4,D-Phe12]BN, two NH containing ring modes are observed, appearing at 1441 and 891 cm−1, 1437 and 887 cm−1, and 1604 and 1435 cm−1, respectively, close to the bands in the SERS spectrum of BN. Hence, the indole ring nitrogen atom of [D-Phe12]BN, [Tyr4]BN, [Lys3]BN, and Tyr4,D-Phe12]BN is also bound to the silver surface. On the other hand, the indole ring nitrogen atom of [D-Phe12, Leu14]BN and [Leu13-®-Leu14]BN does not seem to interact directly with the metal surface. With [D-Phe12,Leu14]BN, the 1602 cm−1 band in the SERS spectrum is hardly marked. For [Leu13-®-Leu14]BN, a band appears at 1442 cm−1 in its SERS spectrum. Hence, substitution of Met14 by Leu14 in these two analogues produces changes in the indole ring interactions with the electrochemically roughened silver surface. Such spectral specificity for the amino acid replacement in these two peptides probably arises from the lack of the sulphur atom on the C-termini. It is well known that sulphur atoms possess high affinity to silver surfaces and preferentially bind to it (unfortunately, due to the experimental conditions, it was not possible to measure SERS spectra on the electrochemically roughened silver surface below 800 cm−1). Hence, these differences could be due to changes in the proximity of the indole ring nitrogen atom to the surface. This difference in the behavior of [D-Phe12,Leu14]BN and [Leu13-®-Leu14]BN on the silver surface in comparison to the behavior of other peptides investigated here is in agreement with study of the biological activity, which shows that Met14 could only be replaced with its D isomer with a 10% retention of biological activity, while any other substitutions drastically reduce the biological potency of those peptides.31
So, major vibrations could be assigned to Trp,8 with minor stretches originating from both the CO fragment of pGlu1, Asn6, or Gln2,7 and the amide bond(s). However, the acidic groups are not expected to adsorb strongly on a metal surface, due to less charge-transfer character of the enhancement, which further decreases the signal from these groups. Indeed, only the CO stretching vibrations give the most intense SERS bands.
On going from the solid (Figure 2, top trace) to the silver surface-bound (Figure 3, top trace) state of BN, significant spectral changes occur. The SERS spectrum of BN adsorbed onto the electrochemically roughened silver surface is dominated by three bands, at 1508, 1398, and 1274 cm−1. Among these, only the former band is due to the vibrations of the Trp residue (W4). It is only slightly enhanced in the Raman spectrum of BN and moves from 1491 cm−1 for solid to 1508 cm−1 for adsorbed BN. Its strong SERS enhancement could indicate that the indole ring of Trp8 directly interacts with the macroscopic silver surface, whereas the 17 cm−1 upshift could point out that this interaction goes through the dearomatized CC bond(s). However, W4 exhibits a similar enhancement not only for BN, but also in the case of the other six peptides (see Table I for bands position), whilst previously it was stated that these peptides interact with the macroscopic silver surface in a very similar, but not identical, fashion. Hence, its high relative intensity should be explained in another way. Actually, it has been reported that the amide II vibrations should also be expected around this frequency (1488–1518 cm−1).70 The calculated frequencies of the amide II mode of L-phenylalanine-containing dipeptides were substantially downshifted in comparison to that observed in the corresponding Raman spectra, which the Author ascribed to changes in the bond order of the CANA and CAOA bonds of the amide moiety (∼1.5).70 This could explain the occurrence of the very strong band around 1508 cm−1 in the SERS spectra of all peptides, and also the absence of the amide I band (mainly ν(CO)). Based on these data, it is reasonable to propose that the 1508 cm−1 band of very strong relative intensity observed in the SERS spectra of bombesin and its analogues deposited on the electrochemically roughened silver surface could be a result of overlapping of W4 and the amide II modes. Additionally, it could suggest that the amide bond(s) directly interact with the macroscopic silver surface. The broadness of this band (24 cm−1) may support this conclusion.
The second of the most intense bands of the BN SERS spectrum, 1398 cm−1, is due to the symmetric stretching vibration of the CO fragment of the pGlu1, Asn6, or Gln2,7 residues. Its relative intensity is comparable to the intensity of the 1508 cm−1 spectral feature. This enormous enhancement and broadness (FWHM = 30 cm−1) points out that the oxygen lone electron pair of the CO bond shows high affinity to the electrochemically roughened silver surface and directly and strongly binds to it. In the SERS spectra of all of the analogues investigated here, the ν(CO) mode is also enhanced (see Figure 3). However, its intensity substantially differs between the spectra. For [Tyr4]BN and [D-Phe12, Leu14]BN adsorbed on the macroscopic silver surface, this band is considerably enhanced at 1400 and 1409 cm−1, respectively, which states that the CO unit of these two analogues strongly interacts with this surface, similar to BN. The frequency of this band for [Tyr4]BN is very close to that for BN (Δ = 2 cm−1), while [D-Phe12,Leu14]BN is shifted by 11 cm−1 in comparison to that of BN. These differences in the band positions could suggest that the CO fragment of [D-Phe12,Leu14]BN directly interacts with the silver surface, like BN, whereas the CO unit of [Tyr4]BN is slightly dismissed from this surface. In the SERS spectra of the other four analogues, [D-Phe12]BN, [Tyr4,D-Phe12]BN, [Leu13-®-Leu14]BN, and [Lys3]BN, the ∼1399 cm−1 (see Table I for detailed band positions) exhibits medium-low intensity, suggesting direct but relatively weak interactions between CO and the silver surface, probably due to the distance effect.
As mentioned above, the most obvious difference between the Raman and SERS spectra of bombesin and its analogues is a lack of an amide I band near 1663 cm−1 (disordered structure), which is usually the most prominent spectral feature in a peptide spectra, and a strong enhancement of the ∼1274 cm−1 band (see Figure 3). The absence of the amide band could indicate that only side-chain groups are enhanced. However, it is unlikely that all of the peptide backbone was oriented parallel to the electrochemically roughened silver surface or was too far away from this surface to be enhanced. However, the exclusive enhancement of the side groups may be a consequence of specific adsorption of these side groups on the macroscopic silver surface, generating a charge-transfer character of enhancement. If no such adsorption occurred between the molecules forming the amide bonds of the backbone and the silver surface, no enhancement of this character would result, even if the backbone was in close proximity to this surface. Probably, the specific adsorption of the investigated peptides on the electrochemically roughened silver surface induces changes in the electron distribution of the amide bond. As mentioned earlier, it is likely that upon adsorption, the bond order of the CO bond of the CONH fragment decreases. This is why the amide I, due mainly to ν(CO), is not observed in the SERS spectra of all investigated molecules adsorbed onto the macroscopic silver surface.
The enormous observed increase of the relative intensity of the amide III vibrations (∼1274 cm−1) indicates that the amide bond of the peptide binds to the electrochemically roughened silver surface. Additionally, the movement of its position from 1256 to 1274 cm−1 in the BN SERS spectrum, the frequency that is characteristic for the α-helical conformation of the peptide backbone, could suggest a direct interaction between the amide bond and the silver surface, and that the amide bond(s) are strongly hydrogen bonded, resulting in the high wavenumber amide III modes. An optional explanation of such a huge upper shift (Δ = 18 cm−1) is the possibility of changes in the BN secondary structure upon adsorption on the macroscopic silver surface, as it takes place for BN attached to the phospholipid bilayer.58 This could be supported by the enhancement of the weak amide I band at around 1641–1648 cm−1 and by stronger enhancement of the higher-wavenumber W7 band (the indole ring buried in the globin). However, at the moment, this explanation is questionable.
In the SERS spectra of the six analogues of bombesin, the 1287 cm−1 spectral feature is also enhanced at similar frequencies (1274–1287 cm−1). However, its relative intensity decreases for all analogues in comparison to the relative intensity exhibited for BN. Careful analysis of the SERS spectra shows that relative intensity of this band decreases in the direction: BN > [Tyr4]BN ≈ [Tyr4,D-Phe12]BN ≈ [Lys3]BN >[D-Phe12]BN ≈ [D-Phe12,Leu14]BN ≈ [Leu13-®-Leu14] BN. This finding indicates that bombesin analogues, like BN, bind directly to the electrochemically roughened silver surface, although the strength of this interaction is slightly weaker for the analogues than for BN and decreases in the same direction.
Besides the indole ring, CO bond, and amide bond bands, a few bands assignable to the methylene group vibrations are seen in the SERS spectra presented in Figure 3. In the BN SERS spectrum, a shoulder at 1290 cm−1 is seen on the tail of the strong band at 1274 cm−1 due to the CH2 twisting mode. Because bands due to the indole ring of Trp8, CO group, and amide bond vibrations are predominantly enhanced in the SERS spectra for bombesin and its analogues, it seems rational to propose that most of the intensity of the 1290 cm−1 shoulder is certainly ascribed to the methylene group vibration of the Trp8 residue. Around this frequency in the SERS spectra of the remaining peptides, the 1292–1296 cm−1 is seen, except in [D-Phe12,Leu14]BN. It is slightly more enhanced for [D-Phe12]BN, [Tyr4,D-Phe12]BN, [Leu13-®-Leu14]BN, and [Lys3]BN than for [Tyr4]BN and BN. This phenomenon points out that the CH2 unit of Trp8 disturbs the vertical orientation of the indole ring of [D-Phe12]BN, [Tyr4,D-Phe12]BN, [Leu13-®-Leu14]BN, and [Lys3]BN on the macroscopic silver surfaces. That is why the indole ring of [Tyr4]BN and BN may more easily “stand up” on this surface.
Also, another common feature that possesses the CH2 character is enhanced at 1452–1463 (the CH2 scissoring deformations) and 1072–1084 cm−1 (the CH2 twisting deformations) in the SERS spectra of all investigated peptides adsorbed on the electrochemically roughened silver surface, pointing out that, in fact, this group participates in the peptide-silver surface interaction. This interaction is supported by slight broadening and shifting of the frequencies for these modes.
CONCLUSIONS
The results described above demonstrate the feasibility of using SERS spectroscopy to probe protein-metal interactions that mimic the mechanism of substart binding to its receptor. The SERS spectra of BN and its analogues adsorbed on the electrochemically roughened silver surface differ considerably from their Raman spectra and exhibit features that can be used to identify the specific residues in the adsorbed proteins. By analyzing the position and brodness of the enhanced bands due to vibrations of these specific molecular fragments the adsorption mechanism (substrate/receptor mechanism) on this surface was proposed for BN and its analogues. The observed changes in the adsorption mechanism were correlate with their biological activity.
The SERS spectra of BN and its six analogues, [D-Phe12]BN, [Tyr4]BN, [Tyr4,D-Phe12]BN, [D-Phe12,Leu14]BN, [Leu13-®-Leu14]BN, and [Lys3]BN, deposited onto the electrochemically roughened silver surface show bands due to vibrations of the molecular fragments that were in close contact with this surface. These include the Trp8 ring π-system, the electron lone pair of nitrogen in this ring, and the methylene moiety of Trp8, CO bond, and amide bond. Because vibrations of these fragments are predominantly enhanced in the presented SERS spectra, it seems reasonable to allocate the amide bands and carbonyl fragment to the vibrations of the amide bond between the Gln7 and Trp8 residues and the CO fragment of Gln7, respectively.
The carbonyl group of BN, [Tyr4]BN, and [D-Phe12, Leu14]BN is attached to the macroscopic surface, while that of [D-Phe12]BN, [Tyr4,D-Phe12]BN, [Leu13-®-Leu14]BN, and [Lys3]BN is slightly removed from this surface.
It was shown that the indole ring of BN binds to the macroscopic silver surface mainly through a dearomatized pyrrole co-ring and nitrogen atom. It almost “stands up” on this surface, causing the weakening of the interaction between the CH2 group of Trp8 and the silver surface. In the case of the bombesin analogues[D-Phe12]BN, [Tyr4]BN, [Tyr4,D-Phe12]BN, and [Lys3]BN, their Trp8 residue also interacts with the silver surface via the dearomatized co-pyrrole ring and nitrogen lone electron pair as well as the methylene group, whereas the Trp8 of [Leu13-®-Leu14]BN and [D-Phe12, Leu14]BN is attached to the macroscopic silver surface through the dearomatized indole ring and methylene unit. The indole ring for all analogues, except [Tyr4]BN, adopts a tilted orientation on the silver surface, while [Tyr4]BN only slightly leans out from the position characteristic for bombesin. Additionally, upon adsorption the indole ring changes its arrangement from exposed characteristic for soild state to buried in the globin.
Also, it seems that the secondary structure of bombesin and its six analogues change upon adsorption onto the electrochemically roughened silver surface, from a disordered structure, which is characteristic for the solid state, to the α-helical structure. The huge up-shift of the amide III band and movement of the amide I band to the lower wavenumber could make this hypothesis convincing; however, it could be questionable.




