Conformational transition and liquid crystalline state of regenerated silk fibroin in water
Abstract
The conformational transition of molecular chains of regenerated silk fibroin (SF) aqueous solution is systematically investigated by circular dichroism, Raman, IR, and UV–vis spectroscopies. It is found that an initial random coil conformation of the SF can be readily changed into an ordered β-sheet structure by optimizing the solution conditions, such as the SF concentration, pH, temperature, or metal-ion content. Circular dichroic spectra quantitatively confirm a steadily decreased content of the random coil conformation but a significantly increased β-sheet content after an ultrasonic or extruding treatment. Furthermore, the extrusion is more powerful to achieve high β-sheet content than the ultrasonic. It is interesting that the polarized optical micrographs of the SF aqueous solution extruded by injection illustrate the formation and existence of liquid crystalline state. A study of extrusion in vitro could be used as a model system to understand the natural silk spinning process in silkworm. © 2007 Wiley Periodicals, Inc. Biopolymers 89: 497–505, 2008.
This article was originally published online as an accepted preprint. The “Published Online” date corresponds to the preprint version. You can request a copy of the preprint by emailing the Biopolymers editorial office at [email protected]
INTRODUCTION
Silk fibroin (SF) is one of the most familiar and useful proteins around us. The study on its structures and properties has a long history.1 Various fibroin-type proteins exist in natural silks, among them Bombyx mori silkworm silk has been extensively investigated.2, 3 The primary structure of its crystalline region largely consists of repetitive hexameric units: (Gly-Ala-Gly-Ala-Gly-Ser).4 It has been accepted that there are two distinct structural forms of the crystalline domains in Bombyx mori SF, namely: silk I and II. The organized crystalline form (silk I) of the β-sheet precursor found in spinning dope may adopt multiple local secondary structures before transforming to silk II. A careful perusal of the spectroscopic and crystallographic data for silk I suggests that at least two distinct polymorphs are observed and reported as “silk I,”5, 6 while the silk II form is characterized by an antiparallel β-sheet structure.7, 8
During the natural silk spinning from the spinneret of silkworm, the conformation of SF converts from random coil or α-helical to β-sheet. Although it has been reported that the impressive mechanical properties of the silk are closely related to the primary amino acid sequence, the silk spinning conditions, such as pH, temperature, metal ion, SF concentration, and shear stress of the SF solution, are possibly more important.9-11 Indeed, it is interesting to note that a silkworm or spider produces such strong fibers merely at ambient temperature and pressure from weakly acidic aqueous solution and in the presence of a small amount of metal ions.12-14 It has been known that the mechanism of fiber formation from the liquid silk in the silk gland of the silkworm is unique for nearly a century.15-23 It is clear that during the process of spinning, i.e., the liquid silk moving from the posterior to the anterior in silk gland, the SF concentration rises significantly from 12 to 30 wt%, accompanying with evaporation of water as well as transition of SF conformation simultaneously.23 There are several studies on spectroscopy to describe secondary structure of silk protein in solutions, fibers, or films from silkworms.15-18 Little report of the formation of the liquid crystalline state of the regenerated SF aqueous solution was found till now.20-22
In this article, circular dichroic (CD) spectroscopy has been used to analyze the secondary structure transition induced by pH, temperature, metal ions, solution concentration, ultrasonic treatment, and extrusion (shear stress), in Bombyx SF solutions. The CD spectra suggest that both ultrasonic shaking and extrusion could induce a remarkable conformational transition from random coil to β-sheet, especially the extrusion is much more powerful. In particular, repetitive shearing action would strongly promote the formation of the liquid crystalline state of the regenerated SF aqueous solution. The possible significance of these findings to the silkworm spinning mechanism is elaborated.
METHODS
Preparation of the Regenerated SF Aqueous Solution
Commercially supplied cocoons of Bombyx mori were boiled for 30 min in an aqueous solution of 0.05M Na2CO3, and then rinsed thoroughly with distilled water to extract the gluelike sericin proteins and wax. The extracted SF was dissolved in a 9.3M LiBr solution and slightly stirred at 60°C for 4 h. This solution was filtered with a paper filter to remove residues, and dialyzed with cellulose tubes (molecular weight cut-off 12,000–14,000) in pure water for 3 days to remove the salts, until an AgNO3 test could detect no trace of bromide ion, and then clarified by spinning in a centrifuge at 3000 rpm for about 4 min. The supernatant of the SF aqueous solution was collected. The fibroin concentrations considered in this study range from about 0.05 to 5 mg mL−1, which was determined by weighing samples before and after totally drying to constant weight at 100°C. There is almost no sericin in the regenerated silk because all sericin should be removed by boiling for 30 min in an aqueous solution of 0.05M Na2CO3, and then rinsed thoroughly with distilled water to extract the gluelike sericin proteins and wax. Ubbelodhe viscosimetry has been used to evaluate the molecular weights of regenerated and original SFs in the spinneret of silkworm. It is found that there is no substantial difference between the molecular weights of the regenerated and original SFs. The fibroin was stored at 4°C for around 10 days.
Thin SF films for IR spectral study were formed by simply dropping the mother solution onto the glass substrates and dried at room temperature. To study the effect of ultrasonic wave on the secondary structure of SF, 15 mL of 0.1 mg mL−1 SF solution was placed in ultrasonic oscillator for ultrasonic wave shaking at frequency 30 kHz for different times. While for the effect of shearing force on the secondary structure of SF, 3 mL of 0.1 mg mL−1 SF solution was repeatedly injected by first sucking and then extruding at the same rate of 4 mm s−1 through the aperture of a 0.6-mm diameter tube, mimicking the duct in the natural silk spinning process. One cycle from sucking to extruding means one-time extrusion.
Measurements
Circular Dichroism Analysis
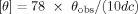
Fourier transform infrared (IR) transmission spectra of the SF films that were prepared by simply dropping the SF solution onto the substrate and dried very quickly with JEOL2000 (Nihon Denshi) Spectrophotometer and UV–vis spectra of SF solution with a UV-2401 (Shimadzu Japan) Spectrophotometer were obtained to investigate the secondary structure. Raman spectra were recorded using a Renishaw 1000 Raman microscope from injected SF solution that had been glued onto the appropriate viewing frames. A He-Ne laser was used to give 1 mW of energy at 632.8 nm red line. The laser beam was polarized to the solution surface. A PXSVI-E zoom stereomicroscope that was fitted with crossed polaroids (made in Shanghai China) was used to observe the liquid crystalline phenomenon of the SF aqueous solution.
RESULTS AND DISCUSSION
Effect of SF Concentration on Conformational Transition
Figure 1a shows the CD spectra in an SF concentration range from 0.05 to 0.3 mg mL−1. It is seen that four curves present the occurrence of a negative Cotton effect at around 195 nm, indicating that the solution is composed predominantly of a random coil conformation.24, 25 However, with increasing SF concentration, a new negative Cotton effect gradually appears at 217 nm (Figure 1a blue and red curves), which ischaracteristic of β-sheet structure.24, 25 It is found from Figure 1b that the content of random coil decreases with an increase in SF concentration, while the content of α-helix (the result of intramolecular hydrogen bonds) and β-sheet (the result of intermolecular hydrogen bonds) increases. This is evidence that the conformation of SF transits from random coil in dilute solution to β-sheet in concentrated solution. It can be inferred that increasing concentration would make the SF macromolecular chains closer to each other due to hydrophobic interactions and hydrogen-bond effects. Finally, an ordered rearrangement of the SF macromolecules occurs.
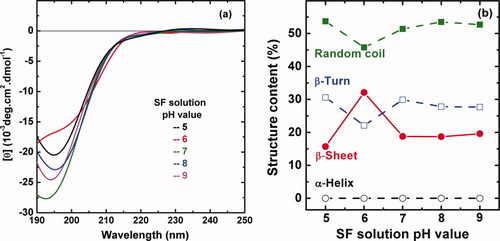
The effect of regenerated silk fibroin (SF) concentration on the conformational transition of SF aqueous solution. (a) The CD spectra of regenerated SF solution of four concentrations at 18°C and pH = 6.82; (b) the structure content in SF solution at different concentrations calculated by the empirical Yang equation.24, 25
By the way, Figure 1b suggests that the regenerated SF contains 10–20% β-sheet and 30–20% β-turn, depending on its concentration. However, the total amount of β-conformation varies slightly from 42.3% to 47.8% that is lower than the random coil content of 49.0%–57.9%. This high β-conformation in the quiescent regenerated silk solutions has been further verified by IR spectroscopy (see Section 6).
Effect of Alkali Metal Ions on Conformational Transition
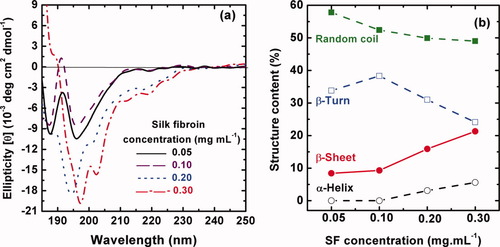
The effect of three alkali ions, Li+, Na+, K+ with the same Cl− anion, on the CD spectra of regenerated SF solution is shown in Figure 2a. The alkali ion concentration is fixed at 10 mM since this value is similar to the salt concentration inside the silk gland estimated for the dilute solution of native SF.26-29 It is seen that all of the CD spectra illustrate a negative Cotton effect around 191–199 nm, confirming the predominance of the random coil conformation,24, 25 but the ellipticity weakens gradually in the order of Na+ > no ion > K+ > Li+. Note that the β-sheet content calculated by the Yang equation24, 25 in Figure 2b increases in the order of no ion < Na+ < Li+ < K+. So K+ produces the strongest effect on inducing the conformational transition of SF. This is supported by the assumption that the K+ ion played an important role in the spinning process of silkworms.26 It is reported that as the silk dope passes through the spinning duct, the addition of K+ ion to dilute dope solution produces a spontaneous formation of nanofibrils.26 The nanofibrils would not be induced by Na+, Ca2+, or Mg2+ of the same concentrations. The conformational transition of SF induced by the metal ions would be ascribed to an electrostatic effect: an interaction between the ions and SF chains because SF is an amphoteric electrolyte. As a result of the electrostatic effect, the initial local movement of a large amount of the macromolecular segments will result in final ordered rearrangement of the whole macromolecular chains. These results are supported by a previous study on the effect of LiBr and Na2CO3 on the chain conformation.30
Effect of alkali metal ions on the conformational transition of silk fibroin aqueous solution at 0.1 mg mL−1 at 18°C and pH = 6.82. (a) CD spectra of SF solution with Li+, Na+, and K+ at the same concentration of 10 mM; (b) the structure content in SF solution calculated by the empirical Yang equation.24, 25
Effect of SF Solution Temperature on Conformational Transition
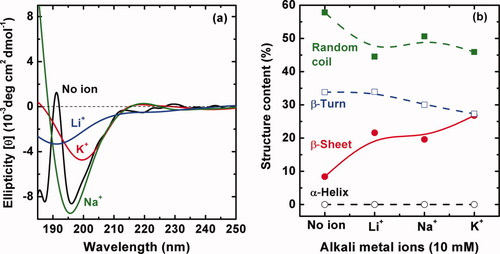
CD spectra in Figure 3a show the conformational transition of SF with the temperature from 20 to 85°C. The CD spectrum of freshly prepared aqueous solution of SF at room temperature 20°C shows the occurrence of a negative Cotton effect around 195 nm, indicating that the solution was composed predominantly of a random coil component. However, with the temperature rise, the negative Cotton effect gradually weakens, while a new small negative peak at 217 nm increases simultaneously that is characteristic of β-sheet structure, although all of these changes are minor. According to the results calculated by the Yang equation24, 25 in Figure 3b, the content of random coil decreases first and then increases with elevating temperature, and the content of β-turn decreases first and then hardly ever increases. Their contents seem to be the minimum at 65°C. In contrast, the content of β-sheet displays the maximum at 65°C. It is especially important to note that the content of α-helix seems to keep constant. That is to say, the conformational transition of the SF chains with elevating temperature mainly occurs among the random coil, β-turn, and β-sheet.
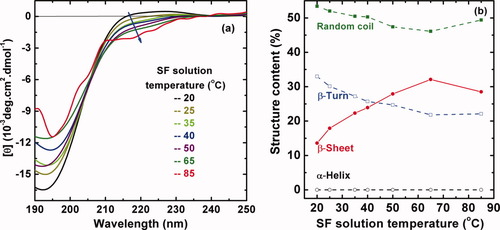
Temperature effect on the conformational transition of 0.1 mg mL−1 silk fibroin aqueous solution. (a) The CD spectra recorded at different solution temperatures of the samples equilibrated at each temperature for 30 min; (b) the content of different structures in SF solution calculated by the empirical Yang equation.24, 25
The UV spectroscopy of Tyr and Trp in SF aqueous solution (Figure 4) was also evaluated under different temperatures in order to investigate the conformational changes and assess the overall microenvironment of the Tyr and Trp residues. There are three absorption peaks centered at 236 nm (1st, sharp), 275 nm (2nd, broad), and 282 nm (3rd, sharp) in Figure 4 at 21°C, which should be contributed to protein chromophore: indole in Trp and phenolic group in Tyr residues. With elevating temperature from 21 to 30°C, the UV spectrum hardly ever changes. However, upon further elevating temperature from 30 to 45°C, an obvious change of UV–vis spectra has been observed, i.e., a slightly hypsochromic shift of the 1st peak to 235 nm and a clear decline and merge of the 2nd and 3rd peaks. After that, the UV–vis spectra change slightly with continuously increasing temperature from 45 to 85°C. That is to say, the electronic structure of the SF macromolecules remains constant in 21–30°C, exhibits a sudden change between 30 and 45°C, and remains nearly constant again in 45–85°C. It could be speculated that the origin of the structural change implied by the decreased UV band around 280 nm might be assigned to weakening π–π* (transition of the indole ring in Trp and phenolic ring in Tyr residues. A correspondent decreased β-turn content but increased β-sheet content have been observed in the CD spectra as the temperature changes over the same range in Figure 3. A sharp change of the absorbance and characteristics of the UV–vis spectra between 30 and 45°C strongly indicates that the conformation of SF in dilute aqueous solution was thermally unstable. The influence of temperature on the conformational transition of the proteins should be realized most likely by providing energy to speed up the transition process because the conformational transition is kinetically driven. Higher temperature would accelerate the movement of water molecules and SF macromolecular chains and thus increase the opportunity of the molecular collisions, and finally lead to faster formation of β-sheet structure.30
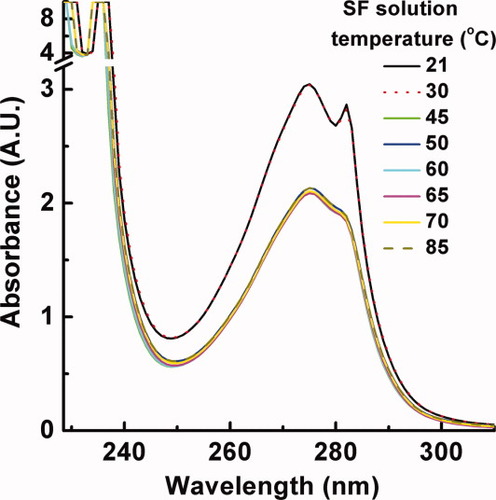
UV–vis spectra of silk fibroin aqueous solution of 0.1 mg mL−1 at different temperatures.
Effect of SF Solution pH Values on Conformational Transition
Here, we studied the SF solution at different pH values5-10 to study the effect of hydrogen ions on the conformational makeup of the regenerated SF solutions. CD spectra in Figure 5a display negative Cotton effect around 195 nm, indicating that the solution was composed dominantly of a random coil. Nevertheless, the absorption intensity exhibits the minimum at pH = 6, while the content of β-sheet structure determined by the Yang equation24, 25 in Figure 5b exhibits the maximum (33%) at pH = 6. This result is basically consistent with that determined by simulation of Cβ peak (δ14.5–22) in15-18C CP-MAS solid state NMR spectrum.28 The conformational transition of SF macromolecules with pH would be ascribed to their structural amphiphilicity just like an amphiphilic block copolymer.26 Lower pH may suppress the negative charges in two regions of the fibroin molecules, allowing a closer approach of neighboring molecules and thus the formation of hydrophobic interactions and later hydrogen bonds between molecules. These two effects make the SF macromolecules rearrange themselves to form a more stable state. These results could be used to explain the conformational transition of the SF chains with pH changes as the silk dope passes through the spinning duct in Bombyx mori silkworms.31 It is certain that the pH in the lumen of the Bombyx mori silkworm changes gradually from neutral (pH 6.9) in the posterior division to weakly acidic (pH 4.8) in the anterior division adjacent to the spinneret.
Effect of Ultrasonic Treatment on Conformational Transition
Figure 6 shows the effect of ultrasonic wave shaking on conformation of SF macromolecules for different times. A typical random coil conformation is revealed for a freshly prepared SF solution,24, 25 showing a negative Cotton effect around 195 nm and a positive Cotton effect at about 202 nm during the ultrasonic time of 0.5–4 h. The positive Cotton effect would be the result of a combination of α-helix and β-sheet structures. The unstable α-helix structure as the result of intramolecular hydrogen bonds would easily transform to β-sheet as the result of intermolecular hydrogen bonds, so SF may first transform from random coil or β-turn into α-helix and finally into β-sheet. Therefore, maximal α-helix content appeared at the ultrasonic treatment time of 2 h (Figure 6b). With prolonged shaking time, the negative Cotton effect around 195 nm gradually weakens, whereas another negative peak at 217 nm as characteristic of the β-sheet structure becomes stronger synchronously.24, 25 In short, Figure 6b shows that a transition from random coil to β-sheet took place and the content of β-sheet increased steadily with the prolongation of shaking time. At the shaking time of 4 h, the content of the β-sheet conformation in the SF solution is up to 32.2% that is the highest value of the four structure contents. Apparently, the ultrasonic wave with high frequency has a significant effect on dispersion and disaggregation on SF molecular chains. The high frequency wave shakes the SF solution, accelerates the movement of water molecules and SF macromolecules, and thus intensifies molecular collisions. Consequently, the β-sheet structure would form facilely.
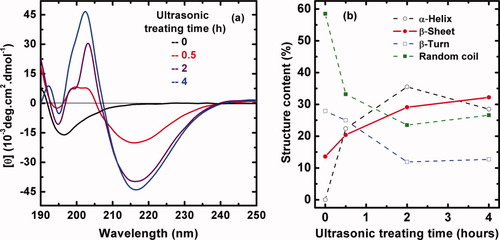
Effect of ultrasonic wave treatments on the conformational transition of 0.1 mg mL−1 regenerated silk fibroin solution. (a) The CD spectra were recorded at different shaking times of 0, 0.5, 2, and 4 h. The samples were equilibrated at each temperature for 30 min and measured at 18°C, pH = 6.82; (b) Different structure content in silk fibroin solution according to (a) calculated by the empirical Yang equation.24, 25
Effect of Extrusion on SF Solution Conformational Transition
The variation of the CD spectra of SF solution with the numbers of extrusions is shown in Figure 7. The freshly prepared SF solution before extrusion looks clear (Inset of Figure 7a) and illustrates typical strong negative Cotton effect around 195 nm, confirming the predominance of the random coil conformation. With increasing the numbers of extrusions from zero to 31, the negative Cotton effect around 195 nm weakens gradually but a positive one at 197 nm gets stronger, as indicated by a purple arrow. Simultaneously, the clear solution becomes uniformly opaque after extrusion of 31 times, as shown in the inset of Figure 7a. There is a drastic change with increasing numbers of extrusions from 7 to 15. At the same time, a new weak negative peak at 217 nm appears, as pointed out by a purple arrow. In particular, a dramatic enhancement of peak intensity at 217 nm is observed with increasing numbers of extrusions from 22 to 31. Both of the Cotton effects at positive 197 nm and negative 217 nm are characteristic of the β-sheet structure. That is to say, a steady and rapid transition from random coil to β-sheet conformation has been facilely accomplished by a simple extrusion (Figure 7b).
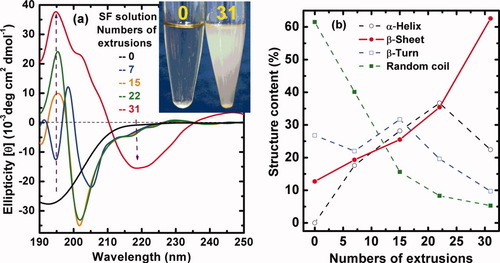
(a) Effect of extrusion (shearing force) on the conformational transition of regenerated SF aqueous solution recorded by circular dichroism. Silk fibroin solution was extruded through the aperture of a 0.6-mm diameter tube 0–31 times and all the samples were measured at 18°C, pH = 6.82; (b) Different structure content in silk fibroin solution according to (a) calculated by the empirical Yang equation.24, 25
Raman spectroscopy under a microscope, as a powerful tool to investigate the structure of protein solution of both spiders and silkworms31-33 has been used to confirm the remarkable conformational transition. Figure 8 shows the Raman spectrum of the SF solution after 31 extrusions. The peaks at 1085, 1227, 1268 (amide III) and 1665 cm−1 (amide I) arise from the β-sheet conformation, while the shoulder absorptions at 1099 and 1653 cm−1 imply the existence of a small amount of random coil or a helical conformation, based on a careful comparison with other Raman spectra in literature.34-36
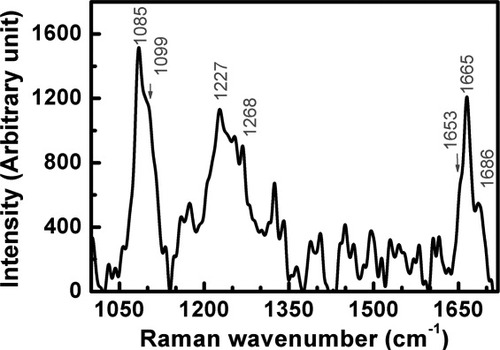
Raman spectrum of regenerated silk fibroin solution after extrusion 31 times at 18°C and pH = 6.82.
IR spectra of the regenerated SF films in Figure 9 are further utilized to confirm the conformational changes of SF molecules. The SF film before extrusion shows strong absorption bands at 1645 cm−1 (amide I: CO stretching), 1537 cm−1 (amide II: NH deformation and CN stretching), and 1235 cm−1 (amide III: CN stretching and NH deformation), which are attributed to the random coil conformation.4 On the contrary, the SF film after extrusion exhibits red shifts: amide I at 1639 cm−1, amide II at 1511 cm−1 and amide III at 1230 cm−1, which are all assigned to the β-conformation.37-39 These results are coincident with those of CD and Raman spectra. It can be concluded that the shearing force induced by extrusion efficiently makes the random coil part in regenerated SF transit to β-sheet and the conformational transition is more complete by the extrusion (Figure 7b) than the other methods (Figures 1b, 2b, 3b, 5b, 6b). Note that the SF film before extrusion also exhibits a shoulder band due to amide I at 1625 cm−1 as well as strong band to amide II at 1517 cm−1, implying that a high β-conformation content in the SF film from quiescent regenerated silk solutions has been confirmed by FTIR spectroscopy, as mentioned earlier.
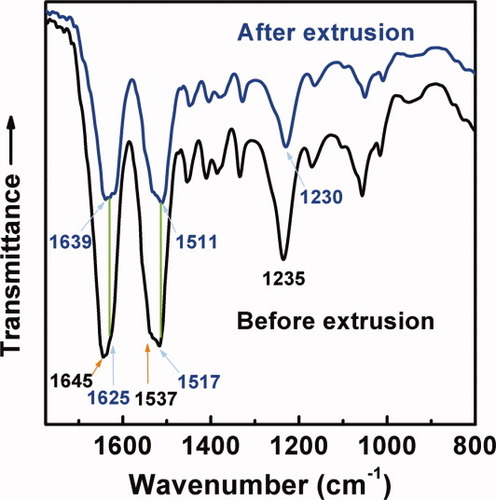
IR spectra of the dried films of regenerated silk fibroin aqueous solutions before and after extrusion 31 times at 18°C and pH = 6.82.
Liquid Crystalline Phase in Injected SF Solution
In this study, the SF solution after being extruded 20–31 times presents an homogeneously turbid and opaque state (Inset of Figure 7a), but neither precipitation nor phase separation occurs, although the extrusion more than 31 times would induce the formation of β-sheet precipitates. Optical polarized micrograph in Figure 10b shows an existence of a liquid crystalline state with a fingerprint texture in solution. However, the liquid crystalline state would disappear in 30 min, accompanying with the formation of a gel state and the vanishment of the fluidity at room temperature of 25°C. During the extrusion, the SF must be subjected to shearing force that results in hydrogen bonding and conformational preferences. Further, the silk protein would form secondary structures (helices or β-sheets) that have enough shape anisotropy (or rigidity) and chain structure order to form liquid crystalline phases. These secondary structures were handed objects that were thus likely to form mesogenic units,40 further orienting in the shearing direction,41 and finally the occurrence of a cholesteric phase.19
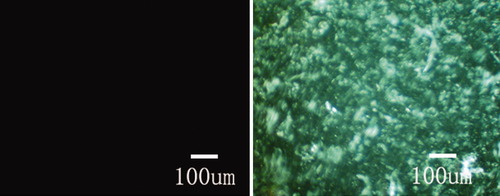
Polarized optical micrographs of the silk-fibroin aqueous solution (left) before and (right) after extrusion through the aperture of a 0.6-mm diameter tube 31 times at room temperature.
CONCLUSIONS
The structural characteristics and conformational transition of regenerated SF under different circumstances can be controlled to some extent by regulating the concentration, pH, and temperature of the SF aqueous solution or particularly applying shearing force onto the solution. The content of β-sheet conformation increases with elevating the concentration and temperature, while the β-sheet content would reach the maximum at pH = 6 because of an amphiphilic characteristics of the SF molecules in aqueous solution. Ultrasonic wave treatment and extrusion shearing force show a very great effect on the conformational transition of SF. CD and Raman spectra and polarized optical micrographs confirm that 91.4% random coil conformation of the SF can be transited into β-sheet structure exhibiting liquid crystalline order upon ultrasonic or especially shearing treatments. It could be concluded that the shearing force plays a very vital role in the formation of the liquid-crystalline ordered β-sheet conformation of SF in aqueous solution.




