Structural insights into cold inactivation of tryptophanase and cold adaptation of subtilisin S41†
Dedicated in memory of the late Prof. Elkan R. Blout who pioneered the art and science of physical protein chemistry and will be remembered for his unique virtue in bringing together wisdom, vision, and “mentchlichkeit.”
Abstract
A wide variety of enzymes can undergo a reversible loss of activity at low temperature, a process that is termed cold inactivation. This phenomenon is found in oligomeric enzymes such as tryptophanase (Trpase) and other pyridoxal phosphate dependent enzymes. On the other hand, cold-adapted, or psychrophilic enzymes, isolated from organisms able to thrive in permanently cold environments, have optimal activity at low temperature, which is associated with low thermal stability. Since cold inactivation may be considered “contradictory” to cold adaptation, we have looked into the amino acid sequences and the crystal structures of two families of enzymes, subtilisin and tryptophanase. Two cold adapted subtilisins, S41 and subtilisin-like protease from Vibrio, were compared to a mesophilic and a thermophilic subtilisins, as well as to four PLP-dependent enzymes in order to understand the specific surface residues, specific interactions, or any other molecular features that may be responsible for the differences in their tolerance to cold temperatures. The comparison between the psychrophilic and the mesophilic subtilisins revealed that the cold adapted subtilisins have a high content of acidic residues mainly found on their surface, making it charged. The analysis of the Trpases showed that they have a high content of hydrophobic residues on their surface. Thus, we suggest that the negatively charged residues on the surface of the subtilisins may be responsible for their cold adaptation, whereas the hydrophobic residues on the surface of monomeric Trpase molecules are responsible for the tetrameric assembly, and may account for their cold inactivation and dissociation. © 2007 Wiley Periodicals, Inc. Biopolymers 89: 354–359, 2008.
This article was originally published online as an accepted preprint. The “Published Online” date corresponds to the preprint version. You can request a copy of the preprint by emailing the Biopolymers editorial office at [email protected]
THE QUESTION POSED
What are the molecular and structural features that govern the divergent tolerance to temperatures of two different groups of proteins–cold labile versus cold adapted?
COLD INACTIVATION
A reversible loss of activity at low temperature is found in a widespread variety of oligomeric enzymes, e.g., pyrophosphatase, pyruvate carboxylase, alcohol dehydrogenase, as well as in pyridoxal phosphate (PLP)-dependent enzymes such as glutamate decarboxylate and tryptophanase (Trpase) (Table I). They all bind to a cofactor and/or ions, needed for their activity. In most cases the loss of activity is followed by dissociation into monomers, a process which can be reversed by rewarming; but in some cases it is followed by an aggregation step.9, 10, 14 Reversible cold inactivation is believed to be of physiological significance. For example, tetramer–dimer equilibrium of phosphofructokinase was suggested as a factor responsible for a relative decrease in the contribution of glycolysis to overall energy production during hibernation in ground squirrels and European hamsters.15
| Enzyme | Source | State | Mw (kDa) | Ion/Cofactor | Pdb Entry |
|---|---|---|---|---|---|
| Inorganic pyrophosphatase1 | E. coli | Homohexamer | ∼20 | Mg2+ | 1ipv;1faj |
| Glutamate decarboxylase2 | E. coli | Homohexamer | 57.2 | PLP | 1pmm; 1pmo; 1xey |
| Tryptophanase3 | E. coli | Homohexamer | 52 | PLP | 2c44;2oqx |
| Chloroplast coupling factor (CF1)4 | Market spinach | Heterohexamer α3β3 | Nucleotides,Mg2+-ATP | ||
| Acetyl-CoA hydrolase | Rat liver | Hexamer | 2nvv (Porhyromonas gingivalis) | ||
| Phosphoenolpyruvate carboxylase | Panicum maximum | Tetramer | SCN− | 1jkn; (E. coli) | |
| Cytosolic3,5,3′ triiodo-L-thyronine-binding proteins5, 6 | Human erythrocyte | Polymeric form tomonomers | |||
| Malic enzyme7 | Pigeon liver | Tetramer | NAD | 2a9f | |
| Alcohol dehydrogenase8 | Dimer (?) | NAD(P) | |||
| Lactate dehydrogenase9, 10 | Porcine | Tetramer (T↔4M; 4M↔T) | NAD | 1ldm (dogfish) | |
| Pyruvate carboxylase11 | Rat liver mitochondria | Tetramer | Biotin | 1ulz; (Aquifex aeolicus) | |
| Xylulose reductase12 | Rodent | Homotetramer | ∼26 (human) | NADPH | 1wnt; 1pr9 |
| L-threonine deaminase13 | Rhodospirillum rubrum | Tetramer | 2gn2; 2gn0, 2gn1 | ||
| Pyruvate carboxylase11 | Chicken liver mitochondria | Tetramer | Biotin | 1ulz; (Aquifex aeolicus) |
Several models for low temperature destabilization of proteins that take into account interactions between segments of polypeptide chains and solvent molecules were suggested.16 These models predict that the pivotal role in cold inactivation is played by hydrophobic interactions. It is believed that at low temperature the entropic term for association becomes less favorable, because the solvent molecules released upon association are more ordered and the enthalpy of dissociation is a strong function of temperature.17
The present work aims at elucidating the structural features that are common and required for reversible cold inactivation and dissociation of the widely distributed bacterial enzyme Trpase from E. coli is as an example of a cold labile enzyme. We compared its crystal structure with three homologues, PLP-dependent enzymes whose crystal structures have been determined: Proteus vulgaris Trpase (PDB entry 1ax418), Citrobacter freundii tyrosine phenol lyase (PDB entry 1tpl19), and E. coli aspartate amino transferase (PDB entry 1cq7).
Tryptophanase (tryptophan indole-lyase, EC 4.1.99.1) catalyzes α,β-elimination and β-replacement reactions of L-Trp and a variety of other β-substituted L-amino acids. Trpase consists of four identical monomers, 52 kDa each. One molecule of the cofactor PLP forms an aldimine bond with Lys27020-23 (Figure 1). Its holo form exhibits a pH-dependent absorption and CD spectra with maxima at 420 and 337 nm typical for covalently bound PLP.24-26 In addition, it requires certain monovalent cations (K+, NH4+, Tl+) for activity and for tight PLP binding.27-29
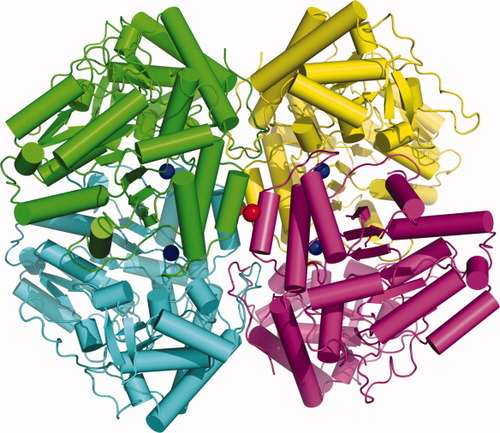
Crystal structure of tetrameric E. coli tryptophanase (pdb entry 2oqx) each chain is colored differently.
Using kinetics methods (HPLC analyses, spectrophotometric activity measurements–steady state and stopped-flow, and time resolved single photon correlation spectrofluorometry) as well as size-exclusion chromatography, with wt E. coli Trpase and several point mutated analogues, we have shown that the enzyme undergoes a reversible inactivation followed by dissociation into dimers after incubation at 2°C. The cold inactivation process is tightly dependent on the buffer used (tricine, tris, or potassium phosphate), and on the sequence of anions, arranged according to their effectiveness in promoting the dissociation which coincides with that of the Hofmeister series of salts.30-32
On the basis of these studies, a model describing the sequence of events, going from “closed” to “open” conformations, was suggested. The reduction in fluorescence lifetime observed for holo wt Trpase and its W330F mutant, upon cooling from 25°C to 2°C, could thus be attributed to the “open” conformation and the higher exposure of PLP in the active site to bulk water.3 When bound to the active site in the “closed” conformation, PLP freedom of rotation and wobbling are restricted by hydrogen bonding and salt bridges. These restrictions are alleviated in the relative more “open” conformation which precedes the aldimine bond cleavage. These suggested conformational changes are further supported by our and others' crystallographic work on apo and holo Trpases presenting different levels of “open” and “closed” conformations, which supports the role of PLP in the maintenance of the quaternary structure.18, 33, 34, 35
The model proposes that low temperature weakens hydrophobic interactions; this leads to a more open conformation that exposes the cofactor PLP, allowing cleavage of thePLP-Lys270 aldimine bond, and release of the cofactor PLP. This results in the concomitant release of the cofactor and dissociation of the tetramer into dimers.3, 35 The suggested sequence for PLP-Trpase interactions is further supported by HPLC-Diode array profiles which could not detect PLP-bound dimers.
Superpositioning of the crystal structures of E. coli Trpase, P. vulgaris Trpase, and Citrobacter freundii tyrosine-phenol lyase (Figure 2a) indicates that these enzymes have the same fold. In Figures 2b and 2c the hydrophobic residues on the monomer subunit of Trpase are highlighted in red, suggesting that hydrophobic contacts between monomers are the major interactions which are called for the tetramer assembly, and are responsible for Trpase cold inactivation and dissociation.
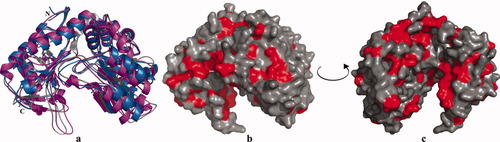
Superposition of three crystal structures of PLP dependent enzymes is shown in (a): E. coli Trpase is shown in gray, P. vulgaris Trpase is shown in magenta and tyrosin phenole lyase is shown in blue; (b) and (c) are two views of the molecular surface of E. coli Trpase (shown in gray); hydrophobic residues are highlighted in red.
COLD ADAPTATION
Psychrophilic organisms grow at a temperature of 15°C or lower and are unable to grow at above 20°C. Thus, cold adapted or psychrophilic enzymes isolated from such organisms display a high catalytic efficiency at low to moderate temperatures which is associated with low thermal stability. Therefore, these enzymes are attractive system for understanding the structure-stability relationship in proteins, as well as a target for biotechnological studies.36-39 They offer economic benefits through energy savings, in skipping the expensive heating steps, as well as minimizing the chemical reactions or degradations of products that occur at higher temperatures.36, 37, 40 Consequently, considerable efforts are directed towards identifying the specific structural features responsible for the activity and stability of these enzymes (reviewed37, 40-44). However, thus far, only a limited number of cold-adapted enzymes have been characterized structurally using X-ray crystallography. On the basis of those crystal structures it was shown that the cold adapted enzymes contain a reduced number of protein stabilization factors, such as salt bridges, hydrogen bonds, and aromatic–aromatic contacts, and have reduced Pro and Arg contents, when compared with their mesophilic counterparts.
The molecular basis of the adaptation of thermophiles to extreme high temperatures also has been a subject of intensive research.45-47 It was shown however, that the adaptation of these proteins to higher temperature is a result of several molecular factors such as better internal packing of nonpolar groups, increased number hydrogen bonds, and more ion pairs.48-60
In general, regardless of whether the research is directed at thermophilic or psychrophilic adaptation, the results show that each protein family adopts its own strategies for coping at extreme temperatures. The most frequently reported features related to adaptation to low temperatures are less compact packing of the hydrophobic core, an increased charged surface area, decreased metal ion affinity, longer surface loops, and a reduced number of prolines in loops.36, 40, 41, 61-66
Many cold-adapted enzymes have a reduced Arg/Lys ratio, while thermostable proteins have higher content of Arg residues that increases the number of salt bridges of the guanidinium groups. Another molecular feature which was considered to be responsible for adaptation to extreme temperatures is metal binding which can stabilize the enzyme by bridging numerous secondary structures or domains. Indeed, recent crystallographic studies revealed that different members of the subtilisin superfamily posses variable multiple calcium binding sites. For example, two binding sites were found for the mesophilic subtilisin BPN' (pdb entry 1sup), three were found for the thermophilic subtilisin thermitase67 (pdb entry 1thm) and the subtilisin-like serine protease from the psychrophilic Vibrio species (pdb entry 1sh7)68. Five sites were found for the mesophilic subtilisin Sph (pdb entry 1ea7)69 and the psychrophilic subtilisin S41 (pdb entry 2gko); and recently, six calcium binding sites were reported for the hyperthermophilic archaeon Tk determined with its propeptide.70 Since multiple calcium-binding sites were found both for themophilic and psychrophilic proteins, it was recently suggested that calcium ions are important for correct folding of subtilisins and not for their thermostability.
COMPARISON BETWEEN THE COLD ADAPTED AND THE COLD LABILE ENZYMES
Previous amino acids sequence analysis suggested that the psychrophilic subtilisin S41 surface is rich in hydrophilic residues, particularly Asp, which may contribute to its adaptation to psychrophilic conditions.71-75 This is further supported by our work on the crystal structure of S41 recently determined (pdb entry 2gko). Hence, we compared the amino acids sequence and the crystal structure of four subtilisins: the psychrophilic subtilisin, S41, the psychrophilic subtilisin-like protease from Vibrio,68 the mesophilic subtilisin BPN′,76, 77 and the thermophilic subtilisin thermitase67 (Figure 3a). In Figure 3b and 3c, the acidic residues of S41 are highlighted in blue creating a charged surface typical for psychrophilic subtilisins.
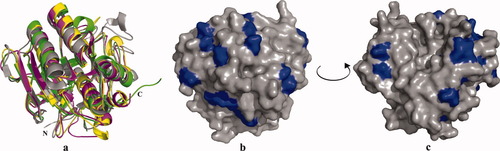
Superposition of four crystal structures is shown in (a); the subtilisin BPN' shown in yellow; thermitase is shown in magenta, the psychrophilic subtilisin-like protease shown in green and subtilisin S41 is shown in grey: (b) and (c) two views of the molecular surface of subtilisin S41 (shown in grey), Asp residues are highlighted in blue.
In Table II we summarized several molecular features for the subtilisins and the PLP-dependent enzymes. The cold adapted enzymes were found to be monomeric and do not depend on a cofactor. Also, they have high content of Asp and Glu compared to their mesophilic and thermophilic counterparts. On the other hand, the cold labile enzymes are multimeric, PLP and ion dependent. They have neither significant high content of the negatively charged Asp and Glu nor high content of the positively charged Arg or Lys. Rather, hydrophobic residues on the surface of monomeric Trpase molecules contribute to the tetrameric assembly.
| Trpase (E.coli) | Trpase (P.vulgaris) | Tyrosine phenol Ivase | Aspartate amino transferase (E.coli) | Thermitase (Thermophilic) | Subtilisin BPN′ (Mesophilic) | Subtilisin-Like (Vibrio) (Psychrophilic) | Cold Adapted Subtilisin S41 (Psychrophilic) | |
|---|---|---|---|---|---|---|---|---|
| Pdb entry | 2oqx | 2ax4 | 1tpl | 1cq7 | 1thm | 1sup | 1sh7 | 2gko |
| R+K | 24+27 | 23+35 | 25+27 | 22+18 | 5+10 | 10+5 | 9+5 | 7+10 |
| E+D | 33+25 | 36+29 | 36+25 | 27+20 | 2+13 | 2+11 | 19+5 | 21+7 |
| E+D/R+K | 58/51 | 64/58 | 61/52 | 40/47 | 15/15 | 13/15 | 24/14 | 28/17 |
| 1.14 | 1.10 | 1.17 | 1.17 | 1.0 | 0.86 | 1.71 | 1.65 | |
| Surface area (Å2) | 20,734 | 20,427 | 18,984 | 17,819 | 9696 | 9930 | 10,309 | 11,027 |
| Total residues | 471 | 467 | 456 | 405 | 279 | 275 | 281 | 309 |
CONCLUSIONS
The present work attempts to compare for the first time between the structural features of two different groups, of cold adapted and cold inactivated proteins. Major structural differences were evident. The cold adapted proteins examined here are monomeric, have a highly charged surface, less exposed surface area and higher Asp residues content when compared with their mesophililc counterparts. On the other hand, the cold labile enzymes presented here have a multimeric quaternary structure, held together by surface hydrophobic interactions and by the cofactor PLP. These interactions are known to reduce at cold temperatures, resulting in dissociation to subunits, and taking apart the inter-subunit active sites.




