Pulsed electromagnetic fields on postmenopausal osteoporosis in southwest China: A randomized, active-controlled clinical trial†
Hui-Fang Liu and Lin Yang contributed equally to this paper.
Abstract
A randomized, active-controlled clinical trial was conducted to examine the effect of pulsed electromagnetic fields (PEMFs) on women with postmenopausal osteoporosis (PMO) in southwest China. Forty-four participants were randomly assigned to receive alendronate or one course of PEMFs treatment. The primary endpoint was the mean percentage change in bone mineral density of the lumbar spine (BMDL), and secondary endpoints were the mean percentage changes in left proximal femur bone mineral density (BMDF), serum 25OH vitamin D3 (25(OH)D) concentrations, total lower-extremity manual muscle test (LE MMT) score, and Berg Balance Scale (BBS) score. The BMDL, BMDF, total LE MMT score and BBS score were recorded at baseline, 5, 12, and 24 weeks. Serum concentrations of 25(OH)D were measured at baseline and 5 weeks. Using a mixed linear model, there was no significant treatment difference between the two groups in the BMDL, BMDF, total LE MMT score, and BBS score (P ≥ 0.05). For 25(OH)D concentrations, the effects were also comparable between the two groups (P ≥ 0.05) with the Mann–Whitney's U-test. These results suggested that a course of PEMFs treatment with specific parameters was as effective as alendronate in treating PMO within 24 weeks. Bioelectromagnetics 34:323–332, 2013. © 2012 Wiley Periodicals, Inc.
INTRODUCTION
Osteoporosis is defined as a systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, leading to enhanced bone fragility and consequently an increased risk of fractures [Consensus Development Conference, 1993]. Not only incident fractures, but osteoporosis itself can also lead to long-lasting pain, reduced mobility, impaired quality of life, etc. Moreover, it was estimated that in the year following a fracture, medical, and hospitalization costs were 1.6–6.2 times higher than pre-fracture costs and 2.2–3.5 times higher than those for matched controls [Budhia et al., 2012]. Furthermore, according to the National Osteoporosis Foundation (Washington, DC, USA), at least half of the people aged 50 years and older will be affected by osteoporotic fractures. So, with the aging of the world's population, osteoporosis is a major public health threat worldwide.
Apart from healthy lifestyles and proper nutrition, postmenopausal osteoporotic women should receive intensive medical intervention. Bisphosphonates are currently the most commonly prescribed medications for osteoporosis [Strampel et al., 2007]. Among them, alendronate has been shown to be very effective in the prevention and treatment of postmenopausal osteoporosis (PMO) [Felsenberg et al., 1998; Hosking et al., 1998]. On the other hand, complications such as upper gastrointestinal adverse events, renal toxicity, influenza-like illness, and osteonecrosis of the jaw have drawn much attention and are possible reasons for poor patient compliance [Strampel et al., 2007].
Pulsed electromagnetic fields (PEMFs) have been used extensively as a non-invasive treatment for various disorders [Binder et al., 1984; Mooney, 1990; Thamsborg et al., 2005; Martiny et al., 2010; Giannakopoulos et al., 2011; Czyz et al., 2012]. From 1976 to 1979, in Washington, DC, tests under the auspices of the National Aeronautics and Space Administration (NASA), responsible for the civilian space program, aeronautics, and aerospace research, suggested that PEMFs could be equally effective as mechanical stimulation in maintaining or improving bone mass. Since then, much evidence has positively supported the treatment effect of PEMFs for osteoporosis, although with an effective window [Rubin et al., 1989; Tabrah et al., 1990; Eyres et al., 1996; Garland et al., 1999; Giordano et al., 2001]. In addition, various biochemical mechanisms have been associated with its effectiveness [Shankar et al., 1998; Chang and Chang, 2003; Sun et al., 2009; Chen et al., 2010; Shen and Zhao, 2010] and there seems to be no detrimental side effects [Blaszczak et al., 2009] or sufficient amplitude to elicit neuromuscular responses [Radon et al., 2001; Spadaro et al., 2011]. But, negative results were also reported. For example, no significant effect was observed on forearm disuse osteopenia [Spadaro et al., 2011], which was assumed to be attributed to the treatment starting point, duration, daily exposure time, PEMF waveform, and participant-related factors.
To determine the effect of PEMFs on PMO, we conducted a randomized, active-controlled clinical trial in southwest China. It was hypothesized that PEMFs would be as effective as alendronate in improving bone mineral density (BMD), vitamin D status, muscle strength, and balance.
MATERIALS AND METHODS
Participants
From the outpatient department of the West China Hospital of Sichuan University in Chengdu, postmenopausal women residing in southwest China were invited. All participants had to meet the following criteria for entry into the study: (a) postmenopausal women; (b) aged between 45 and 70 years; (c) who had been postmenopausal for at least 1 year and diagnosed with osteoporosis (lumbar spine or femur T-score of −2.5 or less) [Consensus Development Conference, 1993]. Women with suspected confounding disorders (e.g., diabetes mellitus, hypertension, hyperlipidemia, obesity, secondary and idiopathic osteoporosis, fracture history, systemic inflammatory disorders, visual impairment, severe vestibular lesions, cerebellar lesions, peripheral nerve or muscle disease, mental retardation, mental illness, cognitive impairment, obstetrical and gynecological diseases, blood disease, stroke or venous thromboembolic disease, etc.) with or without corresponding treatment, who had received anti-osteoporosis treatment within 6 months before inclusion in the study, and drank alcohol or smoked were all excluded.
Randomization and Procedures
Included participants were randomly assigned with computer-generated random numbers to receive alendronate (70 mg/week) or PEMFs treatment (40 min/treatment, 1 treatment session/day, 6 treatment sessions/week, for a total of 30 times as one course of treatment). It was conducted by an independent researcher not involved in the screening, treatment or outcome assessment.
The PEMFs treatment was conducted using an XT-2000B therapeutic stimulator (Tianjin xtmed, Tianjin, China; Fig. 1). According to the manufacturer's instructions and statement, as well as the purpose of the study, it generated time-varying fields consisting of bursts of asymmetric pulses. Each burst lasted for 0.2 ms and repeated at a frequency of 8 Hz. For the treatment region of the bed, where the lumbar spine of the supine participant is supposed to be, the fields were delivered perpendicular and the flux density within a single burst started with a peak value of 3.82 mT and decreased to 0 mT in 0.2 ms.
Computer-controlled PEMFs treatment device.
In addition, basic osteoporosis treatment, that is, 600 mg/day calcium and alfacalcidol vitamin D supplement 0.25 µg/bid, was received by all participants. After PEMFs treatment, the intervention procedure remained the same for the alendronate group, whereas for the PEMFs group, only basic treatment was maintained. Follow-up visits were scheduled at 5, 12, and 24 weeks after baseline assessment.
Prior to the initiation of the trial, trainings were provided with respect to protocol and measurement procedures and methods used to maintain a blind experiment. Given the nature of the interventions, participants, and therapists could not be blinded to treatment allocation. To reduce bias, apart from independent randomization, this study implemented a single-blind design by separating treatment and assessment. Those who gave treatment were not involved in assessing outcomes, whereas the researchers who assessed and analyzed outcomes were independent and blinded to the participant's treatment allocation.
Since no previous study was ever conducted regarding the effect of PEMFs on PMO, especially with these very parameters, it seems theoretically impractical to calculate the sample size. Instead, we estimated it to be 20 per group, similar to that of the study exploring the treatment effect of PEMFs on forearm disuse osteopenia [Spadaro et al., 2011]. To test the rationality of this estimation, we calculated the statistical power for the primary endpoint, the mean percentage change in bone mineral density of the lumbar spine (BMDL), from baseline at the last visit.
This study was approved by the clinical research and biomedical ethics committee of West China Hospital, Sichuan University, and performed in accordance with the ethical standards in the 1964 Declaration of Helsinki. Informed consent was obtained from all the participants before their inclusion in the study.
Measurements
Our primary endpoint was the mean percentage change in BMDL, and secondary endpoints were the mean percentage changes in left proximal femur bone mineral density (BMDF), serum 25OH vitamin D3 (25(OH)D) concentrations, total lower-extremity manual muscle test (LE MMT) score, and Berg Balance Scale (BBS) score. Demographic measures including participant's age (in years), menopause age (in years), time since menopause (in years), and body mass index (BMI; in kg/m2) were measured at baseline.
To evaluate bone mass, the BMDL and BMDF were measured. They were conducted at baseline and at 5, 12, and 24 weeks, along with the total LE MMT score and BBS score. The last two time points were included to observe a possible delayed effect on maintaining or improving bone mass. At each time point, participants were scanned in the standard manner by an expert technologist using dual energy X-ray absorptiometry (DXA; GE Lunar iDXA with encore 12.0 software, GE Healthcare Lunar, Madison, WI) following recommendations from the manufacturer. Quality assurance calibrations were verified daily. With regard to the left proximal femur, reports of the BMD of the total femur were recorded, and for the lumbar spine the average BMD (aBMD) of the four vertebrae (L1–L4) were extracted. All these data were provided by the corresponding software and no extra calculations were required.
Serum concentrations of 25(OH)D were measured at the first two time points (baseline and 5 weeks) in the morning using the IDS OCTEIA 25(OH)D direct enzyme-linked immunosorbent assay kit (IDS AC-57F1, Immunodiagnostic Systems, Boldon, UK). To obtain serum concentrations, first the biotin-labeled 25(OH)D is bound to a specific sheep 25(OH)D antibody. Then, a horseradish peroxidase-labeled avidin binds the biotin complexes. Finally, using a chromogenic substrate, a characteristic color is developed whose intensity is inversely proportional to the 25(OH)D concentration. The measurements were performed in the biochemical laboratory of West China Hospital.
Manual muscle strength, which indicates the force-generating capacity, was estimated for eight muscle groups bilaterally: hip flexors, extensors, abductors and adductors, knee flexors and extensors, and ankle dorsiflexors and plantar flexors. They were accessed by employing the manual muscle testing (MMT) method with the traditional six-point ordinal Medical Research Council scale (0–5). All tests at each time point were performed by a single examiner under the standard testing positions. The examiner and participants were required to rest for at least 5 min before testing. Half-point scores were used between all grades except 4 and 5, which require manual resistance that cannot be graded reliably [Noreau and Vachon, 1998]. The LE MMT score was used for analysis. It was obtained by summing up scores across 16 muscle groups (8 per side), with a maximum possible score of 80.
The BBS was used to evaluate static and dynamic balance, with a maximum possible score of 56 [Berg et al., 1992]. Another examiner performed the tests for all the participants following the standard procedure at each time point. The examiner and participants were also required to rest for at least 5 min before testing.
Statistical Analyses
All participants with a baseline value and at least one measurement after randomization were included in the efficacy analyses (intention-to-treat analysis) using the last observation carried forward (LOCF) method.
Variables were expressed as means and standard deviations (SD). The comparability at baseline between the two groups was investigated using the Mann–Whitney's U-test, and treatment differences were assessed on the percentage change from baseline to the three follow-up visits. For variables at multiple time points, that is, BMDL, BMDF, LE MMT score (total), and BBS score, we made comparisons using a mixed linear model [Cleophas et al., 2012] with treatment group as a fixed factor and time as a random factor. Adjustments were made for covariables including participant's age, menopause age, time since menopause, BMI, and the corresponding baseline value. In regards to 25(OH)D concentrations, comparisons were made for mean percentage changes and values at 5 weeks using the Mann–Whitney's U-test. Furthermore, we applied the Wilcoxon signed rank test for intragroup analysis using all the variables mentioned above.
Finally, to explore relationships among variables we calculated Spearman's correlation coefficients along with their P-values. A two-sided value of P < 0.05 was considered statistically significant. All statistical analyses were carried out using 19.0 SPSS software (SPSS, Chicago, IL).
RESULTS
Of the 105 eligible candidates, 44 participants were randomized, 39 participants completed all four visits, and 41 participants entered the final analysis (Fig. 2). The two groups were comparable at baseline (Table 1). Among those entered into the analysis, compliance was good because all participants completed the treatment course of PEMFs and received pills as scheduled. In addition, there were no serious adverse events reported.
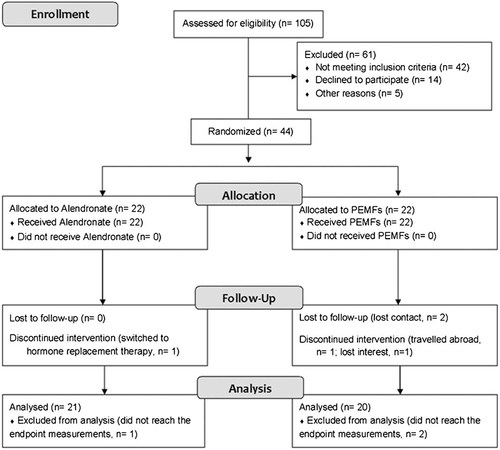
Participant flow chart.
| Variables | All patients | Treatment | P-value | |
|---|---|---|---|---|
| Alendronate | PEMFs | |||
| Number (%) | 41 | 21 (51.22) | 20 (48.78) | — |
| Age (year) | 62.44 ± 5.19 | 63.14 ± 4.32 | 61.70 ± 1.34 | 0.57 |
| Menopause age (year) | 48.54 ± 4.05 | 49.05 ± 3.80 | 48.00 ± 4.33 | 0.45 |
| Time since menopause (year) | 13.90 ± 6.61 | 14.10 ± 6.12 | 13.70 ± 7.25 | 0.85 |
| Body mass index (kg/m2) | 23.13 ± 3.32 | 23.33 ± 3.33 | 22.93 ± 3.37 | 0.58 |
| BMDL (g/cm2) | 0.78 ± 0.08 | 0.79 ± 0.08 | 0.76 ± 0.08 | 0.77 |
| BMDF (g/cm2) | 0.75 ± 0.08 | 0.74 ± 0.07 | 0.76 ± 0.10 | 0.85 |
| 25(OH)D (nmol/L) | 43.73 ± 13.44 | 43.21 ± 16.32 | 44.28 ± 9.96 | 0.30 |
| LE MMT | 70.85 ± 5.86 | 71.26 ± 4.33 | 70.43 ± 7.22 | 0.86 |
| BBS | 53.66 ± 2.64 | 53.29 ± 2.94 | 54.05 ± 2.31 | 0.38 |
- BMDL, bone mineral density of the lumbar spine (L1–4); BMDF, bone mineral density of the left proximal femur; 25(OH)D, 25OH vitamin D3; LE MMT, lower-extremity manual muscle test score (total); BBS, Berg Balance Scale score.
- P-value refers to intergroup comparison.
The mean percentage changes in the BMDL, BMDF, LE MMT score (total), and BBS score from baseline to 5, 12, and 24 weeks are shown in Table 2. Intergroup comparisons were conducted using a mixed linear model with adjustments for the covariables mentioned above. It demonstrated that treatment differences in the BMDL (the primary endpoint) of the PEMFs group were lower than those of the alendronate group, especially at the last visit (P = 0.05), although not statistically significant. However, by intragroup analysis at each follow-up visit, the BMDL in the alendronate group was significantly higher than the baseline value (P < 0.01), whereas the increases were not significant in the group treated with PEMFs (P-values were 0.37, 0.28, and 0.44, respectively). For the BMDF, we also found no statistically significant comparison (Table 2). By intragroup analysis, the results were also inconsistent. At each time point, compared to baseline, the P-values were 0.01, 0.10, and 0.02, respectively, for the alendronate group, and 0.17, 0.33, and 0.72 for the PEMFs group. In addition, there was no significant interaction of intervention group with observation time point, either for BMDL or BMDF, of which P-values were 0.324 and 0.394, respectively.
| Variables | Alendronate | PEMFs | P-value |
|---|---|---|---|
| BMDL (%) | |||
| 5 weeks | 1.60 ± 0.71 | 0.78 ± 0.69 | 0.41 |
| 12 weeks | 2.36 ± 0.71 | 1.05 ± 0.74 | 0.21 |
| 24 weeks | 2.98 ± 0.83 | 0.53 ± 0.88 | 0.05 |
| BMDF (%) | |||
| 5 weeks | 1.61 ± 0.69 | 1.06 ± 0.71 | 0.58 |
| 12 weeks | 1.07 ± 0.65 | 0.82 ± 0.68 | 0.79 |
| 24 weeks | 1.79 ± 0.65 | 0.62 ± 0.69 | 0.23 |
| LE MMT (%) | |||
| 5 weeks | 6.89 ± 1.02 | 7.74 ± 1.05 | 0.57 |
| 12 weeks | 11.85 ± 0.85 | 12.83 ± 0.89 | 0.43 |
| 24 weeks | 12.57 ± 0.62 | 13.38 ± 0.66 | 0.39 |
| BBS (%) | |||
| 5 weeks | 3.25 ± 0.36 | 4.00 ± 0.37 | 0.16 |
| 12 weeks | 3.63 ± 0.32 | 4.21 ± 0.33 | 0.22 |
| 24 weeks | 4.48 ± 0.40 | 4.22 ± 0.43 | 0.67 |
- P-value refers to intergroup comparison.
As for the total LE MMT score and BBS score, we observed similar results (Table 2). No significant comparison or interaction of intervention with time (P = 0.99 and 0.10 for the two variables, respectively) was detected. Intragroup comparisons, however, were all statistically significant (P < 0.01; data not shown).
Moreover, we should notice the effect of covariables such as the BMI and corresponding baseline value on the outcome data. In some cases, P-values of these covariables were less than the 0.01 level of significance, which further supported the decision to control for the influence of covariables.
According to Table 3, serum 25(OH)D concentrations in the alendronate group were significantly higher at 5 weeks, whereas for the PEMFs group specifically, the effects were non-significant. But there was no significant difference between the two groups either for the observed value or mean percentage changes from baseline.
| Alendronate | P1 | PEMFs | P2 | P3 | P4 | |||
|---|---|---|---|---|---|---|---|---|
| 5 week (nmol/L) | MPCB (%) | 5 week (nmol/L) | MPCB (%) | |||||
| 25(OH)D | 48.15 ± 14.46 | 16.47 ± 27.75 | 0.03* | 46.07 ± 8.12 | 6.76 ± 18.60 | 0.17 | 0.84 | 0.38 |
- MPCB, mean percentage change from baseline to 5 weeks.
- P1 refers to intragroup comparison in the alendronate group; P2 refers to intragroup comparison in the PEMFs group; P3 refers to intergroup comparison at 5 weeks after enrollment; P4 refers to intergroup comparison of mean percentage changes from baseline to 5 weeks.
- * P-value < 0.05.
The main results of correlation analyses were as follows (Tables 4 and 5): (1) age was inversely associated with BMDL and BBS score, and time since menopause was also inversely related to BMDL; (2) high BMI could positively predict high BMDL and BMDF and may lead to a low BBS score; (3) concentrations of 25(OH)D showed a positive relationship with muscle strength, which was highly correlated with the BBS score; and (4) finally, and unexpectedly, we found an inverse association between BMDF and BBS score.
| Variables | Age | MA | TSM | BMI | 25(OH)D | BMDL | BMDF | LE MMT | BBS |
|---|---|---|---|---|---|---|---|---|---|
| MA | 0.07 | — | — | — | — | — | — | — | — |
| TSM | 0.76 | −0.55 | — | — | — | — | — | — | — |
| BMI | 0.02 | 0.03 | 0.08 | — | — | — | — | — | — |
| 25(OH)D | −0.16 | −0.10 | −0.03 | 0.12 | — | — | — | — | — |
| BMDL | −0.32 | 0.14 | −0.36 | 0.45 | −0.02 | — | — | — | — |
| BMDF | −0.20 | 0.06 | −0.15 | 0.67 | −0.02 | 0.61 | — | — | — |
| LE MMT | −0.31 | −0.17 | −0.13 | 0.16 | 0.34 | 0.08 | 0.04 | — | — |
| BBS | −0.37 | −0.13 | −0.20 | −0.41 | −0.01 | −0.11 | −0.16 | 0.53 | — |
- MA, menopause age; TSM, time since menopause; BMI, body mass index.
- Correlations that were significant at the 0.05 level are shown in bold type.
| Variables | Age | MA | TSM | BMI | 25(OH)D | BMDL | BMDF | LE MMT | BBS |
|---|---|---|---|---|---|---|---|---|---|
| MA | 0.70 | — | — | — | — | — | — | — | — |
| TSM | <0.01 | <0.01 | — | — | — | — | — | — | — |
| BMI | 0.90 | 0.86 | 0.65 | — | — | — | — | — | — |
| 25(OH)D | 0.32 | 0.55 | 0.84 | 0.49 | — | — | — | — | — |
| BMDL | 0.05 | 0.41 | 0.03 | <0.01 | 0.87 | — | — | — | — |
| BMDF | 0.21 | 0.73 | 0.35 | <0.01 | 0.88 | <0.01 | — | — | — |
| LE MMT | 0.06 | 0.29 | 0.43 | 0.92 | <0.01 | 0.33 | 0.65 | — | — |
| BBS | 0.02 | 0.44 | 0.22 | 0.01 | 0.98 | 0.19 | 0.04 | <0.01 | — |
- Correlations that were significant at the 0.05 level are shown in bold type.
DISCUSSION
Therapeutic Effects
The present study demonstrated for the first time that compared with alendronate, a course of PEMFs treatment with specific parameters (field frequency of 8 Hz, intensity of magnetism of 3.82 mT, and 40 min/day) was effective for PMO in southwest China. It was achieved by improving bone mineral density (BMD), vitamin D status, muscle strength and balance, and was maintained for at least 19 weeks after treatment ceased.
Nowadays, antiresorptive agents such as bisphosphonates are the most commonly prescribed medications for osteoporosis [Strampel et al., 2007] and have been proven to be able to normalize bone turnover, restore the balance of bone remodeling, prevent bone loss and deterioration of bone structure, and reduce fracture risk [Heaney et al., 1997; Reszka and Rodan, 2003; Boonen et al., 2008]. Among these agents, alendronate was reported to produce significant, progressive increases in BMD for PMO [Felsenberg et al., 1998; Hosking et al., 1998] and to be of high antifracture efficacy [Black et al., 2000]. Thus, from this point of view the anti-osteoporosis efficacy of PEMFs observed in this randomized, alendronate-controlled clinical trial was of clinical significance and further confirmed the application of PEMFs for PMO.
The effect of PEMFs for osteoporosis was first introduced by NASA, which showed that they could be equally effective as mechanical stimulation in maintaining or improving bone mass. In vitro studies have provided positive evidence from different aspects. For example, PEMFs were observed to accelerate the apoptotic rate in osteoclasts derived from primary osteoblasts and bone marrow cell cocultures [Chang et al., 2006]. With low-frequency, they were able to stimulate osteoblast growth, release transforming growth factor beta1 (TGFbeta1), and increase alkaline phosphatase (ALP) activity [Li et al., 2007]. As for its effect on intracellular signaling events, it was revealed that the anabolic effects of PEMFs may be mediated through the activation of endothelial nitric oxide synthase (eNOS), insulin receptor substrate-1 (IRS-1), and S6 ribosomal subunit kinase [Schnoke and Midura, 2007]. When applied to human bone marrow mesenchymal stem cells (BMMSC), PEMFs (4.5 ms bursts repeating at 15 Hz) could enhance BMMSC proliferation [Sun et al., 2009]. Its multilineage differentiation potential, on the other hand, was similar to that of the control group. Furthermore, it was observed that in the marrow culture system, PEMFs could regulate the expression of nuclear factor kappa B (RANK) [Chen et al., 2010], an important receptor for the activation of osteoclasts and the development of osteoporosis.
Clinical reports of PEMFs for treating bone loss, however, showed controversial results. Using 72 Hz pulsating electromagnetic fields (PEMFs) for the radii of osteoporosis-prone women, the BMD of the treated radii increased significantly during the exposure period and a “cross-talk” effect on the non-treated radii was also detected [Tabrah et al., 1990]. When PEMFs were used to treat osteoporosis at the knee for 6 months, the BMD was observed to increase 5.1% in the stimulated knees and decrease 6.6% in the control knees at 3 months. However, it returned to near baseline values at 6 months and both knees had lost bone at a similar rate at 12 months [Garland et al., 1999]. Moreover, one double-blind study found that PEMFs could prevent bone loss adjacent to the distraction gap for participants undergoing limb lengthening [Eyres et al., 1996]. Another single-blind, randomized pilot study, however, did not detect a significant increase in BMD in both groups, although serum osteocalcin and serum procollagen type I C-terminal propeptide were observed to increase significantly in the treatment group [Giordano et al., 2001]. Most recently, a randomized, sham-controlled study also did not observe significant effect of PEMFs on forearm disuse osteopenia [Spadaro et al., 2011]. All these trials were conducted under different clinical settings using PEMFs with different parameters, and none of them were performed among women with PMO, the majority of people with osteoporosis.
In the present study, we explored the effects of PEMFs on PMO in southwest China. The parameters applied were based on previous findings [Chang and Chang, 2003; He et al., 2007; Huang et al., 2008; Yang et al., 2008; Jing et al., 2010]. For example, effects of different intensity, 0.77, 3.82, and 9.87 mT, were compared for the prevention of osteoporosis and the most effective one was proved to be that of 3.82 mT [Yang et al., 2008]. Additionally, we explored the efficacy of anti-osteoporosis treatments using variables given much attention to, that is, mean percentage changes from baseline in BMDL, BMDF, 25(OH)D concentrations, total LE MMT score and BBS score. Furthermore, we applied a mixed linear model [Cleophas et al., 2012] with adjustments for covariables, taking into account the interaction of intervention group with observation time point.
The inconsistent results between the intergroup analysis and intragroup analysis may be due to the different baseline characteristics, even though they were found to be non-significant (Table 1), which may have resulted from the small sample size. When using the Wilcoxon signed rank test, baseline characteristics were not taken into account, whereas in the mixed linear model they were shown to be important covariables. Therefore, we concluded that a course of PEMFs treatment was as effective as alendronate in improving either the primary endpoint (BMDL) or the secondary endpoint (BMDF), and the effect could be maintained for at least 19 weeks after treatment ceased. However, although there was no significant comparison detected between the two groups, we should notice that the mean percentage changes of BMD in the PEMFs group were consistently lower than that of the alendronate group (Table 2), especially for the lumbar spine at the last visit (P = 0.05). Therefore, the non-significant comparisons may be due to the small sample size, the specific population selected, or potential confounding factors out of our consideration. Moreover, even though the outcome assessor was blinded to the intervention, results might still be biased because participants and caregivers were not blinded. Thus, these findings should be interpreted with caution and more investigations are warranted.
Furthermore, it is generally accepted that increasing bone mass and reducing the risk of fracture are the optimal treatments for osteoporosis. Deterioration in bone mass and quality as well as deterioration in muscular performance, balance, and coordination increases the risk of fracture [Gregg et al., 2000]. In this study, apart from BMD, we assessed the effect by further checking the following variables: vitamin D status (serum 25(OH)D concentrations), which is essential for bone metabolism, muscle function and prevention of falls in elderly people [Janssen et al., 2002], lower-extremity muscle strength (total LE MMT score) and static and dynamic balance (BBS score). All these are associated with fall risks and are thus related to the risk of fracture [Janssen et al., 2002; Menz et al., 2005].
Normal vitamin D status is essential for human health [Janssen et al., 2002]. This study was the first attempt to explore the effect of PEMFs in improving the status. The explanation for the inconsistent results between intergroup and intragroup analyses was similar to that of the BMD, that baseline characteristics might be the confounding factors. Another possible reason was that the effects in the two groups might all be attributed to the vitamin D supplements. Although the mechanisms were unclear, and is was unknown whether the effects were due to the two treatments or vitamin D supplements, we could conclude that the effect in improving vitamin D status was at least comparable between the two groups.
Muscular performance, balance, and coordination are essential factors for preventing falls [Gregg et al., 2000; Janssen et al., 2002; Menz et al., 2005]. For the first time, we also assessed the potential effects of alendronate and PEMFs regarding the total LE MMT score and BBS score. The comparable effect of the two groups revealed that both treatments might play a role in reducing fracture risk other than increasing BMD. We assumed that the improved vitamin D status might be the reason, which was supported by correlation analyses showing that 25(OH)D concentrations were positively related to muscle strength, which in turn was highly correlated with the BBS score (Table 4). Although the mechanism was unclear, these findings were of great clinical significance.
From the significant relationships detected in the correlation analyses (Tables 4 and 5), the following four facts were further confirmed: First, BMDL and the ability to keep balance decrease with age. Second, people with higher BMI are probably not prone to osteoporosis. Third, vitamin D status is important for muscular performance, balance, and coordination. Fourth, one may improve their balance ability by improving muscle strength, especially the strength of the lower extremity. We should note that the BMI was observed to be adversely associated with the BBS score. Since a high BMI was beneficial for keeping bone mass, at the same time came the question of whether a high BMI was a protective factor in preventing incident fractures. It was impossible to be examined in this study because none of the participants experienced fractures. But, from previous reports it seems that a low BMI confers a risk of substantial importance for all fractures that are largely dependent on BMD [De Laet et al., 2005]. Another unexpected result is the inverse association between the BMDF and BBS score, which we assumed occurred by chance since it was unreasonable and only marginally significant.
Limitations of the Study
One of the limitations of this study is the failure to blind participants and therapists to treatment allocation. Confounding factors such as the psychological status of the participants might have also contributed to the outcomes. Moreover, even though outcome assessors were blinded to the allocation, bias could still exist since participants might divulge information about the allocation information during visits. On the other hand, the primary outcome, BMDL, an objective variable obtained by instrumentation, is not susceptible to the bias mentioned above. Furthermore, the results were obtained by an independent technologist and the assessing process involved almost no contact with participants. So, from this point of view this limitation might not be so significant.
Another limitation is the sample size, which seems small. However, the power based on the primary outcome in a linear mixed model was calculated post hoc. The retained sample size of 41 participants with a SD of 0.86 has a power of more than 0.99 to detect a 2.45% between-intervention difference in the mean percentage change from baseline using a two-sided alpha of 0.05. Therefore, this limitation once again might not be a significant influence on the conclusions.
CONCLUSIONS
This randomized, active-controlled clinical trial for first the time revealed that a course of PEMFs treatment with specific parameters was as effective as alendronate in treating PMO in southwest China. The effect could be maintained for at least 19 weeks after treatment ceased compared to the active control, alendronate, which was taken orally throughout the entire study period.




