The effects of moderate-intensity gradient static magnetic fields on nerve conduction
Abstract
Whether exposure to static magnetic fields (SMF) for medical applications poses a therapeutic benefit or a health hazard is at the focus of current debate. As a peripheral nerve model for studies of the SMF effects, we have investigated whether exposure of in vitro frog sciatic nerve fibers to moderate-intensity gradient SMF up to 0.7 T modulates membrane excitation and refractory processes. We measured the changes in the amplitudes of the electrically evoked compound action potentials for three groups: a control group without SMF exposure and two exposed groups with continuous inhomogeneous exposure to maximum flux densities (Bmax) of 0.21 and 0.7 T SMF for 6 h. The values of the nerve conduction velocity of C fibers were significantly reduced by Bmax of 0.7 T SMF during the 4- to 6-h exposure period but not by Bmax of 0.21 T SMF during the entire exposure period of 6 h, relative to the unexposed control. From these findings, we speculate that exposure to moderate-intensity gradient SMF may attenuate pain perception because the C fibers are responsible for pain transmission. Although the mechanistic reasons for this decrease have yet to be clarified, SMF could affect the behavior of some types of ion channels associated with C fibers. Bioelectromagnetics 33:518–526, 2012. © 2012 Wiley Periodicals, Inc.
INTRODUCTION
Several studies have reported on the physiological effects of static magnetic fields (SMF) on nerve conduction [Reno, 1969; Schwartz, 1978, 1979; Edelman et al., 1979; Gaffey and Tenforde, 1983; Satow et al., 1990, 2001; Cavopol et al., 1995; McLean et al., 1995; Osuga and Tatsuoka, 1999; Eguchi et al., 2003; Coots et al., 2004; Hinch et al., 2005; Sekino et al., 2006; Yeh et al., 2008]. There are only a few earlier studies on the effects of SMF exposure on nerve conduction in which significant effects were found in frog sciatic nerves. Reno [1969] found a significant increase in the nerve conduction velocity (NCV) of compound action potentials (CAP) in sciatic nerves exposed to a uniform SMF of 1.16 T. Edelman et al. [1979] observed a significant increase in the amplitude of CAP in frog sciatic nerves when a uniform SMF of 385 or 600 mT was applied perpendicular to the axis of the nerve fibers.
In the study of the effects of very high-intensity and high-gradient SMF on nerve conduction using an 8 T superconducting magnet, Eguchi et al. [2003] reported that the NCV of CAP was not affected by the 8 T SMF. Instead, they revealed that under SMF exposure an optimal time interval exists in the relative refractory period (1.0–1.1 ms) during which some ions move dynamically through specific ion channels. They speculated that the SMF-induced changes in the membrane permeability and membrane potential may cause a decrease in the stimulus threshold, resulting in enhanced membrane excitation during the recovery process.
By measuring muscle tension of bullfrog sartorius muscle by electrical stimulation of the sciatic nerve, Satow et al. [1990, 2001] found that spatially homogeneous SMF of 0.65 T increased excitability during the recovery period in a conditioning-test stimulation paradigm, when sciatic nerves were exposed to the SMF for more than 3 h. However, it has been reported that the NCV of CAP was not affected by SMF ranging from 0.65 to 8.0 T [Schwartz, 1978; Gaffey and Tenforde, 1983; Satow et al., 1990, 2001; Osuga and Tatsuoka, 1999; Eguchi et al., 2003]. Therefore, it has been believed that action potential propagation does not constitute a major factor in the SMF effects on muscle tension developed by nerve stimulation.
In contrast, Coots et al. [2004] reported that exposure to a homogeneous SMF of 0.5 T induced a decrease in the amplitude of CAP without a change in the response latency during exposure of excised adult guinea pig spinal cords. A maximum effect was evident 1–2 min after the SMF was turned on, with a return to baseline within 1 min after the SMF was turned off. These results were explained by a conduction block in the small axon subpopulation owing to the SMF effect on voltage-activated Na+ channels. The relative selectivity of the SMF was believed to occur because of the relatively greater density of Na+ channels present in smaller axons. According to the theoretical estimation, however, it was assumed that only a very strong SMF of 24 T or more changed the NCV by producing sufficient Lorentz forces for slowing axonal conduction by 10% [Wikswo and Barach, 1980].
Thus, the experimental results and theoretical investigations of SMF effects on nerve conduction have been equivocal and the underlying mechanisms have not been clearly elucidated in lower-intensity levels, particularly less than 1 T SMF. Accordingly, this study focuses on the effect of moderate-intensity inhomogeneous SMF up to 0.7 T on the membrane excitation and refractory processes of frog sciatic nerve fibers.
MATERIALS AND METHODS
Animals
The sciatic nerve of the frog has been used as one of the best study models for nerve behavior, and its easy stimulation and separation has been shown to be suitable for experiments [Eckert et al., 2002]. Adult male African clawed frogs (Xenopus laevis, weight ∼55–70 g, length ∼6–7 cm) were used in this study. Frogs were purchased from Hamamatsu Seibutsu Kyozai (Hamamatsu, Japan). The animal experiments were carried out with the approval of the Animal Ethics Committee of Chiba University (Chiba, Japan). The frogs were euthanized in a 0.1% solution of anesthetic FA-100 (10% solution of eugenol; Tamura Pharmaceutical, Osaka, Japan) and a sciatic nerve bundle was excised, ligated at both ends with a cotton suture thread (USP No. 1, diameter 0.80–0.899 mm; Akiyama Seisakusho, Tokyo, Japan) and soaked in Ringer's solution. After soaking in Ringer's solution for 1 h at 25 ± 0.5 °C, the dissected sciatic nerve bundle (diameter ∼2.5 mm, length ∼60 mm) of a Xenopus frog was placed on 12 platinum electrodes spaced 5 mm apart in an acrylic moist nerve chamber (70 mm × 20 mm × 25 mm) with a transparent lid.
Exposure System
The SMF was generated by two neodymium–iron–boron (NdFeB) magnet assemblies (TDK, Tokyo, Japan). One magnet assembly was constructed by a pair of rectangular NdFeB magnets [15 cm × 15 cm × 2.4 cm; maximum flux density (Bmax) 0.26 T]. The magnetic plates, with opposite polarities vertically attracting each other through a 5 cm air gap, were fixed parallel to both sides of the stainless frame. In the other magnet assembly, a pair of stronger NdFeB magnetic plates (15 cm × 15 cm × 6 cm; Bmax 0.75 T) were used with the same air gap of 5 cm.
The chamber was centered on the lower magnetic plate in one of the two magnet assemblies (Fig. 1). The SMF was oriented vertically allowing a chamber to rest on one pole (north-seeking pole). A nerve bundle was placed on a ladder of electrodes in the chamber. The direction of the long axis of the nerve bundle was oriented orthogonal to the attracting force of the magnetic plates. The spatial distribution of the SMF was measured along the y-axis using a Gauss/Teslameter (Model 4048, Hall probe A-4048-002; Bell Technologies, Orlando, FL). An entire nerve bundle in the chamber was exposed to spatially inhomogeneous SMF (Fig. 1A,B). The Bmax in the center of the nerve bundle was 0.21 T (Fig. 1A) or 0.7 T (Fig. 1B) at the mid-pole field in each magnet assembly.
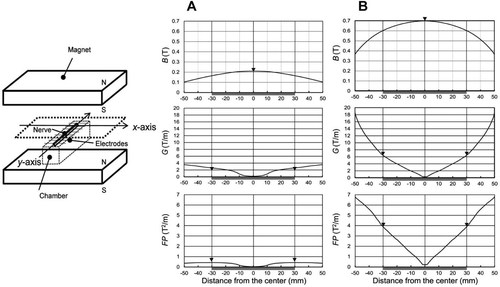
Schematic view of exposure of a nerve bundle to a magnet assembly with a vertically oriented and spatial distribution of SMF in two types of magnet assemblies along the y-axis. The entire nerve bundle was exposed to a spatially inhomogeneous SMF of Bmax 0.21 T (A) or 0.7 T (B) at the mid-pole field along the y-axis. Horizontal gray bar indicates a nerve bundle (length ∼60 mm) placed ∼30 mm from the center of each magnet assembly. A,B: Upper row, magnetic flux density (B) values; middle row, magnetic gradient (G) values; lower row, force product (FP) values, and ▾, respective peak values.
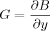 (1)
(1)The force product (FP) was defined as:(2)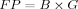
In the magnet assembly of Bmax = 0.21 T, the maximum values of the magnetic gradient (Gmax) and force product (FPmax) were 2.60 T/m and 0.43 T2/m, respectively, in the periphery of the nerve bundle (Fig. 1A). In the magnet assembly of Bmax = 0.70 T, the Gmax and FPmax values were 6.47 T/m and 3.90 T2/m, respectively, in the same portion of the nerve bundle (Fig. 1B).
The nerves were maintained under stable physiological conditions for 6 h. The ambient temperature in the magnet was maintained at 25 ± 0.5 °C with a relative humidity of 50 ± 10%.
Experimental Protocol
The nerve bundle was electrically stimulated and then exposed to a non-homogeneous SMF of Bmax 0.21 T or Bmax 0.7 T. Except for a strong SMF generated by a superconducting magnet, detailed procedures have been described elsewhere [Eguchi et al., 2003]. Briefly, a stimulus current was applied to the sciatic nerve bundle through two stimulating electrodes at the proximal end and the values of CAP (i.e., the summed electrical activity resulting from action potentials occurring simultaneously in several motor neuron axons) were measured across paired recording electrodes at the distal end. A measurement instrument (MEB-5508, Neuropack Σ; Nihon Kohden, Tokyo, Japan) was used to stimulate the nerve bundle and record the CAP. The reference electrode was connected to the earth terminal of the power source to eliminate stray current interference. All electrodes were made of pure platinum, which is an inert and non-ferromagnetic material (molar magnetic susceptibility χmol (cgs) = 193 × 10−6 cm3/mol at 295 K) [Martienssen and Warlimont, 2005] and, therefore, is apparently neither attracted nor repelled by a magnet.
Relative Amplitude During the Refractory Period
To evaluate the possible SMF effects on refractory processes, double-pulse stimulation with varying interpulse intervals ranging from 0.6 to 10.0 ms and a selected stimulus current intensity of 1.0 mA was applied to the nerve bundle because the wave form of the CAP was clear at the stimulus intensity of 1.0 mA. The threshold current was estimated to be 0.2–0.3 mA from our preliminary studies. When the stimulus intensity of 0.2–0.3 mA was applied, a very small CAP appeared. These values are consistent with the experimental values obtained by Iramina and Ueno [2007]. The values of CAP and action potentials of Aα fibers were recorded at 5 and 50 mm from the proximal stimulating electrode, respectively. According to the method of Eguchi et al. [2003], we estimated the duration of the refractory period and analyzed the changes in the relative amplitude, which refers to the amplitudes of the action potentials during the refractory period normalized to the maximal peak of the action potentials at varying interpulse intervals.
Data were collected from the sciatic nerves in either the right or left sides of 14 animals, in 10 of which stability was sufficient to allow detailed analysis. The animals were randomly divided into two groups: the control (sham exposure) group without SMF exposure (n = 5) and the exposed group with Bmax 0.7 T SMF exposure (n = 5) for up to 6 h. The values of CAP and action potentials of Aα fibers were measured for the two groups.
Nerve Conduction Velocity (NCV)
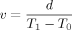 (3)
(3)Data were collected from the sciatic nerves of either the right or left sides of 20 animals, in 15 of which stability was sufficient to allow detailed analysis. The animals were randomly divided into three groups: the control (sham exposure) group without SMF exposure (n = 5) and two exposed groups with SMF exposure to Bmax 0.21 T (n = 5) or Bmax 0.7 T (n = 5) for up to 6 h. We measured the peak latency of multiple peaks in the recorded CAP and analyzed the different kinds of NCV, depending on their structure and function. The NCV values were compared for the three groups.
Statistical Analysis
Comparisons over time between groups were made using two-way analysis of variance with repeated measures, followed by the Wilcoxon rank sum test for pairwise comparisons.
RESULTS
Compound Action Potentials (CAP)
We found and could discriminate multiple peaks in the recorded CAP. Nerve fibers were classified into several types (Aα, Aβ, Aγ, Aδ, B, and C) according to their NCV and function (Fig. 2). These different kinds of fibers have different peak latencies in the recorded waveforms because of their different NCV. We identified the largest and smallest peaks corresponding to the Aα and C fibers. In general, the thick myelinated Aα fibers (large diameter and fast conducting) are associated with motor function, while the thin unmyelinated C fibers (small diameter and slow conducting) are responsible for pain transmission. The respective NCV of the Aα and C fibers typically recorded are 38 and 0.985 m/s.
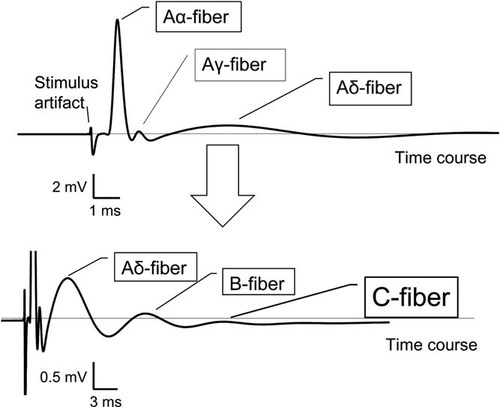
Typical multiple peaks in the recorded action potentials. Depending on their structure and function, nerve fibers with different velocities are classified into several types.
Relative Amplitude During the Refractory Period
Regarding Bmax 0.7 T SMF effects on relative amplitude during the refractory period for exposures up to 6 h, the CAP (at a distance of d = 5 mm between the stimulus and measurement points) and action potentials of Aα fibers (d = 50 mm) were measured using double-pulse stimulation with interpulse intervals ranging from 0.6 to 10 ms and a selected stimulus intensity of 1.0 mA. Here, the relative amplitude was calculated as the ratio of the amplitudes of the action potentials during the refractory period (V2) normalized to the maximal peak of the action potentials (V1) at varying interpulse intervals. We analyzed the relative amplitude ratios (V2/V1) of CAP and action potentials of Aα fibers and estimated the duration of the refractory period. The duration of the absolute refractory period, when a new action potential cannot be initiated, was estimated to be ∼0.6–0.8 ms with and without exposure (data not shown). The duration of the relative refractory period, which is the amount of time that it takes a neuron to recover from stimuli, was estimated to be ∼1.0–3.0 ms and its duration was not changed by Bmax 0.7 T SMF (data not shown). From the absolute refractory period to 10 ms, there were no changes in the relative amplitude ratios of CAP and action potentials of Aα fibers during exposure to Bmax 0.7 T SMF for 6 h (data not shown).
Nerve Conduction Velocity (NCV)
The NCV values of C fibers were significantly reduced by Bmax 0.7 T SMF during the 4- to 6-h exposure period compared with the unexposed control (Fig. 3, Table 1). Six hours after the onset of exposure to Bmax 0.7 T SMF, the NCV value of C fibers was decreased by 4.8% (0.985–0.938 m/s; Fig. 3, Table 1). However, the NCV values of C fibers were not significantly changed by Bmax 0.21 T SMF during the entire exposure period of 6 h, relative to the unexposed control (Fig. 3, Table 1). The results, as summarized in Table 1, demonstrate a significant decrease in the NCV values of C fibers during exposure to Bmax 0.7 T SMF alone. The NCV values of Aα fibers (Table 1) and other nerve fibers (Aβ, Aγ, Aδ, and B fibers; data not shown) were not affected by both exposure intensities.
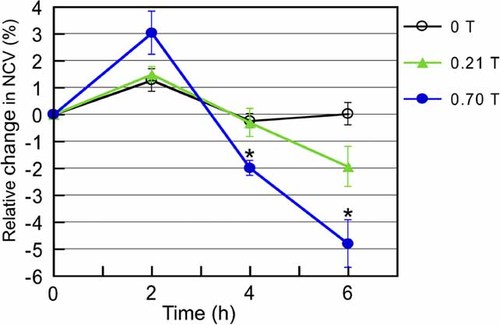
Time course of relative change in the nerve conduction velocity (NCV) of C fibers under a stimulus intensity of 1.0 mA with a pulse repetition rate of 10 Hz and a pulse width of 0.01 ms in exposed Bmax 0.7 T SMF, Bmax 0.21 T SMF, and control groups. Solid blue circles represent the exposed Bmax 0.7 T SMF group; solid green triangles represent the exposed Bmax 0.21 T SMF group; and open black circles represent the control group (0 T). Values are expressed as mean ± SEM. NCV values are significantly reduced by 0.7 T SMF exposure for 4–6 h compared with the control group. *P < 0.05 is considered statistically significant. Baseline value = 0%.
| Exposure | Types of fibers | Exposure duration (h) | |||
|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | ||
| 0 T | C | 0.990 ± 0.014 | 1.003 ± 0.009 | 0.988 ± 0.006 | 0.992 ± 0.013 |
| Aα | 37.82 ± 6.99 | 37.68 ± 6.70 | 37.77 ± 6.87 | 37.94 ± 7.45 | |
| 0.21 T | C | 0.992 ± 0.012 | 1.007 ± 0.013 | 0.989 ± 0.014 | 0.973 ± 0.016 |
| Aα | 37.78 ± 7.02 | 37.94 ± 7.33 | 38.03 ± 7.11 | 38.31 ± 7.95 | |
| 0.7 T | C | 0.985 ± 0.012 | 1.015 ± 0.016 | 0.965 ± 0.007* | 0.938 ± 0.017* |
| Aα | 38.04 ± 7.20 | 37.86 ± 6.98 | 38.13 ± 7.24 | 38.43 ± 7.14 | |
- Values represent mean ± SEM of five animals per group.
- * P < 0.05 compared with the control group (0 T).
DISCUSSION
Electrical stimulation for the frog sciatic nerve has served as an electrical nociceptive stimulus [Shrager, 1987, 1988; Levenson and Rosenbluth, 1990; Dugandzija-Novaković et al., 1995]. Therefore, our findings of reduced NCV of C fibers by exposure to Bmax 0.7 T SMF imply that moderate-intensity gradient SMF contributes to their preferential analgesic action against C fiber-mediated electrical current nociception. In contrast, exposure to Bmax 0.21 T SMF did not change the NCV of C fibers.
We speculate that the action of moderate-intensity gradient SMF involved in this process may be similar to that of Na+ channel-blocking anesthetics. Even if the duration from anesthesia to the first NCV measurements (0-h exposure period) was approximately 1 h, the Na+ channel-blocking anesthetic used in this study could interfere, at least in part, with the reduction in the NCV induced by the SMF. Therefore, we cannot rule out the effect of Bmax 0.21 T SMF on the NCV in intact neurons.
Regarding very strong SMF effects on nerve conduction in frog sciatic nerves, the previous experimental results obtained by Eguchi et al. [2003], which were obtained by exposure to high-intensity and high-gradient SMF of Bmax 8 T and FPmax 400 T2/m, showed that the NCV of CAP was not affected. However, the membrane excitation during the recovery process in the relative refractory period (just after Na+ channels were inactivated) of the maximal peak of nerve excitation was enhanced by 10% during the 3-h exposure period. In this study, conducted by exposure to inhomogeneous SMF up to 0.7 T, these phenomena have not been observed. Therefore, we suppose that the mechanistic effects of SMF on the nervous system appear to be substantially different, depending on the intensity and force.
A series of studies have been conducted to explore the effect of brief exposure to moderate-intensity homogeneous SMF on biological membranes, that is, changes in the molecular structure of the cell membrane and ion-specific (mainly Na+, K+, and Ca2+) voltage-gated channels [Rosen, 1994, 1996, 2003a, b; Prina-Mello et al., 2006; Petrov and Martinac, 2007; Shen et al., 2007]. In particular, concerning Na+ channels, Rosen [2003a] examined the effect of a homogeneous 125 mT SMF on the kinetics of Na+ channels in GH3 cells using the whole-cell patch clamp method. The results indicated that the peak values of the inward voltage-activated Na+ current were reduced by less than 5% during exposure to a 125 mT SMF for 150 s at 35 and 37 °C, and the activation rate for lower activation voltages decreased, which was indicated by an increase in the value of the activation time constant, τm [Rosen, 2003a]. The significant increases in τm values were found during SMF exposure and at least 100 s after cessation of SMF exposure without changing the inactivation time constant, τh [Rosen, 2003a]. Rosen's experimental observations support his own hypothesis that the slow reorientation of diamagnetic phospholipid molecules within the cell membrane is capable of distorting imbedded ion channels sufficiently to alter their function or activation kinetics because it is the structural properties of biological membranes that allows for the summation of diamagnetic anisotropies of individual molecules, and, therefore, the preferred orientation of the ensemble even at lower field strengths [Rosen, 2003a, b].
With regard to the SMF effects on nerve conduction, we suggested that the SMF did not affect large diameter and fast conducting Aα fibers (Table 1) but only C fibers with a small diameter and slow conduction (Table 1, Fig. 3). The most parsimonious explanation might be that C fibers possess a relatively greater density of voltage-gated Na+ channels. In myelinated Aα fibers, Na+ channels are clustered in the axon membrane at the node of Ranvier and are present in the internodal axon membrane (under the myelin sheath) at lower densities [Ritchie and Rogart, 1977; Waxman and Ritchie, 1985]. In contrast, for unmyelinated C fibers the distribution and density of Na+ channels are relatively uniform and may equally contribute to impulse conduction [Meiri et al., 1985]. Thus, the SMF effects on nerve conductivity are different between unmyelinated nerves and myelinated nerves, depending on the distribution and density of Na+ channels in the nerve fibers.
Moreover, Coots et al. [2004] speculated that the relative selectivity of the SMF could occur in smaller axons, such as in C fibers, because the relatively greater density of Na+ channels, which are essential for the propagation of action potentials, is present in these axons. We partially support their hypothesis after finding that moderate-intensity SMF reduced the NCV of C fibers with a greater density of Na+ channels, relative to Aα fibers, presumably via Na+ channel inactivation.
In our present study, there were no changes in latencies during the entire exposure period of SMF up to 0.7 T (data not shown). These results are consistent with the results of Coots et al. [2004], indicating that a conduction block in the subpopulation of axons would not be expected to influence the latency of the CAP because the latency would be determined by the larger and faster conducting axons.
Effects of moderate- to high-intensity SMF on individual neurons have been studied in several invertebrate species [Todorović et al., 2007; Nikolić et al., 2008; Yeh et al., 2008; Spasić et al., 2011; Yang et al., 2011]. However, inconsistent and contradictory results have been reported concerning the neural activities. Yang et al. [2011] speculated that the inconsistent changes in neuronal activities induced by the SMF may be related to the intensity of the SMF and the different membrane properties of neurons.
The SMF effects on analgesia (antinociception) via the nervous system have recently attracted considerable attention and have been reported in clinical and experimental studies [Choleris et al., 2002; Weintraub et al., 2003; Harlow et al., 2004; Prato et al., 2005; Colbert et al., 2008]. More recently, a series of studies have reported on moderate-intensity and spatial gradient SMF effects on acute pain perception, and the response to analgesia in mice [László et al., 2007, 2009; Sándor et al., 2007; Gyires et al., 2008; Antal and László, 2009] and humans [Kuipers et al., 2007; László and Pivec, 2010; Kovács-Bálint et al., 2011]. However, evidence concerning the SMF effects on nociceptive or pain sensation processes is inconsistent and contradictory in the literature, probably due to differences in species, intensities, spatial gradients, and durations of exposure. To date, it is likely that in vivo nerve conduction studies, including a review by Laakso et al. [2009], have failed to establish a link between in vitro effects and the analgesic responses observed in pain studies.
There are two basic mechanical mechanisms of high-intensity SMF (>1 T) on biological materials or systems, which are made up of diamagnetic water, proteins, and organic molecules: first, the magnetic torques on objects; and second, the mechanical force effects [Ueno and Shigemitsu, 2007; Ueno and Okano, 2011]. When biological materials or systems are exposed to a spatially homogenous SMF, they tend to rotate in a stable direction, which is determined by the anisotropy of the magnetic susceptibility of the materials and the magnetic torque acting on the materials. Next, when biological materials or systems are exposed to a spatially inhomogenous SMF, the materials or systems tend to move in the direction of the steepest gradient of magnetic force. However, in terms of the magnetic force or magnetic torque, their contributions to antinociceptive and neuromodulatory effects have not been clarified.
It has been reported that antinociceptive effects are induced by whole-body exposure to not only homogeneous but also inhomogeneous SMF in mice [László et al., 2007, 2009; Sándor et al., 2007; Gyires et al., 2008; Antal and László, 2009]. In addition, it has been proposed that moderate-intensity SMF effects on the blockade of action potentials in vitro are due to the steep magnetic gradient and not the strength of the SMF [Cavopol et al., 1995; McLean et al., 1995].
More recently, Oliviero et al. [2011] found neuromodulatory effects of a gradient SMF (Bmax 480 mT for 10 min) in healthy humans without changing the resting motor threshold. Here, the resting motor threshold was a possible marker for membrane excitability in pyramidal output cells [Ziemann et al., 1996]. Therefore, regarding the possible mechanism, Oliviero et al. [2011] agreed with the proposed hypothesis of Rosen [2003b] that the changes in cortical excitability induced by SMF may not depend on the membrane excitability of pyramidal neurons but on alterations at the synaptic level. However, a series of Rosen's experimental studies [Rosen, 1994, 1996, 2003a, b; Coots et al., 2004] and the aforementioned earlier studies with significant results [Reno, 1969; Edelman et al., 1979] were conducted using homogeneous SMF, and, therefore, in some cases, the magnetic torque may play a key role in modulating ion channels of excitable membranes.
With regard to uniform and high-intensity SMF effects on cortical excitability, Schlamann et al. [2010] did not find any neuromodulatory effect of SMF (1.5 and 7 T for 63 min) in healthy humans. Oliviero et al. [2011] speculated that if diamagnetic ion movements in membrane ion channels are an important factor for the gradient SMF effects, these gradient differences might explain why their experimental procedure was relatively effective in inducing excitability changes.
We have opted to use gradient SMF rather than homogeneous SMF. Although not specifically addressed in the aforementioned studies, including our own (Bmax 0.70 T, Gmax 6.47 T/m, FPmax 3.90 T2/m), for future research it is important to determine whether these changes are due to the effects of magnetic intensity, gradient, or force. We speculate that the reduction in the NCV per se is sufficient to explain one of the possible mechanisms for the increase in the pain threshold and this is ensured from the current study. A breakthrough in studying the molecular mechanism could provide the framework for understanding the effects of moderate-intensity gradient SMF on nerve conduction. Thus, further experiments are required to examine the effects of SMF on the nervous system to resolve the existing discrepancies and to clarify the mechanisms responsible for moderate-intensity gradient SMF effects in more detail.
CONCLUSION
The NCV values of C fibers were significantly reduced by Bmax 0.7 T SMF during the 4- to 6-h exposure period but not by Bmax 0.21 T SMF during the entire exposure period of 6 h compared with the unexposed control group. These results implicate that exposure to moderate-intensity and inhomogeneous SMF may attenuate pain perception because the C fibers are responsible for pain transmission. Taking these findings into consideration, although the mechanistic reasons for this decrease have yet to be clarified, SMF could affect the behavior of some types of ion channels associated with C fibers.




