No effects of short-term GSM mobile phone radiation on cerebral blood flow measured using positron emission tomography
Abstract
The present study investigated the effects of 902.4 MHz Global System for Mobile Communications (GSM) mobile phone radiation on cerebral blood flow using positron emission tomography (PET) with the 15O-water tracer. Fifteen young, healthy, right-handed male subjects were exposed to phone radiation from three different locations (left ear, right ear, forehead) and to sham exposure to test for possible exposure effects on brain regions close to the exposure source. Whole-brain [15O]H2O–PET images were acquired 12 times, 3 for each condition, in a counterbalanced order. Subjects were exposed for 5 min in each scan while performing a simple visual vigilance task. Temperature was also measured in the head region (forehead, eyes, cheeks, ear canals) during exposure. The exposure induced a slight temperature rise in the ear canals but did not affect brain hemodynamics and task performance. The results provided no evidence for acute effects of short-term mobile phone radiation on cerebral blood flow. Bioelectromagnetics 33:247–256, 2012. © 2011 Wiley Periodicals, Inc.
INTRODUCTION
A large number of behavioral and neurophysiological studies have investigated possible effects of mobile phone radiation on cognition and brain function. There is growing evidence of no effects on cognitive performance, whereas neurophysiological studies have found inconsistent results on the electroencephalogram (EEG) [Kwon and Hämäläinen, 2011]. Other approaches such as brain hemodynamic response measurements using positron emission tomography (PET) with the 15O-water tracer are promising, but currently there are only a few studies, with inconsistent results [Huber et al., 2002, 2005; Haarala et al., 2003; Aalto et al., 2006; Mizuno et al., 2009].
The 15O-labeled water is a traditional cerebral blood flow (CBF) tracer in PET studies [Fox et al., 1984]. Local CBF responses to external stimuli or cognitive tasks in such studies are taken as indicators of local neural activity. Due to the mechanisms of neurovascular coupling, it takes only a few seconds for a local CBF to reach its peak and return to background levels after onset and end of stimulus-related activity, respectively [Girouard and Iadecola, 2006]. Therefore, the CBF–PET method is appropriate for registration of neurally mediated CBF changes even when using short exposures (e.g., 5 min as in the present study). However, concerning mobile phone radiation, there are no known mechanisms as to how it would affect brain function.
In two previous CBF–PET studies, Huber et al. [2002, 2005] acquired [15O]H2O–PET scans after a 30 min unilateral (left) exposure to 900 MHz Global System for Mobile Communications (GSM) signals emitted from a planar antenna. Subjects performed a counting task during scanning. Both studies reported increased CBF in the left dorsolateral prefrontal cortex ipsilateral to the exposure to a handset signal, but the specific areas of activation were inconsistent between the two studies. In addition, even though effects of mobile phone exposure could be transient due to a low power level, the scans were acquired 10 min after the exposure period.
Haarala et al. [2003] and Aalto et al. [2006] acquired [15O]H2O–PET scans during 45 and 51 min unilateral (left) exposures, respectively, to a 902 MHz GSM handset signal emitted from a modified mobile phone. Subjects performed a working memory task (n-back, 0–3 items) during scanning under exposure. Haarala et al. [2003] first reported decreased CBF in the auditory cortex bilaterally, but this finding was probably confounded by an auditory signal emitted from the battery of the active phone. Aalto et al. [2006] controlled the auditory confound and subsequently found no CBF changes in the auditory cortex. Instead, they reported decreased CBF in the left fusiform gyrus ipsilateral to the exposure and increased CBF in the bilateral prefrontal cortex.
A recent PET study reported increased cerebral metabolic rates of glucose in the brain regions closest to the active handset after a 50 min mobile phone exposure [Volkow et al., 2011]. However, the methodology of the study and the reporting of the results have evoked considerable criticism and discussion [Davis and Balzano, 2011; Kosowsky et al., 2011; Nordström, 2011; see also reply by Volkow et al., 2011], and therefore further studies are called for. In addition, cerebral glucose metabolism and CBF are closely coupled [Fox et al., 1988] but represent different physiological processes.
Despite similar exposure locations, previous CBF–PET studies reported non-overlapping brain regions being affected by phone radiation. Differences in study design and exposure parameters could be the reasons for such a discrepancy. The present study investigated the effects of a pulse-modulated 902.4 MHz GSM handset signal on CBF, using three different phone locations (left ear, right ear, forehead) to test for possible exposure effects on brain regions close to the exposure source. This study was particularly stringent in exposure and dosimetry of the phone radiation. Whole-brain [15O]H2O–PET images were acquired during exposure while subjects performed a simple visual vigilance task. Temperature of the face skin and in the ear canals was recorded because temperature rise in adjacent tissues due to phone radiation has been reported [Paredi et al., 2001; Pau et al., 2005].
MATERIALS AND METHODS
The study protocol was approved by the Ethics Committee of the Hospital District of Southwest Finland in Turku, and the study was performed according to the Declaration of Helsinki. Subjects were 15 right-handed male volunteers aged 20–28 years (mean ± SD = 24.3 ± 2.6) without a history of neurological or psychiatric problems, or permanent medications. They gave informed consent and were paid for their participation.
Prior to the experiment, a high-resolution T1-weighted anatomical magnetic resonance imaging (MRI) image was acquired from each subject using a Philips Gyroscan Intera 1.5 T CV Novo Dual Scanner (Philips Healthcare, Best, The Netherlands) with a standard head coil (repetition time (TR) = 25 ms, echo time (TE) = 4.6 ms, flip angle = 30°, voxel size = 1.09 mm × 1.09 mm × 0.5 mm, matrix size = 256 × 256, and field of view (FOV) = 27.9 cm2). MRI images were used to check for brain abnormalities and for anatomical references in PET data processing. PET data were acquired in a 3D acquisition mode for 25.2 cm axial FOV with a high-resolution PET scanner (ECAT HRRT, Siemens CTI, Knoxville, TN) [Wienhard et al., 2002].
An intravenous catheter was inserted into the left antecubital vein for tracer injection. To monitor possible temperature rise in external head tissue due to phone radiation, five skin/surface temperature probes (YSI 409A, Measurement Specialties, Hampton, VA) were placed on the face: one on the forehead, two at the outer eye corners, and two on the cheeks in the middle between the outer eye corner and the corner of the mouth. These probes were 1.5 mm thick, 5 mm diameter pressed-disc ceramic sensors, connected to a 0.75 mm thick flat ribbon cable. Two flexible catheter probes (YSI 555, Measurement Specialties) of 0.7 mm diameter were inserted into the ear canals. The temperature was measured at 1 min intervals during the experiment and recorded in data loggers (Veriteq Instruments, Type 1400, Richmond, Canada) placed on the waist. Possible effects of metallic parts of the temperature probes (and the PET scanner) on the field distribution and heating were assessed and validated by carrying out numerical simulations.
The head of the subject was restrained by the headrest of the scanner with an individual thermoplastic mask made before the experiment (Fig. 1). For the task presentation, an 18-inch LCD flat panel display connected to a laptop computer was placed outside the scanner at a distance of 40 cm from the face and at a 45 degree angle between the lying body of the subject and the scanner in an upright position.
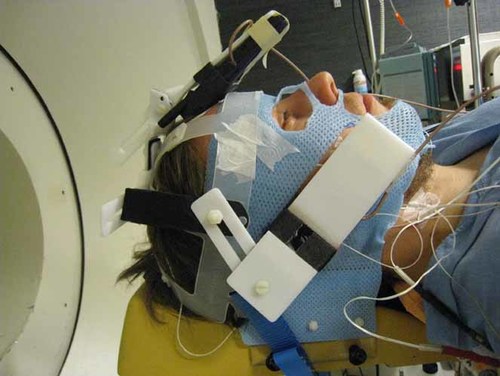
Lateral view of the subject's head prepared to be placed into the PET scanner on the left. Two phones are shown on the forehead and the right ear, and the third phone was placed symmetrically on the left ear. The head was restrained by the headrest with a thermoplastic mask, which was partly cut under the two phones on the ears and around the top of the head for direct contact between the phones and the skin. The wires around the neck were connected to the skin temperature probes under the mask. [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/bem]
Three modified mobile phones with battery and electronic parts removed were attached to a plastic helmet and placed against the left and right ears and on the forehead of the subject in the scanner (Fig. 1). Three different phone locations (LEFT, RIGHT, FRONT) and a sham exposure (OFF) were included in order to systematically test for possible effects on neural tissues receiving the highest exposure. Only one phone in the corresponding location transmitted at a time, while the other two were inactive. In the sham exposure, none of the phones transmitted the signal. Each subject was scanned 12 times for emission data, 3 scans per condition, in a counterbalanced order across subjects (Latin square design). One experimenter (MSK) controlled the exposure equipment outside the scanner room while a subject and the other experimenter (VV), who gave instructions and administered the task to the subject, were unaware of the exposure conditions (double-blind design).
Subjects were exposed to phone radiation for 5 min in each scan, which was followed by a 10 min interscan interval with no exposure (Fig. 2). After a 3 min exposure, 308 ± 15 MBq (1.5 ml) of 15O-water was injected as a 15 s bolus, which was then flushed with 7.5 ml of isotonic saline. The saline flow rate was 10 ml/min and the total injection time was 60 s. Emission data acquisition started with the injection and continued for 2 min until the end of the exposure. The 15O-water radiotracer was produced in a continuously working water module (Hidex RWG, Turku, Finland) using a diffusion membrane technique [Sipilä et al., 2001] to trap radioactive water vapor into the sterile saline.
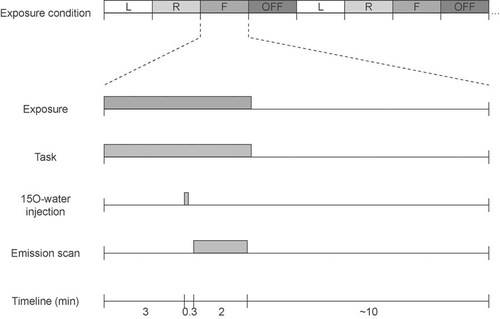
Timeline of the experimental session. The top row shows an example of the initial part of a counterbalanced sequence of left (L), right (R), frontal (F), and sham (OFF) exposure conditions. The lower part of the figure shows the timing of a single block of a given exposure condition. A bolus was injected 3 min after onset of the exposure/task period, followed by a 10 min interscan interval.
During the 5 min exposure, subjects performed a simple visual match-to-sample (0-back) task (Fig. 3) to minimize inter-condition differences in cognitive states. Circles (2 cm in diameter) of four different colors (red, green, blue, yellow) were presented in a random order in the center of the screen for 0.5 s at random 2–5 s onset-to-onset intervals with a central fixation cross. Subjects responded to the target color (red, probability = 0.25) by pressing a mouse button with the right index finger as soon as possible. Twelve different stimulus sequences were used, one for each scan, in a counterbalanced order across subjects. Stimulus presentation and response registration (reaction time (RT), and error rate) were controlled by the presentation software (Neurobehavioral Systems, Albany, CA).
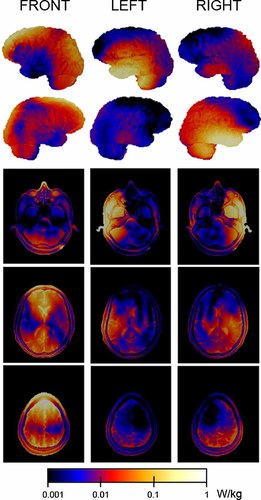
SAR distribution on the lateral surface of the brain (two upper rows) and three horizontal head slices (three lower rows) for the forehead (FRONT) and two natural talking positions (LEFT, RIGHT) of the active phones. SAR distribution takes into account the geometry of the PET scanner and temperature probes. [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/bem]
The experiment was conducted in a quiet, dimly lit room starting with a 10 min transmission scan. Because of the long PET session (15 min × 12 scans = 3 h), subjects, when needed, were released from the scanner for a short break (0–2 times per subject), preferably between the cycles of four different conditions. The experiment was then resumed after repositioning the head in the same position as in previous scans and conducting a new 10 min transmission scan. Altogether, the experiment took about 4–5 h including preparation time and breaks.
Exposure System
The phone used for the exposure was a Nokia 6110 GSM phone (Espoo, Finland) with its transmitter deactivated, battery removed, and antenna input replaced by a coaxial cable. The signal was taken from an identical phone, which was controlled by a service software (WinTesla v. 6.03, Nokia) to continuously transmit a typical GSM voice call signal. The signal consisted of 0.577 ms bursts of a 902.4 MHz carrier frequency (GSM channel # 62) repeated every 4.615 ms. The power level was adjusted with an amplifier (R720F, RF Power Labs, Woodinville, WA) and the signal was fed to the antenna of the exposing phone with a coaxial cable. Transmitted and reflected power levels were monitored during exposures with an RF power meter (NAS Z7, Rohde & Schwartz, Munich, Germany).
The exposure level is generally expressed in terms of specific absorption rate (SAR), a measure of microwave power dissipated in a unit of mass. To assess the correct input power, SAR values for the exposing phones were measured as specified by International Electrotechnical Commission (IEC) 62209-1 [IEC, 2005]. The phones were placed against the ear of a standard anthropomorphic mannequin (SAM) and SAR was measured with a dosimetric scanning system (DASY4, Schmid and Partner Engineering AG, Zurich, Switzerland). The SAM phantom was filled with head tissue simulating liquid (σ = 1.023 S/m, εr = 42.01, ρ = 1000 kg/m3). The aimed exposure level was 1 W/kg as the 10 g averaged SAR (SAR10g). The corresponding input power was 240 mW, which was similar to the maximum output power of standard GSM phones. The final measured SAR10g values were 1.0 W/kg (RIGHT) and 1.1 W/kg (LEFT), and 1 g averaged SAR (SAR1g) values were 1.4 W/kg (RIGHT) and 1.5 W/kg (LEFT).
Prior to exposures, the return loss for each exposing phone was measured with a network analyzer (HP 8752C, Hewlett-Packard, Santa Clara, CA) to ensure that the external antenna feeding was properly attached. The phones were measured at both sides of the SAM phantom and the return losses varied from 15.9 to 24.7 dB at 902.4 MHz. Hence, the feeding was operating as intended and the antenna matching was adequate.
SAR Evaluation
Numerical simulations were carried out to evaluate the SAR distribution. Possible effects of metallic parts of the PET scanner and temperature probes on the SAR distribution were also examined. Simulations were carried out at the same frequency of 902.4 MHz used in the exposure with commercial finite-difference time-domain (FDTD) software (SEMCAD X v. 14.2, Schmid and Partner Engineering AG). The results were visualized with open-source software (ParaView 3.8, Kitware, Clifton Park, NY).
Numerical models of the exposure setup consisted of 63–87 × 106 non-uniformly sized and spaced voxels. The human model used in the calculations was an MRI-based model (Duke, 34-year-old European male) of the Virtual Family [Christ et al., 2010] that was cut at the shoulder level. The basic voxel size of the head model was 2 mm × 2 mm × 2 mm. Dielectric parameters for the 45 tissues in the head model were taken from the data in Gabriel [1996].
The source model was based on the CAD data of the exposing phone provided by the manufacturer. The three phones were placed in the same locations as in exposures. The calculation grid was reduced to 0.2 mm for the antenna element of the exposing phone. The two dummy phones were modeled with a coarser grid. Dielectric properties of the phones were the same as in Boutry et al. [2008].
A simplified model of the PET scanner was generated by connecting two concentric hollow metallic cylinders consecutively with a flange. The cylinder surrounding the head was 345 mm in diameter and 315 mm in length, and the corresponding dimensions of the cylinder surrounding it were 265 mm and 500 mm. The temperature probes were modeled as 2 mm thick metallic wires. The probes inside the ear canals were coated with an insulator. All the metallic parts in the numerical model were treated as perfect electric conductors.
The numerical phone model was validated by simulating the exposure on a numerical SAM model. The phone model was placed against the ear of the SAM model and the SAR distribution was evaluated. The simulation was carried out for both sides of the head. The simulated SAR1g and SAR10g values differed from the measured values by less than 7.6% and 4.9%, respectively. Moreover, the simulated return losses and center frequencies agreed well. The quality of the source model was therefore considered adequate.
The uncertainty of the dosimetry was assessed by running multiple simulations with the model modified according to actual positioning accuracies and anatomical variations between individuals. The uncertainty of the instrumentation was also analyzed. The main factors contributing to the uncertainty of the dosimetry are postural changes during exposure, differences in head anatomy, uncertainty in incident power, and numerical uncertainties [Kuster et al., 2004]. The uncertainty due to phone positioning was assessed to be ±9%. The variation in SAR due to different head sizes was assessed by scaling the head model ±10%. Based on the simulations, differences in head anatomy produced an uncertainty of ±9%. The uncertainty in incident power was assessed to be ±10%. The numerical uncertainties result from, for example, uncertainty in dielectric properties and staircasing errors. The order of magnitude of these errors was estimated to be ±10%. The expanded uncertainty for the dosimetry was ±20% at a 95% confidence interval.
Data Analysis
In the data of each emission scan, a 90 s frame was selected starting from the moment when the true coincidence rate exceeded the random coincidence rate. The selected frames were reconstructed into images with 1.22 mm isotropic voxels using an optimized version of the ordinary Poisson ordered subset expectation maximization (OP-OSEM) 3D algorithm with 8 iterations [Hong et al., 2007] after checking the data for the tolerance to statistical bias [Johansson et al., 2007]. All images were smoothed with a 2.5 mm 3D Gaussian filter and corrected for attenuation, scattering and random events, scanner dead time, and detector normalization, and finally calibrated to kBq/ml with decay correction.
The PET data were analyzed with the SPM5 version of statistical parametric mapping (Wellcome Department of Cognitive Neurology, London, UK) [Friston et al., 2007] implemented in Matlab 7.4.0 (The Mathworks, Natik, MA). First, in order to eliminate possible differences in the head position between the scans, within-subject realignment was performed using a trilinear interpolation for both parameter estimation and volume reslicing. Each image was realigned to the first image of the series and the mean realigned image was created. All images were then realigned to the mean image. The realigned images were spatially normalized to the Montreal Neurological Institute (MNI) PET brain template using both affine (regularization factor = 1) and nonlinear (7 × 9 × 7 basis functions) interpolation and resliced to a voxel size of 2 mm × 2 mm × 2 mm using trilinear interpolation. The normalized PET images were smoothed with an isotropic 16 mm full width at half maximum (FWHM) Gaussian filter.
In statistical processing, both explorative fixed-effect group analysis and second level random-effect analysis were performed. The fixed-effect analysis of inter-condition differences was performed by fitting the PET data to a flexible factorial model implemented in SPM5 including 15 subjects and 3 repetitions of 4 conditions, while using proportional scaling for global signal correction and a series of t-tests to estimate the contrasts. The same analysis was repeated including mean RT values for each scan into the model as a covariate in order to account for the possible influence of task performance on inter-condition contrasts and to check for a possible linear relationship between local CBF and task performance.
In addition to the group fixed-effect analysis, subject-specific contrasts were obtained using a subject-by-condition interaction design with similar processing parameters and no covariate. The subject-specific contrasts were entered into the second level random-effect analysis allowing a population-level inference [Penny and Holmes, 2004]. All the real versus sham exposure contrasts (LEFT vs. OFF, RIGHT vs. OFF, FRONT vs. OFF) and the combined real versus sham exposure contrasts (0.33 × LEFT + 0.33 × RIGHT + 0.33 × FRONT vs. OFF) were analyzed along with the reversed variants of all those contrasts. The results of both fixed- and random-effect analyses were thresholded at the P < 0.05 voxel level with family-wise error (FWE) corrections for multiple comparisons.
In addition, because the brain areas with highest SAR values might show the most prominent CBF changes, separate fixed-effect analyses of two symmetric volumes of interest (VOIs) representing the left and right temporal lobes were performed. The VOIs were made using MARINA software (provided by B. Walter, Bender Institute of Neuroimaging, University of Giessen, Germany) based on the brain structure atlas by Tzourio-Mazoyer et al. [2002]. Each VOI (180 cm3) included the superior, middle, inferior, Heschl's and parahippocampal gyri, hippocampus, amygdala, and part of the fusiform gyrus. The VOI was limited posteriorly at an MNI coordinate of about y = −70 mm. Each VOI was analyzed for contrasts involving relevant conditions, that is, the left VOI for the LEFT versus OFF and OFF versus LEFT contrasts, and the right VOI for the RIGHT versus OFF and OFF versus RIGHT contrasts. The P < 0.05 voxel threshold with FWE corrections was applied for the VOI.
Performance data were lost for one scan of one subject. The RT was compared among the four conditions and tested as a function of time using a two-way repeated-measures analysis of variance (ANOVA) with condition (LEFT, RIGHT, FRONT, OFF) and cycle (three repetitions of four conditions) as independent variables. Error rates (miss, false alarm) were compared among the four conditions using one-way repeated-measures ANOVA with the condition as an independent variable.
Temperature data were compared among the four conditions and tested as a function of time for each probe using three-way repeated-measures ANOVA with condition, cycle, and time (1-min intervals of the 5-min exposure) as independent variables. Greenhouse-Geisser corrections were made for violations of sphericity, providing corrected P values (note that the reported degrees of freedom are always uncorrected). Pairwise comparisons were adjusted for multiple comparisons with Bonferroni corrections. Temperature data from 1 to 2 subjects were lost for the left (n = 14) and right (n = 14) ear canal and forehead (n = 13) due to technical problems such as bad connections between the probes and loggers.
RESULTS
The SAR distribution was slightly asymmetric between LEFT and RIGHT exposures (Fig. 3), and between the two hemispheres in the FRONT exposure. The SAR was maximal in the temporal lobe ipsilateral to the exposure source for the lateral active phone locations (LEFT, RIGHT), and in the frontal and occipito-parietal regions surrounding the interhemispheric fissure and the adjacent mesial cortices for the forehead location (FRONT). The SAR values of selected tissues for the three different exposure locations are shown in Table 1.
| SAR1g | SAR10g | SARavg | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Left | Right | Front | Left | Right | Front | Left | Right | Front | |
| Gray matter | 337.5 | 400.3 | 359.4 | 222.1 | 270.2 | 263.3 | 17.7 | 24.4 | 34.8 |
| White matter | 157.5 | 183.8 | 182.3 | 74.6 | 107.3 | 128.2 | 6.8 | 11.4 | 22.5 |
| Cerebellum | 153.2 | 77.1 | 63.1 | 87.6 | 51.6 | 33.8 | 14.3 | 10.9 | 13.2 |
| Midbrain | 21.4 | 23.9 | 17.6 | 12.2 | 16.5 | 10.7 | 10.4 | 13.9 | 9.6 |
| Thalamus | 9.5 | 14.9 | 13.5 | a | a | a | 8.2 | 13.1 | 12.1 |
| Brain avg.b | 334.4 | 392.6 | 340.4 | 212.0 | 244.9 | 234.2 | 12.8 | 17.4 | 27.0 |
| Total head | 2786.7 | 1884.9 | 1046.8 | 1007.8 | 715.5 | 666.7 | 27.7 | 29.1 | 24.5 |
- SAR1g and SAR10g are the spatial maxima of SAR, and SARavg is SAR averaged over the tissue in question. The output power of the transmitting mobile phone was 240 mW.
- a Mass of thalamus was less than 10 g.
- b Brain avg. includes gray matter, white matter, cerebellum, midbrain, and thalamus.
In the PET data, neither random-effect (t < 6.9, P > 0.13) nor fixed-effect (t < 4.21, P > 0.15) whole-brain analyses revealed significant CBF differences in any of the analyzed contrasts between the conditions. Separate analyses for the left and right temporal VOIs did not reveal significant changes either (t < 3.37, P > 0.19). Inclusion of the RT covariate into the fixed-effect model did not affect the results. A significant (t = 4.77, P = 0.023) negative correlation between RT and CBF was revealed in the left cerebellar pyramid at MNI coordinates (x, y, z) of −28, −64, and −36, respectively, but it was irrelevant to the exposure and related rather to motor preparation [Horwitz et al., 2000].
Exposure had no influence on task performance. The two-way ANOVA with condition (LEFT, RIGHT, FRONT, OFF) and cycle (three repetitions of four conditions) factors revealed no main effects or interactions for RT (mean = 420.2–433.2 ms, SEM = 13.6–15.1). The one-way ANOVA also revealed no main effects or interactions for misses (mean = 0.1–0.3, SEM = 0.1–0.2) or false alarms (mean = 0.5–1.3, SEM = 0.1–0.3).
In the temperature data, the main effect of time was significant for the left (F4,52 = 11.813, P = 0.001; subscripts represent degrees of freedom) and right (F4,52 = 4.912, P = 0.021) ear canals, and right cheek (F4,56 = 8.735, P = 0.002), indicating an overall temperature rise as a function of time, regardless of exposure locations. The main effect of condition was also significant for the left (F3,39 = 20.227, P < 0.0005) and right (F3,39 = 3.123, P = 0.037) ear canals, indicating a temperature rise during the corresponding LEFT (mean ± SEM = 0.04 ± 0.01 °C) and RIGHT (0.02 ± 0.01) exposures respectively. FRONT exposure also induced a smaller temperature rise in the left ear canal (0.02 ± 0.01). Sham exposure induced no temperature rise (mean = −0.02–0.01, SEM = <0.01) in any measurement locations. A significant condition × time interaction for the left ear canal (F12,156 = 10.895, P < 0.0005) indicated a temperature rise as a function of time during LEFT exposure (mean increment ± SEM = 0.06 ± 0.01 °C) and a smaller increase during FRONT exposure (0.03 ± 0.01).
DISCUSSION
The present study did not reveal any significant influence of the 902.4 MHz GSM phone radiation on CBF, either close to the emitting phone or elsewhere in the brain. The results of no effects on CBF cannot be explained by any inter-condition differences in the task performance because neither RT nor error rates were dependent on exposure. Moreover, inclusion of the RT into the analysis did not change the negative PET results. Even the VOI analyses for the temporal lobes failed to reveal any CBF changes, although Aalto et al. [2006] previously observed decreased CBF in the left inferior occipito-temporal cortex at the coordinates (x, y, z) −50, −48, and −20, respectively, which was covered by the left VOI in the present study.
Although these two studies were similar in terms of the exposure signal (902 MHz GSM), Aalto et al. [2006] acquired seven sequential scans for real and sham exposure conditions, allowing a 51 min constant exposure for each condition. Thus, exposure effects could accumulate throughout the scanning. The present study employed three different phone locations at the expense of exposure duration, allowing three repetitions of 5 min exposures for each of the four conditions. Thus, the results of no effects could be due to the short, intermittent exposure. Indeed, the previous studies that revealed effects of phone radiation on CBF [Huber et al., 2002, 2005; Haarala et al., 2003; Aalto et al., 2006] or glucose metabolic rate [Volkow et al., 2011] used longer exposure periods. In addition, considering possible retention of CBF changes after exposure [Huber et al., 2002, 2005], we cannot rule out possible carryover effects that could contaminate our data due to the short 10 min interval between scans with different exposure conditions.
On the other hand, we aimed at studying non-thermal effects of GSM exposure that could be mediated by changes in neural activity. Local CBF responds quickly to neural activity and fades within seconds after stimulation stops [Girouard and Iadecola, 2006]. Therefore, we reduced the exposure time to include three exposure locations and to obtain more scans per condition to get a high signal-to-noise ratio and reliable statistics. The experiment already took about 4–5 h and a prolongation of exposure time (12 scans altogether) would make the time that subjects had to stay in the scanner substantially longer, which was not desirable. However, the fact that we did not observe any CBF changes with a 3 min pre-scan and a 2 min scan exposure calls for further investigations with longer exposure times.
The temperature rise in external head tissue can be a confounding factor that leads to changes in somatosensory input and related brain activation. The phones used in the present study did not contain any electronic circuits, battery, speaker, microphone, or light-emitting diodes (LED) as these can be sources of heat during phone operation. This also ensured that the subjects had no clue to the exposure status of the phones, such as sound, vibration, or light. However, thermal effects in living tissue have often been considered as one of most possible consequences of phone radiation exposure [Paredi et al., 2001; Pau et al., 2005]. Poor heat dissipation due to direct contact between the skin and the modified phone could also induce temperature rise in the adjacent skin tissue. Thus, we opted to monitor temperature changes on the face skin as well as in the ear canals, where temperature rise has been observed earlier [Tahvanainen et al., 2007].
Although a systematic temperature rise was observed in the present study in the ear canals according to the exposure location, its magnitude was very small (<0.06 °C). This suggests that phone radiation causes only negligible thermal effects when other potential sources of heat were excluded. In previous studies, thermal effects of GSM exposure was expected to reach its maximum (0.3 K) in the tissue near the antenna ∼12 min after exposure onset [Pau et al., 2005]. Regarding brain tissue, the heating solely due to 900 MHz phone radiation was calculated to be ∼0.01 °C and would never exceed 0.1 °C [Bernardi et al., 2001]. Such temperature changes were below natural fluctuations of the body temperature and might not be accompanied by a noticeable CBF reaction.
Finally, a sample size of 10–20 subjects provides a good statistical power (i.e., reliable detection of brain activation) in 15O-water studies of human cognition [Andreasen et al., 1996; Van Horn et al., 1998]. However, as possible influences of such short-term phone exposure on brain function could be rather weak, a larger sample size would be needed to disclose the effects.
Acknowledgements
The authors are very grateful to the physician (Riitta Parkkola), radiographers (Minna Aatsinki, Heidi Betlehem, Anne-Mari Jokinen, Tarja Keskitalo, Leena Lehtimäki, Hannele Lehtinen, Kaleva Mölsä), and medical laboratory technicians (Eija Nirhamo, Emilia Puhakka, Sanna Suominen, Minna Tuominen) at Turku PET centre. We are also grateful to Tim Toivo and Tuomas Mustonen at STUK for carrying out the SAR and return loss measurements.



 O and positron emission tomography
O and positron emission tomography
