Genome-wide pathway analysis of folate-responsive genes to unravel the pathogenesis of orofacial clefting in man†
The data in this manuscript have not been presented at a meeting.
Abstract
BACKGROUND:
A cleft of the lip with or without the palate (CLP) is a frequent congenital malformation with a heterogeneous etiology, for which folic acid supplementation has a protective effect. To gain more insight into the molecular pathways affected by natural folate, we examined gene expression profiles of cultured B-lymphoblasts from CLP patients before and after the addition of 5-methyltetrahydrofolate (5-mTHF) to the cultures.
METHODS:
Immortalized B-lymphoblasts from five children with CLP were cultured in folate-deficient medium for 5 days. 5-mTHF was added to a concentration of 30 nM. Gene expression patterns were then evaluated before and after supplementation using Human Genome U133 Plus 2.0 arrays. Data analysis was performed with Omniviz and the GEPAS analysis suite. Differential genes were categorized into biological pathways with Ingenuity Pathway systems. Differential expression was validated by quantitative RT-PCR.
RESULTS:
Using supervised clustering, with a false discovery rate <1%, we identified 144 and 409 significantly up-regulated and down-regulated probesets, respectively, after 5-mTHF addition. The regulated genes were involved in a variety of biological pathways, including one carbon pool and cell cycle regulation, biosynthesis of amino acids and DNA/RNA nucleotides, protein processing, apoptosis, and DNA repair.
CONCLUSIONS:
The large variety of the identified folate responsive pathways fits with the modifying role of folate via the methylation pathway. From the present data we may conclude that folate deficiency deranges normal cell development, which might contribute to the development of CLP. The role of these folate responsive genes in CLP development is intriguing and needs further investigation. Birth Defects Research (Part A) 2008. © 2008 Wiley-Liss, Inc.
INTRODUCTION
Clefting of the lip with or without the palate (CLP) is a common congenital malformation that occurs in approximately 14.2 per 10,000 live births in the Netherlands (EUROCAT-Northern-Netherlands, 2006). The etiology of CLP is largely unknown, but is considered multifactorial in origin. As recently reviewed by Krapels et al. (2006) associations between CLP and developmental genes, such as TGFα and MSX1, and linkage disequilibrium of various chromosomal regions, such as 3p21.2, 10p13, and 16p13.3 and CLP emphasize the involvement of genetic components. Of great interest are the findings of the last two decades that environmental factors play a role in CLP etiology as well. Maternal periconception use of folic acid supplements and folate-rich food (Czeizel et al., 1999; van Rooij et al., 2004), medication (van Rooij et al., 2002), and smoking (Shaw et al., 1996; van Rooij et al., 2001) are known to modulate the risk of having a CLP child. We have shown that periconception supplementation with folic acid reduces the CLP birth prevalence rate by approximately 50% (van Rooij et al., 2004), thereby supporting the recommendation of folic acid supplementation in the periconception period, not only to prevent neural tube defects (NTDs) but CLP as well. Other countries have started folic acid fortification programs with beneficial effects on the reduction of NTDs (Mason et al., 2007; Quinlivan and Gregory, 2003). Such programs imply long-term exposure of the total population to folate supplements. It is therefore remarkable that studies investigating the effects of synthetic folic acid and natural folate on biological processes are very scarce (Mason et al., 2007).
After reduction of folic acid to natural folates, folate derivatives serve intracellularly as a one-carbon group donor for the synthesis of purines, pyrimidines, and proteins and the remethylation of homocysteine into methionine. The methionine metabolite, S-adenosylmethionine, is the main methyl donor of the cell and methylates DNA, RNA, proteins, and lipids (Chiang et al., 1996). Folate deficiency induces elevated homocysteine concentrations, uracil accumulation and misincorporation, DNA strand breaks, abnormal DNA and protein methylation patterns, and increased apoptosis (Courtemanche et al., 2004; Duthie and Hawdon, 1998; Kim, 2005; Steegers-Theunissen et al., 2000; Wasson et al., 2006). However, these biological mechanisms cannot explain the protective effects of additional intake of folic acid and food folate on CLP development.
Despite the limited knowledge of the effects of folate on molecular and biological pathways, mothers-to-be and the intrauterine-developing embryo and fetus are supplemented with synthetic folic acid. It is therefore of great interest to elucidate these pathways. In order to explore the possible options for advanced research of specific pathways we performed a pilot study to identify proteomic and genomic changes in response to folate addition. Our previous (unpublished) study on protein changes in response to folate revealed the involvement of glucose metabolism, energy production, nucleocytoplasmic transport, cell cycle regulation, cytoskeleton, protein processing, and DNA transcription and translation. Regarding these findings and the essential role of folate in DNA stability, methylation, and cell death, a clear genomic response is to be expected. To get a first impression of this response, Epstein Barr virus immortalized (EBV) B-lymphoblast cultures were induced with 5-methyltetrahydrofolate. The orientating nature of this approach, and the use of EBV B-lymphoblasts, clearly limits the possible observation of specific developmental functions of folate in facial primordia. However, we expect that there will be mutual consequences of folate supplementation. Therefore the goals of the present study are: (1) to identify folate responsive pathways using gene expression profiling; (2) to identify possible relationships of these differential genes with embryonic pathways involved in palate formation; (3) to compare the folate responsive genes with the earlier identified proteins.
MATERIALS AND METHODS
Sample Selection and Culture Scheme
Five Epstein Barr immortalized B-lymphoblast cultures were established from venous blood samples derived from five Dutch Caucasian children (two male, three female) with a nonsyndromic, complete, unilateral cleft lip, jaw, and palate following a standard protocol (Neitzel, 1986). Blood samples were collected 15 months after birth, during a nationwide case control study on orofacial clefting in the Netherlands, and frozen until use (van Rooij et al., 2003). For this study, early passages (<10) of the EBV immortalized B-cell cultures were used. To achieve folate depletion, B-lymphoblasts were cultured in folate-free RPMI (Gibco-BRL, Gaithersburg, MD) with 10% (v/v) dialyzed fetal calf serum (Perbio; Pierce Biotechnology, Rockford, IL) and 1% (v/v) L-glutamate, sodium-pyruvate, and penicillin/streptomycin (Gibco-BRL) for 5 days. On day 5 the natural folate metabolite, 5-methyltetrahydrofolate (5-mTHF; Sigma-Aldrich, St. Louis, MO) was added to reach the target concentration of 30 nM. Folate concentrations were measured in the medium using the Modular E170 electrochemiluminescence assay (Roche Diagnostics GmBH, Mannheim, Germany). B-lymphoblasts were harvested before (day 5) and after 5-mTHF addition on day 6. The B-lymphoblasts were pelleted and washed once with 10 mL phosphate buffered saline. Pellets were snap-frozen in liquid nitrogen and stored at −80°C until further analysis.
RNA Isolation, Labeling, and Hybridization
After thawing, total RNA was isolated using the RNeasy Midi kit (Qiagen, Valencia, CA) following the manufacturer's instructions. RNA concentrations and quality were measured with the Nanodrop ND-1000 UV-VIS spectrophotometer (NanoDrop Technologies, Wilmington, DE) and the Agilent 2100 BioAnalyzer (Agilent, Palo Alto, CA). RNA integrity was guaranteed by only using samples with an RNA integrity number of 9.2 or higher. RNA was stored at −80°C until further use.
RNA was purified and labeled for hybridization using the One Cycle Target Labeling kit (Affymetrix, Santa Clara, CA) with a starting amount of 5 μg RNA. Labeled cRNA was hybridized to Human Genome U133 Plus 2.0 gene Arrays (Affymetrix) and scanned on an Affymetrix Scanner 3000 (Affymetrix).
Quantitative Real-Time RT-PCR
Validation of microarray expression data was accomplished by quantitative real-time PCR of a selection of 10 genes, that is, ANKRD11, RBBP6, pTEN, BATF3, HSP90AA1, BCCIP, HNRPD, DPP3, TSC22D3, and MTCH1. First strand cDNA synthesis was performed using 2 μg total RNA and Superscript 2 enzyme (Gibco, Carlsbad, CA), according to a standardized protocol supplied by the vendor (protocol is available on request). Real-time PCR was performed using the SYBR Green PCR Kit (Applied Biosystems, Foster City, CA) in the Opticon 2 apparatus (MJ Research, Bio-Rad Laboratories Inc., Waltham, MA). For the PCR reaction, 5 ng cDNA of each sample was used. A melting curve analysis was performed for each reaction following each experiment to ensure the presence of a single amplified product. All PCRs were performed in duplicate. The expression level of each gene was normalized to the expression level of a reference gene, β-actin.
Data Analysis
Raw expression data were normalized using the Robust Multichip Average expression summary consisting of background adjustment, quantile normalization, and summarization (Bolstad et al., 2003).
Unsupervised hierarchical clustering and significance analysis of microarrays (SAM) were performed with Omniviz software version 3.8 (Maynard, MA) using all probe sets. For the clustering analysis the log transformed (base 2 scale) ratio of the expression values relative to the geometrical mean of the probe set was determined. To reveal differentially expressed genes, a SAM analysis was performed using a false discovery rate of less than 1 per 100 probe sets. Additional identification of classifying probe sets was performed with the GEPAS analysis suite, http://www.gepas.org (Montaner et al., 2006). Differential genes were visualized in biological pathways with the mapping software of Ingenuity Pathway systems (Ingenuity Systems, www.ingenuity.com) and in canonical pathways with the KEGG PATHWAY database (www.genome.jp/kegg/pathway.html).
For the quantitative real-time RT-PCR, Wilcoxon Signed Ranked Test was used to calculate significant differences. A p value <.05 was considered statistically significant.
RESULTS
Folate concentrations in the medium were on average 4.4 nM (standard deviation 0.4) and 25.9 nM (standard deviation 4.0), respectively, before and after the addition of 5-mTHF. The distribution of intensities of the raw microarray data showed increased average intensities for one cell line before folate addition because of high background signal. After normalization, gene expression profiles from samples of the same cell line were highly consistent and therefore grouped together prior to clustering by folate status (Fig. 1). However, the cluster plot also showed sets of genes from which the expressional level seemed to alternate corresponding to the folate status. These potential folate-responsive genes were identified with a SAM analysis. From Figure 1 it is also clear that after normalization the “E before” sample, that is, the sample with the high background signals, showed very low expression levels for almost all genes. For this reason, this sample was left out of further analysis, because it did not contain any usable information. The SAM analysis was performed with the standard false discovery rate of less than 1 per 100 probe sets, corresponding to a delta-value of 4.226. This resulted in the identification of 144 significant up-regulated and 409 down- regulated probe sets after addition of 5-mTHF. The median expression ratio was 1.63 (range: 1.2–3.8) for the significant down-regulated genes and 1.58 (range: 1.2–4.2) for the up-regulated genes.
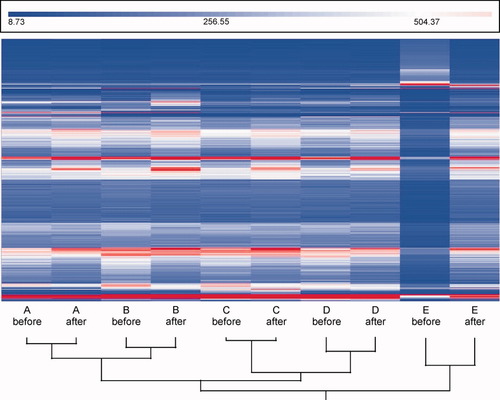
Unsupervised clusterplot of gene expression data including all probe sets of 5 B-lymphoblast cell lines (A–E), determined before and after 5-methyltetrahydrofolate addition. The values represent the log transformed (base 2 scale) ratio of the expression values relative to the geometrical mean of the probe sets. Though the data sets (all probe sets) were primarily clustered on originating cell line, there were also gene sets from which the expressional level seemed to alternate in correspondence with the folate status.
These potential folate-regulated genes are listed in Table 1 by the pathway or disease in which they are involved. These include cancer development, cell cycle checkpoint regulation, DNA replication, recombination and repair, biosynthesis of amino acids and DNA/RNA nucleotides, protein processing, and cell death. Furthermore, genes that best discriminated between pre- and postfolate-supplemented cell lines were evaluated. Figure 2 shows the 100 best classifying genes arranged by biological function. Of these classifiers, 64% were found to be significantly differentially expressed with the SAM analysis and had functions in similar pathways.
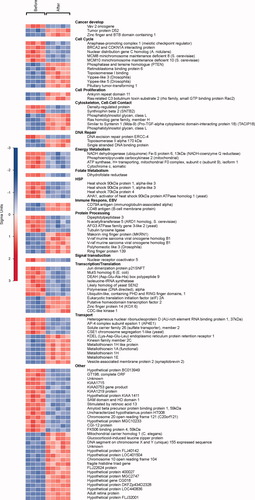
Hundred most differential genes grouped by function in B-lymphoblast cell lines before and after 5-methyltetrahydrofolate addition. The scale is presented in sigma units.
| Gene symbol | Description | Mean ratio | 95% CI |
|---|---|---|---|
| Cell cycle/cancer | |||
| BRCA1 | Breast cancer 1, early onset | 0.615 | (0.537–0.693) |
| CCNE2 | Cyclin E2 | 0.266 | (0.216–0.317) |
| CDC7 | Cell division cycle 7 homologue (S. cerevisiae) | 0.611 | (0.529–0.693) |
| FH | Fumarate hydratase | 0.707 | (0.667–0.747) |
| 0.669 | (0.638–0.701) | ||
| FUS | Fusion (involved in t(12;16) in malignant liposarcoma) | 0.469 | (0.426–0.512) |
| GART | Phosphoribosylglycinamide formyltransferase | 0.546 | (0.471–0.621) |
| HDAC2 | Histone deacetylase 2 | 0.781 | (0.728–0.833) |
| MSH2 | mutS homologue 2, colon cancer, nonpolyposis type 1 | 0.528 | (0.448–0.608) |
| MSH3 | mutS homologue 3 (E. coli) | 0.785 | (0.744–0.826) |
| MSH6 | mutS homologue 6 (E. coli) | 0.570 | (0.512–0.628) |
| PHB | Prohibitin | 0.554 | (0.485–0.623) |
| RAD51C | RAD51 homologue C (S. cerevisiae) | 0.471 | (0.405–0.536) |
| SVH | Armadillo repeat containing 10 | 0.671 | (0.603–0.738) |
| TUBG1 | Tubulin, gamma 1 | 0.572 | (0.505–0.639) |
| ZWINT | ZW10 interactor | 0.702 | (0.649–0.755) |
| AURKA | Aurora kinase A | 1.550 | (1.441–1.659) |
| CCDC28A | Coiled-coil domain containing 28A | 1.409 | (1.307–1.511) |
| CCNB1 | Cyclin B1 | 1.564 | (1.433–1.695) |
| CCNB2 | Cyclin B2 | 1.570 | (1.375–1.765) |
| CCNG1 | Cyclin G1 | 1.582 | (1.418–1.745) |
| CDC25C | Cell division cycle 25 homologue C (S. pombe) | 1.573 | (1.437–1.709) |
| CDCA8 | Cell division cycle associated 8 | 1.637 | (1.506–1.768) |
| CDKN3 | Cyclin-dependent kinase inhibitor 3 (CDK2-associated dual specificity phosphatase) | 1.541 | (1.256–1.826) |
| CSE1L | CSE1 chromosome segregation 1-like (yeast) | 0.489 | (0.433–0.546) |
| FHIT | Fragile histidine triad gene | 2.180 | (1.942–2.418) |
| MDM2 | Mdm2 p53 binding protein homologue (mouse) | 2.218 | (1.370–3.066) |
| NEK2 | NIMA (never in mitosis gene a)-related kinase 2 | 2.135 | (1.880–2.390) |
| PRDM2 | PR domain containing 2, with ZNF domain | 1.841 | (1.600–2.083) |
| PTEN | Phosphatase and tensin homologue (mutated in multiple advanced cancers 1) | 2.144 | (1.848–2.441) |
| 2.101 | (1.761–2.442) | ||
| PTTG1 | Pituitary tumor-transforming 1 | 1.592 | (1.494–1.69) |
| TPX2 | TPX2, microtubule-associated, homologue (Xenopus laevis) | 1.609 | (1.452–1.766) |
| TTC3 | Tetratricopeptide repeat domain 3 | 1.354 | (1.276–1.432) |
| Cell death | |||
| MSH2 | mutS homologue 2, colon cancer, nonpolyposis type 1 | 0.528 | (0.448–0.608) |
| PDCD8 | Apoptosis-inducing factor, mitochondrion-associated, 1 | 0.656 | (0.609–0.702) |
| SOD1 | Superoxide dismutase 1, soluble (amyotrophic lateral sclerosis 1 [adult]) | 0.679 | (0.610–0.749) |
| BCL2 | B-cell CLL/lymphoma 2 | 1.671 | (1.548–1.795) |
| PPP1R15A | Protein phosphatase 1, regulatory (inhibitor) subunit 15A | 2.194 | (1.612–2.777) |
| PTEN | Phosphatase and tensin homologue (mutated in multiple advanced cancers 1) | 2.144 | (1.848–2.441) |
| 2.101 | (1.761–2.442) | ||
| VEGF | Vascular endothelial growth factor A | 2.129 | (1.783–2.474) |
| Cellular assembly, organization, and proliferation | |||
| TTL | Tubulin tyrosine ligase | 0.380 | (0.326–0.433) |
| 0.423 | (0.366–0.480) | ||
| TOP2A | Topoisomerase (DNA) II alpha 170 kDa | 1.561 | (1.428–1.695) |
| PAFAH1B1 | Platelet-activating factor acetylhydrolase, isoform Ib, alpha subunit 45 kDa | 0.510 | (0.428–0.592) |
| VEGF | Vascular endothelial growth factor A | 2.129 | (1.783–2.474) |
| DNA replication, recombination | |||
| BCAS2 | Breast carcinoma amplified sequence 2 | 0.686 | (0.62–0.752) |
| BRCA1 | Breast cancer 1, early onset | 0.615 | (0.537–0.693) |
| CDC45L | CDC45 cell division cycle 45-like (S. cerevisiae) | 0.491 | (0.447–0.535) |
| CDC7 | Cell division cycle 7 homologue (S. cerevisiae) | 0.611 | (0.529–0.693) |
| DFFA | DNA fragmentation factor, 45 kDa, alpha polypeptide | 0.646 | (0.572–0.720) |
| DHX9 | DEAH (Asp-Glu-Ala-His) box polypeptide 9 | 0.539 | (0.479–0.599) |
| EBNA1BP2 | EBNA1 binding protein 2 | 0.422 | (0.354–0.49) |
| EXO1 | Exonuclease 1 | 0.606 | (0.538–0.673) |
| FEN1 | Flap structure-specific endonuclease 1 | 0.460 | (0.391–0.529) |
| HELLS | Helicase, lymphoid-specific | 0.471 | (0.403–0.539) |
| HNRPAB | Heterogeneous nuclear ribonucleoprotein A/B | 0.560 | (0.483–0.636) |
| MCM3 | Minichromosome maintenance complex component 3 | 0.598 | (0.528–0.669) |
| MCM4 | Minichromosome maintenance complex component 4 | 0.474 | (0.399–0.549) |
| MCM5 | Minichromosome maintenance complex component 5 | 0.598 | (0.535–0.661) |
| MCM6 | Minichromosome maintenance complex component 6 | 0.554 | (0.472–0.636) |
| NAP1L1 | Nucleosome assembly protein 1-like 1 | 0.468 | (0.409–0.527) |
| ORC5L | Origin recognition complex, subunit 5-like (yeast) | 0.482 | (0.416–0.547) |
| PAXIP1 | PAX interacting (with transcription-activation domain) protein 1 | 0.585 | (0.527–0.643) |
| POLA | Polymerase (DNA directed), alpha 1 | 0.586 | (0.553–0.618) |
| POLD2 | Polymerase (DNA directed), delta 2, regulatory subunit 50 kDa | 0.665 | (0.600–0.731) |
| POLD3 | Polymerase (DNA-directed), delta 3, accessory subunit | 0.670 | (0.600–0.740) |
| POLE2 | Polymerase (DNA directed), epsilon 2 (p59 subunit) | 0.507 | (0.449–0.565) |
| PRPF19 | PRP19/PSO4 pre-mRNA processing factor 19 homolog | 0.595 | (0.531–0.658) |
| PSMC3IP | PSMC3 interacting protein | 0.643 | (0.578–0.708) |
| 0.489 | (0.385–0.593) | ||
| RAD51C | RAD51 homologue C (S. cerevisiae) | 0.471 | (0.405–0.536) |
| RFC4 | Replication factor C (activator 1) 4, 37 kDa | 0.652 | (0.583–0.721) |
| RPA2 | Replication protein A2, 32 kDa | 0.692 | (0.629–0.755) |
| RUVBL2 | RuvB-like 2 | 0.751 | (0.704–0.798) |
| 0.763 | (0.707–0.818) | ||
| SRPK1 | SFRS protein kinase 1 | 0.582 | (0.518–0.645) |
| CD48 | CD48 molecule | 1.575 | (1.520–1.630) |
| CENPA | Centromere protein A | 1.644 | (1.423–1.866) |
| HNRPA1 | Heterogeneous nuclear ribonucleoprotein A1 | 1.492 | (1.342–1.642) |
| KIF2C | Kinesin family member 2C | 1.592 | (1.493–1.690) |
| MBD1 | Methyl-CpG binding domain protein 1 | 1.517 | (1.354–1.679) |
| PTTG1 | Pituitary tumor-transforming 1 | 1.592 | (1.494–1.690) |
| TOP2A | Topoisomerase (DNA) II alpha 170 kDa | 1.561 | (1.428–1.695) |
| Energy production | |||
| NDUFB2 | NADH dehydrogenase 1 beta subcomplex, 2, 8 kDa | 0.682 | (0.623–0.741) |
| NDUFB6 | NADH dehydrogenase 1 beta subcomplex, 6, 17 kDa | 0.709 | (0.643–0.776) |
| NDUFB9 | NADH dehydrogenase 1 beta subcomplex, 9, 22 kDa | 0.728 | (0.667–0.789) |
| NDUFS3 | NADH dehydrogenase Fe-S protein 3, 30 kDa (NADH-coenzyme Q reductase) | 0.746 | (0.695–0.797) |
| NDUFS6 | NADH dehydrogenase Fe-S protein 6, 13 kDa (NADH-coenzyme Q reductase) | 0.525 | (0.487–0.562) |
| Gene expression | |||
| BRCA1 | Breast cancer 1, early onset | 0.615 | (0.537–0.693) |
| POLA | Polymerase (DNA directed), alpha 1 | 0.586 | (0.553–0.618) |
| POLD3 | Polymerase (DNA-directed), delta 3, accessory subunit | 0.670 | (0.600–0.740) |
| MDM2 | Mdm2 p53 binding protein homologue (mouse) | 2.218 | (1.370–3.066) |
| RBBP6 | Retinoblastoma binding protein 6 | 1.310 | (1.225–1.396) |
| ZNF42 | Myeloid zinc finger 1 | 1.304 | (1.227–1.382) |
| Molecular transport | |||
| IPO11 | Importin 11 | 0.739 | (0.68–0.798) |
| KPNB1 | Karyopherin (importin) beta 1 | 0.653 | (0.614–0.692) |
| NUP205 | Nucleoporin 205 kDa | 0.812 | (0.775–0.850) |
| NUP214 | Nucleoporin 214 kDa | 0.468 | (0.407–0.529) |
| SLC36A1 | Solute carrier family 36 (proton/amino acid symporter), member 1 | 0.693 | (0.629–0.757) |
| Nucleic acid metabolism | |||
| AK2 | Adenylate kinase 2 | 0.610 | (0.545–0.675) |
| NME1 | Protein (NM23A) expressed in nonmetastatic cells | 0.498 | (0.435–0.561) |
| Post-translational modification/protein folding | |||
| BAG2 | BCL2-associated athanogene 2 | 0.642 | (0.565–0.718) |
| CCT3 | Chaperonin containing TCP1, subunit 3 (gamma) | 0.573 | (0.495–0.65) |
| CCT6A | Chaperonin containing TCP1, subunit 6A (zeta 1) | 0.466 | (0.389–0.544) |
| 0.475 | (0.402–0.548) | ||
| CCT7 | Chaperonin containing TCP1, subunit 7 (eta) | 0.621 | (0.544–0.699) |
| DNAJA1 | DnaJ (Hsp40) homologue, subfamily A, member 1 | 0.531 | (0.430–0.632) |
| HSP90AA1 | Heat shock protein 90 kDa alpha (cytosolic), class A member 1 | 0.579 | (0.528–0.630) |
| 0.490 | (0.401–0.578) | ||
| 0.511 | (0.430–0.593) | ||
| 0.624 | (0.600–0.648) | ||
| HSPA8 | Heat shock 70 kDa protein 8 | 0.738 | (0.692–0.784) |
| HSPD1 | Heat shock 60 kDa protein 1 (chaperonin) | 0.772 | (0.725–0.818) |
| HSPE1 | Heat shock 10 kDa protein 1 (chaperonin 10) | 0.417 | (0.340–0.493) |
| RUVBL2 | RuvB-like 2 (E. coli) | 0.751 | (0.704–0.798) |
| 0.763 | (0.707–0.818) | ||
| TCP1 | t-Complex 1 | 0.531 | (0.467–0.594) |
| 0.487 | (0.370–0.604) | ||
| RNA post-translational modification | |||
| DDX52 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 52 | 0.569 | (0.475–0.664) |
| DDX56 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 56 | 0.640 | (0.568–0.712) |
| NOL5A | Nucleolar protein 5A (56 kDa with KKE/D repeat) | 0.488 | (0.426–0.550) |
| SSB | Sjogren syndrome antigen B (autoantigen La) | 0.633 | (0.566–0.699) |
| Small molecule biochemistry | |||
| ADH5 | Alcohol dehydrogenase 5 (class III), chi polypeptide | 0.826 | (0.784–0.868) |
| CYCS | Cytochrome c, somatic | 0.587 | (0.535–0.639) |
| DHFR | Dihydrofolate reductase | 0.530 | (0.454–0.606) |
| 0.493 | (0.445–0.542) | ||
| FABP5 | Fatty acid binding protein 5 (psoriasis-associated) | 0.427 | (0.347–0.506) |
| RARS | Arginyl-tRNA synthetase | 0.737 | (0.687–0.788) |
| TSTA3 | Tissue-specific transplantation antigen P35B | 0.786 | (0.739–0.833) |
| 0.708 | (0.667–0.749) | ||
| UMPS | Uridine monophosphate synthetase | 0.578 | (0.501–0.655) |
| UNG | Uracil-DNA glycosylase | 0.531 | (0.468–0.594) |
| ABCB7 | ATP-binding cassette, subfamily B (MDR/TAP), member 7 | 1.578 | (1.401–1.754) |
| CD79A | CD79a molecule, immunoglobulin-associated alpha | 1.426 | (1.312–1.541) |
| 1.469 | (1.395–1.542) | ||
| DHPS | Deoxyhypusine synthase | 1.551 | (1.411–1.691) |
| DPM3 | Dolichyl-phosphate mannosyltransferase polypeptide 3 | 1.665 | (1.496–1.834) |
| RHOQ | Ras homologue gene family, member Q | 1.769 | (1.568–1.97) |
| 1.929 | (1.697–2.161) | ||
- Certain genes were represented by multiple probe sets, which are given separately.
Projection of the data on known canonical pathways reveals involvement of various pathways in cell cycle regulation and phases of the cycle. DNA replication control is represented by regulation of various DNA polymerases and genes from the MCM family and CDC7 involved in the initiation of genome replication. The G2/M DNA damage checkpoint was represented by the CDC25/CDC2/Cyclin B pathway, associated with DNA damage processing via the P53 tumor suppressor.
The regulation of mitosis was represented with, for example, NEK2, a centriole division gene, and AURKA, which formats and stabilizes microtubules at the mitotic spindle pole during chromosome segregation. Furthermore, the CENPA gene, a methylated variant of histone H3 involved in centrosome formation, was found to be significantly up-regulated.
Other pathways linked to cell cycle regulation, such as the nucleotide excision repair pathway, showed diminished activity after folate addition. This was demonstrated with decreased expression of DNA binding proteins ERCC4 and DDB1 and increased expression of single stranded DNA binding protein and various DNA polymerases.
The apoptosis pathway was represented by BCL-2, FHIT, DFFA, and PDCD8. Interestingly, FHIT encompasses the FRA3B fragile site, which is expressed in a folate-deficient environment.
Besides cell cycle regulation, protein processing pathways were represented by various genes of the chaperonin-containing TCP1 complex family, which were down-regulated after folate addition. This complex folds various polypeptides in an ATP-dependent manner into active proteins, including actin and tubulin.
Other known functions of folate, such as the one-carbon group cycle and nucleotide synthesis, were mainly unregulated, although several genes of the purine synthesis pathway were found to be down-regulated.
The results of quantitative real-time RT-PCR on 10 genes belonging to the 100 best classifying probe sets are shown in Table 2. The reference gene β-actin showed no differential expression (ratio after/before folate addition is 1.01). For 8 out of 10 tested genes the direction of regulation found with the RT-PCR experiments was identical to the direction found with the microarrays. Moreover, from the eight directionally correct genes, five reached statistical significance, that is, RBBP6, pTEN, ANKRD11, BATF3, and HSP90AA1. The directions of the HNRPD and the TSC22D3 genes were opposite to the microarray data.
| Gene | Forward primer sequence | Reverse primer sequence | Ratio after/before folate addition | p-value |
|---|---|---|---|---|
| Internal control | ||||
| ACTB | gcgggaaatcgtgcgtgacatt | gatggagttgaaggtagtttcgtg | 1.018 | 0.401 |
| Up-regulated genes | ||||
| RBBP6 | acagcctagaccctcagcaa | ctcctggagcgttttcactc | 2.037 | 0.005 |
| PTEN | accaggaccagaggaaacct | gctagcctctggatttgacg | 2.591 | 0.005 |
| ANKRD11 | gacaaggagcccagagacag | cactgaggctctgtccttcc | 1.614 | 0.039 |
| MTCH1 | gaccactgaggctcttttcg | cttggcgtaggtgaagaagc | 1.015 | 0.818 |
| TSC22D3 | accagaccatgctctccatc | cagggtcttcaacagggtgt | 0.914 | 0.589 |
| Down-regulated genes | ||||
| BATF3 | agccctgaggatgatgacag | ttcagtgcctctgtcaggtg | 0.511 | 0.005 |
| HSP90AA1 | atgaaactgcgctcctgtct | ttcttccatgcgtgatgtgt | 0.515 | 0.005 |
| BCCIP | atgaggagcagggaaaacct | ccagccttcagagaaaccag | 0.818 | 0.347 |
| DPP3 | acgaggggtatgcaacagtc | gcctcgtattccagaagctg | 0.914 | 0.347 |
| HNRPD | gatcctaaaagggccaaagc | gttgtccatggggagctcta | 1.117 | 0.818 |
DISCUSSION
In the present study we show the results of a genome-wide expression analysis in B-lymphoblasts derived from CLP patients to identify folate-responsive genes and associated pathways and their relevance in lip and palate development. The forced clustering of the data revealed significant up-regulation of 144 and down-regulation of 409 genes in response to folate. Differential expression was confirmed with quantitative RT-PCR, which showed comparable regulation in 8 out of 10 tested genes, from which five genes reached statistical significance. The regulated genes were not concentrated in specific functions or pathways, but covered several functions at a low level. This indicates a general modifying role of folate in physiological processes, which might be connected with the extensive role of folate as a one-carbon group donor. One-carbon groups are used for the synthesis of purines and pyrimidines, proteins, and the remethylation of homocysteine into methionine, the main methyl group donor of the cell. Interruption of these basal functions leads to various types of cellular and chromosomal damage, such as uracil accumulation and incorporation, abnormal protein and CpG methylation, incorrect imprinting patterns, DNA strand breaks, aneusomy, and cell death (Chiang et al., 1996; Duthie and Hawdon, 1998; Kim, 2005; Steegers-Theunissen et al., 2000; Wang et al., 2004). As a result, altered folate status might influence cell cycle progression. This is supported by our data showing modified expression of a relatively high number of genes involved in cell cycle regulation, especially G2/M checkpoint regulation, S-phase initiation, and regulation of mitosis.
The modest number of genes regulated by folate was confirmed by the unsupervised clustering, which demonstrated that the primary clustering of samples was to the original cell line instead of folate status. The similarities between the samples from the same culture were thus larger than the similarities in folate response. One explanation may be the low number of folate-responsive genes or the low number of samples or even different responses to the folate intervention of the separate cell lines.
In our unpublished study we identified folate-responsive proteins in 30 B-lymphoblast cell lines from CLP and control children using a new proteomic method based on peptide fingerprinting. These proteins involved glucose metabolism, energy production, nucleocytoplasmic transport, cell cycle regulation, cytoskeleton, protein processing, and DNA transcription and translation. The present results confirm the responsiveness of these pathways to folate supplementation. This was especially true with respect to various heat shock proteins and heterogeneous nuclear ribonucleoprotein.
Spiegelstein et al. (2004) studied gene expression in the anterior neural tube of Theiler stage 13/14 FOLBP1 knock-out mice after feeding them a folate-deficient diet and using a 5700 gene array. Biological functions identified as being regulated by folate were comparable with those identified in our study and comprised processes such as proliferation, apoptosis, transcription, and translation. Courtemanche et al. (2004) performed a study using a 695 gene targeted microarray focusing on pathways involved in cellular aging and stress. Interestingly, they also identified cell cycle and DNA damage-related expression as a consequence of folate deficiency. However, their limited array size made it impossible to explore various other pathways.
Developmental genes such as TGFβ and MSX1 and their receptors, which are known to contribute to CLP development (Carinci et al., 2007) and thus might be target genes for folate, were not identified as folate-responsive genes. In the case of TGFβ it was reported earlier that this gene is an important regulator of apoptosis in B-cell precursors (Lanvin et al., 2003) and thus it seems likely that the used B-lymphoblasts were expressing TGFβ. The lack of differential expression of TGFβ in response to folate might therefore indicate that there is no interaction. However, in theory, the possibility remains that interaction is selectively and/or temporarily present in the developing facial structures. Additional testing is needed to clarify these possible gene-specific effects of folate.
An interesting observation is that the expression of genes that code for oncogenes and tumor-suppressors was altered in the present study. Although recent reports on the association of folate and the development of certain cancers are still inconclusive, there are increasing concerns that folate deficiency as well as folate excess might contribute to cancer development (Ames and Wakimoto, 2002; Kim, 2007; Mason et al., 2007). The deregulating effects of folate on normal cell development as shown by our data corroborate with this hypothesis and these results might add new starting points to unravel this apparent gene-nutrient interaction.
This is one of the first studies using human cell lines for genome-wide profiling of the gene expression in response to folate. We observed >500 significant changes in expression of genes involved in a variety of biological pathways. The selection of a homogeneous group of patients all with an identical nonsyndromic cleft contributed to the validity of the results. The measurements of the folate concentration in the medium of the cultures confirmed the actual folate states and thus folate deficiency and the target folate concentration. The B-lymphoblast culture model is a frequently used model for folate studies and was found to be appropriate for the assessment of folate-responsive gene expression and, as such, informative for folate-sensitive congenital malformations such as CLP. However, we realize that other time- and tissue-specific pathways may be active during palatal development and may contribute to CLP development. Secondly, only five cell lines derived from CLP patients could be profiled for this explorative study. Evidently, higher numbers of cell lines will increase the reliability of the data and prevent false-positive identifications. Inclusion of samples derived from healthy control children would also allow case or control specific gene expression relevant for increasing the understanding of CLP development. Future studies are needed to explore the possible role of the present set of genes in the development of CLP and other folate-sensitive congenital malformations. This may eventually lead to the further understanding of gene-environment interactions in the development of congenital malformations such as CLP.
Acknowledgements
We would like to thank Mr. W. van Gils of the Department of Clinical Genetics at the Erasmus University Medical Centre, Rotterdam for technical assistance.




