Investigations of the genomic region that contains the clf1 mutation, a causal gene in multifactorial cleft lip and palate in mice
Abstract
BACKGROUND
Human nonsyndromic cleft lip and palate, CL(P), is genetically complex, with one contributing gene on chromosome 17q. A potentially homologous gene, clf1 on distal chromosome 11, is part of the digenic cause of the 10–30% CL(P) in the A/WySn mouse strain. Here we report our progress toward identifying the clf1 mutation.
METHODS
Transcription from all of the known and predicted genes in the 1.5-Mb candidate region was examined in A/WySn and control (AXB-4/Pgn) ED10–11 embryo heads. The marker haplotype for 28 inbred strains across the clf1 region was obtained. The entire transcripts of Wnt9b and Wnt3 in A/WySn were sequenced. Using long PCR, the genomic region from Wnt3 throughWnt9b was screened in A/WySn for an inserted retrotransposon.
RESULTS
Gosr2, Wnt9b, Wnt3, Nsf, Arf2, Crhr1, Mapt, Cdc27, Myl4, Itgb3, chr11_20.152, chr11_20.154, chr11_20.155, and chr11_20.156 are expressed in ED10–11 heads. None is absent or detectably reduced in A/WySn. The ancestral pre-clf1 mutation haplotype was found in CBA/J mice. By a test-cross, CBA/J was confirmed to lack the clf1 mutation. Three single-nucleotide variants in A/WySn (vs. C57BL/6J) were found in each of the 3′ untranslated regions (3′UTRs) of Wnt3 and of Wnt9b, respectively; their presence in CBA/J shows that none are the clf1 mutation. An inserted intracisternal A particle (IAP) retrotransposon located 6.6 kb from the 3′ end of Wnt9b was found in A/WySn and in all clf1 strains tested. This IAP is absent in C57BL/6J and CBA/J.
CONCLUSIONS
The clf1 mutation is a genomic alteration present in A/WySn and absent in the ancestral chromosomal segment in CBA/J. The IAP retrotransposon insertion near Wnt9b in A/WySn fits this criterion; we predict that interference with Wnt9b function by this IAP is the clf1 mutation. Birth Defects Research (Part A) 2005. © 2005 Wiley-Liss, Inc.
INTRODUCTION
The cause of cleft lip and palate in individuals appears to be genetically complex. In some human populations, one of the genes contributing to risk of cleft lip with or without cleft palate, CL(P), appears to be located on chromosome 17q, linked to a marker in the RARA gene (Chenevix-Trench et al., 1992; Peanchitlertkajorn et al., 2003; Moreno et al., 2004). Although RARA was initially considered a candidate gene, the associations detected could also be caused by the presence of a CL(P) gene in the nearby, linked genes.
Study of the orthologous genes in mice has often aided identification of genes involved in genetically complex human traits. A conserved linkage group of genes orthologous to RARA and nearby genes is located on distal chromosome 11 in mice (MGD, 2004; Juriloff et al., 2004). One set of inbred strains of mice, the “A/∼” strains, is homozygous for a CL(P)-causing mutation, clf1, in this region. Like human CL(P), the genetic basis of CL(P) in the A/∼ strains is complex (Davidson et al., 1969), and the primary genetic cause has been shown to be digenic—the combined effects of two genes—the clf1 gene on distal chromosome 11, and the clf2 gene on midchromosome 13 (Juriloff et al., 2001, 2004). A/∼ strains consistently express nonsyndromic CL(P) in some embryos and neonates (Kalter, 1979; Juriloff, 1982; Gong, 2001). Affected pups die in the first day after birth.
Multifactorial threshold traits, such as CL(P) in humans and mice, have a low rate of expression in individuals having the causal genotype (e.g., 10–20% expression in A/WySn mice). This low correlation between genotype and phenotype complicates the process of identifying the causative genetic defects. With focus on identifying the clf1 gene, our genetic recombination studies have narrowed the target region to approximately 1.5 million base pairs, expected to be about 1–3 cM (Silver, 1995), linked to but excluding Rara. Owing to the digenic mechanism, the low rate of CL(P) expression in genetically liable individuals, and the possible suppression of recombination in the region (Juriloff et al., 2004), further reduction of the target region by study of segregants is impractical. However, the sequenced mouse genome (Mouse Genome Sequencing Consortium, 2002) enabled identification of all candidate genes: 10 known genes and up to 17 other, predicted genes (Juriloff et al., 2004). In the context of the probable orthology of clf1 with a human CL(P) gene on 17q, we report our progress toward identifying the clf1 mutation.
From among all the known and predicted genes of more than one exon in the 1.5-Mb recombination-defined candidate region, all candidates for clf1 were identified based on their expression in genetically normal ED10–11 embryonic heads and ranked according to their known or predicted molecular function. The presence of transcripts of all of these candidate genes in ED10–11 A/WySn embryo heads demonstrated that the clf1 mutation does not cause absence of the affected mRNA. A method to discriminate normal polymorphisms from the clf1 mutation was created, based on a strain survey of haplotypes, to identify a strain with the premutation version of the ancestral chromosome on which the clf1 mutation occurred. The normal CBA/J strain, known to be related to the A/∼ strains, was shown to have the ancestral chromosomal segment and was confirmed to be normal at the clf1 locus by test-cross analyses. This ancestral haplotype, without the clf1 mutation, provides a very powerful tool for recognition of the clf1 mutation by comparison of DNA sequence between CBA/J and A/∼ strains. The Wnt3 and Wnt9b transcripts in A/WySn were sequenced and were normal, rejecting the hypothesis of a point mutation within either gene; six single nucleotide differences between A/WySn and C57BL/6J were found in the 3′ untranslated regions (3′UTRs) but were shown to be polymorphisms by the sequence identity between CBA/J and A/WySn. The hypothesis that the clf1 mutation is due to insertion of a transposable element was tested by searching for an insertion of 5–8 kb in A/WySn genomic DNA compared with C57BL/6J, beginning with the genomic region containing Wnt9b. An insertion was found 6.6 kb beyond the 3′ end of Wnt9b in A/WySn and in all related strains known to have clf1, but it is not present in C57BL/6J, CBA/J, or other normal strains. The insertion was identified by sequencing to be an intracisternal A particle (IAP) retrotransposon. IAP retrotransposons often interfere with transcription of nearby genes (Whitelaw and Martin, 2001). The absence of this IAP insertion in CBA/J indicates that its presence close to the 3′ end of the Wnt9b gene in A/∼ strains has a high probability of being the clf1 mutation, and predicts that it interferes with Wnt9b function in early craniofacial development to cause risk of CL(P).
MATERIALS AND METHODS
Strategies to Identify Candidate Genes
Although the normal sequence of the 1.5-Mb chromosomal segment containing clf1 is completely known, not all of the genes have been identified. Segments of DNA sequence that may be part of unidentified genes have been located by gene prediction programs, such as Genscan (Burge and Karlin, 1997), but these are not yet fully accurate. It is likely that some of the DNA sequences predicted to be genes are not in fact genes (Wang et al., 2003). By testing for mRNA transcripts from predicted genes in testis tissue, which generally expresses many genes, we examined the validity of the predictions.
For most of the known genes and all of the predicted genes in the chromosomal segment containing clf1, the specific times and locations of embryonic expression are not known. Since CL(P) in the A/∼ strains arises in abnormal or delayed growth of facial prominences on ED10–11, leading to failure of fusion (Trasler, 1968; Wang et al., 1995), we tested all of the known and predicted genes in the candidate region for their expression in the normal ED10–11 embryonic head. We expected the number of candidates to be reduced by exclusion of genes not expressed in the embryonic head.
Among the products of genes expressed in ED10–11 embryonic heads, some, such as developmental signaling molecules, seem better candidates for clf1 than others that appear to be ubiquitous structural or housekeeping molecules. The cellular function of all candidate, known genes was obtained from the University of California Santa Cruz (UCSC) website (UCSC, 2003). To deduce the possible function of the confirmed (predicted) genes that are expressed in ED10–11 heads, the entire predicted mRNA sequence for each was used in a BLAST search (May, 2004) against the GenBank “nr” database (NCBI, 2004), and the function of the closest matching eukaryote gene was noted.
Reports of mutant and knockout phenotypes for the candidate genes were obtained from the Mouse Genome Database (MGD, 2004) and by review of the literature. Gene knockouts generally have more severe and more highly penetrant phenotypes than spontaneous mutations that have some residual function (hypomorphs). If the very low penetrance and need for the effect of a second locus (clf2) indicates that clf1 is a hypomorph, the lack of craniofacial defects in a knockout mutation would indicate that a candidate gene is probably not clf1.
Strategies to Find the Mutation
The clf1 mutation is recessive (Juriloff et al., 2001, 2004) and this fact points to a loss-of-function type of alteration; mutations causing gain-of-function or ectopic expression are usually dominant (Strachan and Read, 1999). Some types of loss-of-function mutation, such as large deletions in the coding sequence, lead to either alteration of the size of mRNA transcripts or their absence. To detect this type of alteration, if present in the segments of transcripts (cDNA) amplified, we compared A/WySn and the normal C57BL/6J-type control for the size and presence of the PCR products for each candidate gene. Large insertions in the segments of transcripts examined would also be detected this way.
Based on known and predicted gene functions, two genes, Wnt9b (formerly Wnt15) and Wnt3, seemed strong candidates. Therefore we obtained the Wnt9b and Wnt3 mRNA (cDNA) sequence from A/WySn to search for alterations compared with the public sequence for C57BL/6J (UCSC, 2003).
Aspects of the CL(P) trait in the A/∼ strains, such as the presence of alternative phenotypes in an inbred strain (cleft and normal) and the presence of strong genetic maternal effects on phenotype (Juriloff et al., 2001), are characteristics associated with mutations due to an inserted transposable element that variably interferes with a nearby gene's expression (Michaud et al., 1994; Whitelaw and Martin, 2001). These elements are usually 5–8 kb in size, and several types, each present in hundreds of sites, are normally and silently present in genomic DNA. Numerous mutations due to the retrotransposition of various elements into locations that disrupt gene function are known (Baust et al., 2002; Druker and Whitelaw, 2004). We therefore began a search for an inserted element in A/WySn genomic DNA, based on the size of genomic fragments by long PCR of overlapping segments. We screened the entire genomic region from within intron 1 in Wnt3 through the 25-kb of intergenic sequence between the 5′ end of Wnt3 and the 5′ end of Wnt9b (they are aligned “head to head”), through the genomic sequence of Wnt9b, including the 5′UTR, the coding region (4 exons and 3 introns) and the 3′UTR, and extending 7-kb into the intergenic sequence between Wnt9b and Gosr2. Subsequently, one of the segments, from primers L59 (5′-TACCCCTCCCATTTTACGGAT-3′) and L60 (5′-TTACATCACCAAAGGCCTCTG-3′), was amplified from genomic DNA from a second A/WySn individual, A/J, A/HeJ, CL/Fr, AXB-23/Pgn, CBA/J, C57BL/6J, AXB-4/Pgn, LM/Bc, and a segregant embryo (“X115”) with CL(P) from the previous mapping study (Juriloff et al., 2004).
A Strategy for Distinguishing the clf1 Mutation from Normal Polymorphisms
Many strains of laboratory mice are known to share common ancestors (Morse, 1978). They share identical segments of chromosomes, containing many genes and polymorphic markers, derived from the same ancestor (Wade et al., 2002; Wiltshire et al., 2003). The pattern of specific variants at polymorphic markers across the chromosomal segment is termed a “haplotype.” Variants at any individual marker may be evolutionarily ancient and widely dispersed, but a haplotype shared by strains because of their common ancestry is recognizable as a pattern of identical variants across all of several physically linked polymorphic sites. When a new mutation disrupts a gene, it is surrounded by a preexisting haplotype. If the A/∼ -like haplotype on distal chromosome 11 was already dispersed among progenitors of various inbred strains before the clf1 mutation occurred, then some inbred strains will have the same ancestral haplotype as the A/∼ strains, but without the clf1 mutation. This situation is the basis of a powerful strategy for distinguishing the clf1 mutation from normal polymorphisms. Many DNA sequence differences are present in A/WySn relative to the C57BL/6J reference sequence, because they have different ancestral haplotypes, and these differences are normal polymorphisms. In contrast, a genetic alteration affecting a coding or regulatory sequence found in A/∼ strains, but not in its ancestral haplotype, is likely to be the clf1 mutation. In order to identify a normal inbred strain having the same ancestral haplotype as the A/∼ strains around clf1, a panel of 28 strains was typed for 6–12 polymorphic markers from the chromosomal region containing clf1. These markers were previously used in our mapping studies (Juriloff et al., 2004). DNA was obtained from our own stocks and purchased from The Jackson Laboratory (Bar Harbor, ME).
Because of the digenic basis of CL(P), it is necessary to check that any normal strain identified as having the ancestral haplotype does not have an unrecognized clf1 mutation, hidden by the lack of the clf2 factor and lack of the permissive maternal effect. To test for the clf1 mutation in CBA/J, a special test-cross was used. The strategy was to create an F1 that would be heterozygous for both clf1 and clf2, only if CBA/J has clf1, and to test-cross this F1 with A/WySn females to provide the necessary maternal effect and second copy of clf2. AXB-4/Pgn (+/+, clf2/clf2) (Juriloff et al., 2004) was crossed with CBA/J (?/?, +/+) to obtain the F1 (?/+, clf2/+), which was test-crossed with A/WySn females (clf1/clf1, clf2/clf2); their embryos were examined on ED14. If CBA/J had the clf1 mutation, one-fourth of the embryos would have the double homozygous genotype (clf1/clf1, clf2/clf2) with a 12–20% risk of CL(P). If CBA/J did not have the clf1 mutation, no embryos would be homozygous for the clf1 mutation and, as it is recessive, none of the test-cross embryos would have CL(P).
Mice
Mice were maintained under conditions described previously (Juriloff and Mah, 1995). All strains were descendants of mice originally obtained from the Jackson laboratory. AXB-4/Pgn mice have a normal allele at the clf1 locus in a haplotype derived from the C57BL/6J strain, have the A/J allele at the clf2 locus on chromosome 13, and were readily available because of their use in our mapping studies of clf1 (described in Juriloff et al. [2004]). For ED10–11 and ED13 embryos, timed matings from within each strain were obtained as described previously (Juriloff et al., 2001), and the midpoint of the dark cycle was taken as ED0/0 hr.
mRNA
Tissues used for preparation of total RNA were testes obtained from individual adult males, pooled ED10–11 embryo heads, and two pooled E13 A/WySn embryo heads with CL(P). For testis mRNA, adult male mice were euthanized, a testis was immediately removed and homogenized with RLT buffer (Qiagen, Mississauga, Ontario, Canada) and β-mercaptoethanol; RNA extraction then followed the protocol of the RNeasy Protect Mini Kit (Qiagen). For ED10–11 embryo heads, timed pregnant females were euthanized, the uterus was immediately removed, the embryos in decidua and membranes were quickly dissected free of the uterus under cold sterile 1× PBS, then moved to fresh cold 1× PBS for immediate removal of membranes and decidua, then moved to RNAlater (Qiagen), under which their tail somite pairs (TS) and stage of face development were observed, then stored in fresh RNAlater for one to three days. Stored embryos were decapitated by a cut immediately caudal to the second branchial arch, and the heads pooled and stored in RNAlater. Pooled heads from three A/WySn litters (4–10 TS) and four AXB-4/Pgn litters (0–12 TS) of ED10/11 hr to ED11/11 hr embryos were used; both centered on the “oval” to “crescent” stages of nasal placode development as defined by Trasler (Trasler, 1968; Juriloff and Trasler, 1976). The heads from two ED13 A/WySn embryos with CL(P) were similarly obtained and pooled. RNA was extracted from each pool of ED10–11 heads or E13 CL(P) heads by the same protocol as for adult testis (above). RNA was stored in RNase-free water at −80°C.
cDNA was prepared from total RNA following the RT-PCR protocol provided with the Omniscript Reverse Transcriptase kit (Qiagen). Specifically, 2 μg of total RNA was added to 1 μM random primers (Invitrogen, Carlsbad, CA), 10 units RNAguard RNase inhibitor (porcine; Amersham Pharmacia Biotech, Baie d'Urfe, QC, Canada), and 0.5 mM Omniscript Reverse Transcriptase. cDNA was stored in the reaction mixture at −20°C.
Studies of Gene Expression
To test for the presence of transcripts from the known genes and predicted genes in the clf1 candidate region, cDNA samples from ED10–11 heads and testis from each of A/WySn and AXB-4/Pgn, respectively, were screened by PCR with gene-specific primers. The chromosomal interval, previously reported (Juriloff et al., 2004), is shown in Figure 1. Suitable primer sequences were available for two known genes: Myl4 (st96_212) (MGD, 2004) and Crhr1 (Heinrich et al., 1998). For the other eight known genes, segments of 115–620 bp (mean 360 bp) were amplified by PCR from the cDNA. The Web Primer program (Dolinski et al., 2003) was used to design the primers (Table 1). The products spanned at least one intron, so that cDNA products were distinguishable from potential genomic DNA products; the latter would usually be too large to be present in the amplification products. The exon boundaries were located by comparison of the mRNA sequence obtained from GenBank (NCBI, 2004) with an available genomic sequence from GenBank (Crhr1 and Itgb3) or the public DNA sequence database (UCSC, 2003). Similarly, cDNA-specific primers (Table 1) were designed to amplify segments of the 13 predicted genes that contain more than one exon and that are not subsumed by a known gene in the chromosomal interval containing clf1 (UCSC, 2003). PCR products were examined by electrophoresis in agarose gels with ethidium bromide, as described previously (Juriloff et al., 2004). PCR products for each gene from adult testis and ED10–11 embryo head cDNA from A/WySn and AXB-4/Pgn, respectively, were compared for presence/absence, size, and intensity. Duplex reactions amplifying β-actin (Benavides et al., 1995) were used to confirm gene specificity for strain differences in intensity.
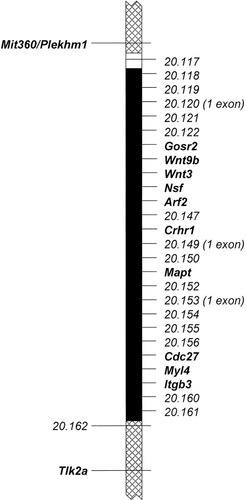
The 1.5-Mb segment of mouse chromosome 11 defined by previous recombination mapping (Juriloff et al., 2004) to contain the clf1 mutation. Genes and predicted genes and their physical order were taken from UCSC (2003); all between Plekhm1 and 20.162 are shown. Hatched regions, marked by polymorphic variants shown to the left, were excluded by linkage mapping. The solid black region represents the segment containing the clf1 mutation, with all genes and predicted genes shown to the right. One predicted gene, 20.117, lies between the excluded and included regions.
| Gene | Number of exons in gene | Exons included in PCR product | Forward | Reverse |
|---|---|---|---|---|
| Known genes | ||||
| Arf2 | 5 | 2–5 | 5′-TGGGGAATGTCTTTGAAAAGC-3′ | 5′-TGGTTTTTGAGCTGGTTGGA-3′ |
| Cdc27 | 11 | 5–7 | 5′-TGCCAAAGGGTCAGAATGTT-3′ | 5′-TCAATGTAAGACACGGAGGAA-3′ |
| Crhr1 | 13 | 3–7a | 5′-TGGACCTCATTGGCACC-3′ | 5′-TGTGTGCAGGTAGCAGC-3′ |
| 3–6a | 5′-CTACGGTGTCCGCTACAAC-3′ | 5′-GCTCACGGTGAGCTGGAC-3′ | ||
| 6–7 | 5′-TGCCTGAGGAACATCATCCA-3′ | 5′-ATGCAGACGAACATCCACTTG-3′ | ||
| 7–8 | 5′-TGGTGACAGCCGCCTACAA-3′ | 5′-TAAAGTTTCCCAATGGCCCA-3′ | ||
| 8–9 | 5′-TTTCCCCATCATTGTGGCTT-3′ | 5′-ATCATGGGGCCCTGGTAGAT-3′ | ||
| 9–12 | 5′-TTGGCAAACGTCCTGGAGTAT-3′ | 5′-AACACAGACACGAAGAAGCCC-3′ | ||
| 12–13 | 5′-GGGCTTCTTCGTGTCTGTGTT-3′ | 5′-TACAGGTCTGCATCCCAGG-3′ | ||
| 1–5 | 5′-TCGTGAAGGCCCTTCTCCT-3′ | 5′-GGCAATGTGGTAGTGCACTTT-3′ | ||
| 2–5 | 5′-TCCCTCCAGGATCAGCAGTGT-3′ | 5′-AGTGGCCCAGGTAGTTGATGA-3′ | ||
| Gosr2 | 6 | 1–6 | 5′-ACCAACAAACGAACAAGCAG-3′ | 5′-TCAGGTACTGTACCACGAGGA-3′ |
| Itgb3 | 15 | 5–7 | 5′-TATGAAGAATGCCTGCTTGC-3′ | 5′-TGGTAAAGGCTGACGACATT-3′ |
| 7–9 | 5′-GGGGCTGATGACTGAGAAA-3′ | 5′-TCACCGTGTCTCCAATCTTGA-3′ | ||
| Mapt | 11 | 2–7 | 5′-TCGGCAGGAGTTTGACACAAT-3′ | 5′-AATTATCTGCACCTTGCCACC-3′ |
| Myl4 | 9 | 8–9b | 5′-ATCATGTCTGGGTAAAGCACG-3′ | 5′-GTGGGTCAGAGAAGCCATGT-3′ |
| Nsf | 21 | 2–8 | 5′-TATGCAAGCTGCGAGATGC-3′ | 5′-CTGGCGATTTTCCTTGGTTT-3′ |
| Wnt3 | 5 | 1–3 | 5′-TCGCTGGCTACCCAATTTG-3′ | 5′-TTCGGCCTGCTTCATTGTT-3′ |
| Wnt9b (Wnt15) | 4 | 2–4 | 5′-TGCTCACCTGAAGCAGTGTGA-3′ | 5′-TTCCAACAGGTACGAACAGCA-3′ |
| Predicted genes | ||||
| chr11_20.117c | 2 | 2 | 5′-ATGACTTCCCTCCGATTTGT-3′ | 5′-TCATTCAGGTTCTGGTCGAGA-3′ |
| chr11_20.118 | 15 | 12–14 | 5′-AACAGCAAAGGAGGGAGATGA-3′ | 5′-TCCATTCCCTTGGTACATCCT-3′ |
| chr11_20.119 | 2 | 1–2 | 5′-TCCTTGGAAGAGATCTACCCG-3′ | 5′-ACCAAGACCAATGTGACCACT-3′ |
| chr11_20.121 | 3 | 1–3 | 5′-AGGACTTGGATGGGTTTGAGG-3′ | 5′-TCGGGGAGCAAAGGATTCT-3′ |
| chr11_20.122 | 5 | 1–5 | 5′-CATCAAGTCCGAGAGCATGAT-3′ | 5′-TTACTAGGTCGGGCTTTTCCA-3′ |
| chr11_20.147 | 6 | 3–6 | 5′-GACAGAGCAACTGGGCTAGAA-3′ | 5′-TAGCTCTTTGCAGGTGGAAA-3′ |
| 1–2 | 5′-TTGTGGAAGTCCAGGACCTTT-3′ | 5′-ATGCTGATATGAAAGCCGGA-3′ | ||
| 2–3 | 5′-AAAAGTGATTCAGGCCAGGG-3′ | 5′-TCTGTCGGTATCAGGCTCCA-3′ | ||
| chr11_20.150 | 5 | 1–5 | 5′-AATCACACGACGAACAAAGCA-3′ | 5′-TTGGCAGCCTCTTCTTCACTT-3′ |
| 2–4 | 5′-AGCCTGGGAACCCAGAAAA-3′ | 5′-TGTGGTCTGACTCTTCAGCAA-3′ | ||
| 4–5 | 5′-GCAAATCAGCTCTCAACACCT-3′ | 5′-AACTGAGTGATGCTCCCACAT-3′ | ||
| chr11_20.152 | 12 | 4–6 | 5′-CACCATGCCTCCTGAAATACA-3′ | 5′-TTTGCTGATGGCACAGCTT-3′ |
| chr11_20.154 | 2 | 1–2 | 5′-TTCTGCGCCTCTGTTTGC-3′ | 5′-TTGGCAGCTATCCATTCTGT-3′ |
| chr11_20.155 | 2 | 1–2 | 5′-TTCTGCGGGTCAGGATTTCT-3′ | 5′-TGATGCTACCACAAGCCCTCT-3′ |
| chr11_20.156 | 10 | 7–10 | 5′-CAAATTTCACAGAGCCTCGGT-3′ | 5′-AAATTCGTCACTTTCCGCC-3′ |
| chr11_20.160 | 3 | 1–3 | 5′-GGCAGAATATGACACAGGCAA-3′ | 5′-TGAGTTGACCATCCTTGATGA-3′ |
| chr11_20.161 | 36 | 3–7 | 5′-TTTACGTGAACGACTTGCCAG-3′ | 5′-TTTTTGGTGTGCTAGGTTCGG-3′ |
Sequencing
The Wnt3 coding region was sequenced from A/WySn adult testis cDNA; the 5′ end of the 5′UTR and the 3′ end of the 3′UTR were sequenced from A/WySn adult genomic DNA. The Wnt9b coding region from mid-exon 2 to the first 75 bp of exon 4 was sequenced from A/WySn E13 CL(P) embryo head cDNA; the remaining coding region (exon 1, all of exon 2, and the coding region of exon 4) and the 3′UTR (3.4 kb) were sequenced from A/WySn adult genomic DNA. Regions containing differences between A/WySn and C57BL/6J sequence in Wnt3 and Wnt9b were also sequenced in CBA/J cDNA from testis or genomic DNA.
For Crhr1, sequences were obtained for an abnormal-sized product for exons 1–5 amplified from A/WySn testis cDNA, the normal-sized product for the same exon 1–5 interval amplified from A/WySn and from AXB-4/Pgn testis cDNA, and the intron4/exon5 region amplified from A/WySn and AXB-4/Pgn adult genomic DNA.
To sequence cDNA, primers based on C57BL/6J mRNA sequence (UCSC, 2003) were used to amplify overlapping segments 300–680 bp in size, straddling at least one intron (to exclude amplification of genomic products by their much larger size). For genomic DNA, primers based on C57BL/6J genomic sequence (UCSC, 2003) were used to amplify segments ranging between 375 and 875 bp in size.
The PCR products to be sequenced were isolated by excision and elution of bands from agarose gels after electrophoresis, using the QIAquick Gel Extraction Kit (Qiagen). Sequencing was done by the Nucleic Acid and Protein Service at the University of British Columbia. Sequence obtained was compared with the C57BL/6J public sequence (UCSC, 2003) by eye and by BLAT (Kent, 2002).
Long PCR
The public mouse C57BL/6J genome database (UCSC, 2003) and the Web Primer program (Dolinski et al., 2003) were used to design primers to amplify overlapping segments through genomic DNA. A total of 27 segments were amplified; products were between 0.9 and 3.2 kb in length, with most being between 1.8 and 2.5 kb. Long PCR was performed using the Qiagen kits, ProofStart DNA Polymerase and Qiagen Taq DNA Polymerase, following the manufacturer's instructions for amplification of long PCR products in their ProofStart PCR Handbook. All long PCR was performed simultaneously on adult genomic DNA from A/WySn and from AXB-4/Pgn or C57BL/6J controls. PCR products were visualized in agarose gels as described previously (Juriloff et al., 2001).
RESULTS
Gene Expression and Candidate Genes for clf1
There are 10 known genes in the chromosomal interval containing clf1 (Fig. 1). Among the total of 29 Genscan gene predictions in the interval (UCSC, 2003), 11 are completely included within the known genes, 2 are largely within known genes (7/9 and 9/10 exons), 3 have only one exon and therefore could not be tested by our methods, and 13 were tested for transcripts.
Adult testes expressed all 10 known genes and 6 of the 13 predicted genes (Table 2). The ED10–11 embryonic heads expressed all 10 known genes and 4 of the 13 predicted genes (Table 2; Fig. 2). All of the genes and predicted genes expressed in embryonic heads were also expressed in testis. Two predicted genes were expressed only in testis. Thus the candidate genes for clf1 were defined as: Gosr2, Wnt9b, Wnt3, Nsf, Arf2, Crhr1, Mapt, Cdc27, Myl4, Itgb3, chr11_20.152, chr11_20.154, chr11_20.155, and chr11_20.156, and a further two predicted genes (chr11_20.118 and chr11_20.161) were confirmed to produce cDNA.
| Known genea | Genscan gene predictionab | Testis cDNAc | E10-E11 embryo head cDNAc | Number of exons in gene | Exons included in PCR product(s) |
|---|---|---|---|---|---|
| chr11_20.117 | − | − | 2 | 1–2 | |
| chr11_20.118 | + | − | 15 | 12–14 | |
| chr11_20.119 | − | − | 2 | 1–2 | |
| chr11_20.121 | − | − | 3 | 1–3 | |
| chr11_20.122 | − | − | 5 | 1–5 | |
| Gosr2 | + | + | 6 | 1–6 | |
| Wnt9b | + | + | 4 | 2–4 | |
| Wnt3 | + | + | 5 | 1–3 | |
| Nsf | + | + | 20 | 2–8 | |
| Arf2 | + | + | 5 | 2–5 | |
| chr11_20.147 | − | − | 6 | 1–6 | |
| Crhr1 | + | + | 13 | 1–13 | |
| chr11_20.150 | − | − | 5 | 1–5 | |
| Mapt | + | + | 11 | 2–7 | |
| chr11_20.152 | + | + | 12 | 4–6 | |
| chr11_20.154 | + | + | 2 | 1–2 | |
| chr11_20.155 | + | + | 2 | 1–2 | |
| chr11_20.156 | + | + | 10 | 7–10 | |
| Cdc27 | + | + | 11 | 5–7 | |
| Myl4 | + | + | 9 | 8–9 | |
| Itgb3 | + | + | 15 | 5–9 | |
| chr11_20.160 | − | − | 3 | 1–3 | |
| chr11_20.161 | + | − | 36 | 3–7 |
- a Genes are listed in physical order on the chromosome, proximal to distal.
- b Genscan Gene Predictions according to UCSC October, 2003 Freeze.
- c No detectable difference in expression between A/WySn and AXB-4/Pgn strains; + indicates PCR product present; − indicates no PCR product detectable.
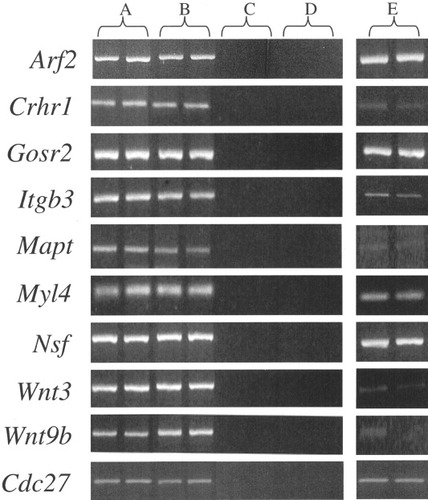
Expression in ED10–11 embryo heads and testes of the known genes in the chromosomal segment containing clf1. Bands are ethidium bromide-stained PCR products. A: A/WySn ED10–11 embryo head cDNA. B: AXB-4/Pgn (control strain) ED10–11 embryo head cDNA. C: H2O controls (no DNA). D: Genomic DNA controls. E: A/WySn testis cDNA.
No differences between the A/WySn and AXB-4/Pgn (normal) strains in PCR product size or quantity from ED10–11 embryonic head cDNA were detected for any known gene (Fig. 2) or predicted gene, indicating that the clf1 mutation does not lead to an absence of transcript, is not a moderate-sized deletion or insertion in the cDNA segments amplified, and does not cause ectopic expression of a gene normally not expressed in the embryonic head. For several genes that are expressed in ED10–11 head, deletions or insertions in exons not present in the segment screened cannot be ruled out (Table 2).
No differences between A/WySn and AXB-4/Pgn were detected for products from testis cDNA, with the exception of products incorporating exons 1–5 and 2–5 from Crhr1 that consistently demonstrated less product (faint bands) from A/WySn. The amplification of a segment spanning from within exon 2 into exon 5 (312 bp) produced an A/WySn-specific additional approximately 800-bp product. As this variant is not expressed in embryonic heads, it does not seem likely to be the clf1 mutation. It was nevertheless defined further by sequencing. The sequence of the first 600 bp of the abnormal 800-bp PCR product from testis consisted of the 3′ end of exon 4, part (499 bp) of the 3′ end of intron 4, and the 5′ end of exon 5, apparently reflecting a misspliced mRNA. In A/WySn genomic sequence compared with C57BL/6J, two alterations near the extraneous intron 4 material were found: a single base deletion (GG → G) 22 bp upstream of the normal intron 4/exon 5 splice junction consensus sequence, and a (C → G) substitution 13 bp downstream of the junction between the 3′ end of exon 4 and the 5′ end of the intron. Amplification by primers specific to each of the two versions of genomic sequence (Strachan and Read, 1999) at each of the two variants, applied to a panel of inbred strains, demonstrated that for both variants, SWR/J, PL/J, CBA/J, and SWV/Bc are like A/WySn, whereas 129/J, C3H/HeHaJ, BALB/cBc, and AXB-4/Pgn are like C57BL/6J, indicating that the pair of Crhr1 alterations seen in A/WySn that lead to an abnormal cDNA in testis are polymorphisms and are therefore not the clf1 mutation.
Gene Function
The current understanding of gene function of all of the candidate genes expressed in ED10–11 embryo heads is summarized in Table 3. Some of the genes—Wnt3, Crhr1, Mapt, and Itgb3—have been observed in null mutants, none of which express CL(P). Among these, null mutants for Crhr1, Mapt, and Itgb3 are viable adults with mild defects that do not result from craniofacial embryology, reducing the probability that these genes are the site of the clf1 mutation. The Wnt3 null mutant dies before the lip develops, which does not exclude it from potentially causing CL(P) in a mutant with some residual gene function.
| Gene symbol | Gene name (MGI) | Gene function | Mutant phenotype | |
|---|---|---|---|---|
| Gosr2 | Golgi SNAP receptor complex member 2 | Intracellular protein transport (golgi) | Not known | |
| Wnt9b | Wingless-type MMTV integration site 9B | Extracellular signaling | Not known | |
| Wnt3 | Wingless-related MMTV integration site 3 | Extracellular signaling | Homozygotes fail to form a primitive streak or undergo gastrulation; die by E10.5. | Liu et al. (1999) |
| Nsf | N-ethylmaleimide sensitive fusion protein | Intracellular protein transport (golgi) | Not known | |
| Arf2 | ADP-ribosylation factor 2 | Intracellular protein transport (golgi) | Not known | |
| Crhr1 | Corticotropin releasing hormone receptor 1 | Transmembrane receptor, signaling | Homozygotes are developmentally normal, but adults have rudimentary adrenal medullas and reduced stress-induced release of ACTH and corticosterone. Exploratory behavior is increased and anxiety-related behavior reduced. | Timpl et al. (1998); Smith et al. (1998) |
| Mapt | Microtubule-associated protein tau | Cytoskeletal stabilization | Homozygotes are developmentally normal, with microscopic reduction of microtubules in cerebellar axons. | Harada et al. (1994) |
| 20.152 | Orthologous to human KIAA1267 | An uncharacterized human protein | Not known | |
| 20.154 | Not known | Not known | Not known | |
| 20.155 | Similar to G protein coupled receptor Lgr8 in mouse | Signal transduction | Not known | |
| 20.156 | Part of Cdc27 in rat | Cell division | Not known | |
| Cdc27 | Cell division cycle 27 homolog (S. cerevisiae) | Cell division | Not known | |
| My14 | Myosin, light polypeptide 4, alkali; atrial, embryonic | Calcium ion binding in cytoskeleton | Not known | |
| Itgb3 | Integrin beta 3 | Cell-cell and cell-matrix adhesion | Most homozygotes are viable and fertile, but have platelet defects, prolonged bleeding times, skin, and gastrointestinal bleeding, and anemia, with reduced survival. Placental defects and fetal hemorrhage cause some prenatal loss. | Hodivala-Dilke et al. (1999) |
Some of the genes are involved in basic functions such as intracellular protein transport (Gosr2, Nsf, Arf2) or cell division (Cdc27 and 20.156), which seem unlikely to cause a specific nonsyndromic defect, like the CL(P) of the A/∼ strains, although they cannot be ruled out. Some have no identified function (20.152 and 20.154).
Some genes seem to be more plausible candidate genes for clf1 to begin with. Myl4, an embryonic myosin polypeptide may affect the cytoskeleton. Gene 20.155 may be involved in signal transduction. Wnt3 and Wnt9b are extracellular signaling molecules in a family of genes that function importantly in embryonic morphogenesis, determining cranial neural crest cell identity (Garcia-Castro et al., 2002; Katoh, 2002; Lee et al., 2004). The known expression domain of Wnt3 during ED9.5–10.5 includes cranial dorsal neuroepithelium with an anterior expression boundary at the diencephalon-telencephalon boundary and lateral diencephalic patches (Roelink and Nusse, 1991; Parr et al., 1993). These domains could influence the neural crest cells that populate the facial prominences. The ED9–11 embryonic expression domains of Wnt9b have not been published, but there is a peak of expression in whole embryo mRNA at ED10.5 (Qian et al., 2003). Preliminary in situ hybridization studies indicate that Wnt9b is expressed in the facial prominences during the development of the upper lip in mice (Andrew C. Lidral, personal communication). Based on the importance of the Wnt gene family in embryonic morphogenesis, the Wnt loci seem the best candidates for the clf1 mutation.
Haplotype Survey of Strains
The 28 strains surveyed grouped into nine haplotypes across the chromosomal region containing clf1 (Fig. 3). As expected, the three A/∼ strains (A/WySn, A/HeJ, and A/J) and the RI strain (AXB-23/Pgn) with the candidate region inherited from A/J, all share the same haplotype. The CBA/J strain shares the A/∼ haplotype for nearly all of the chromosomal segment, switching to a new haplotype distal to D11Mit10 that continues below the segment, as indicated by the Tlk2 marker. Notably, there are only two possible genes in the “non-A” portion of the segment in CBA/J (20.160 and 20.161), and they were both excluded from candidacy for the clf1 locus by the gene expression studies (Table 2). CBA/J, therefore, has the A/∼ haplotype across all possible candidate genes. The LM/Bc strain and its relative, SWV/Bc, appear to have part of the A/∼ haplotype, below Wnt3. All other strains have haplotypes distinct from the A/∼ strains, including 6 that share the C57BL/6J haplotype.
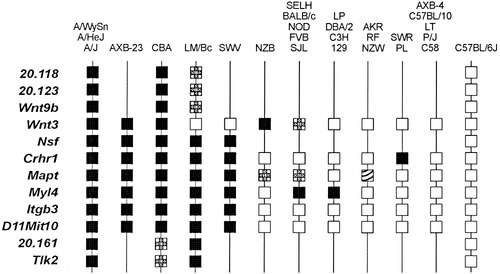
Haplotypes of simple sequence length polymorphism (SSLP) markers in inbred strains across the chromosomal segment containing clf1 (20.118–20.161). Black boxes represent the A/WySn “A”-type; white boxes represent the C57BL/6J “B” marker type; plaid boxes represent a non-A, non-B, “C”-type; hashed boxes represent a non-A, non-B, non-C, “D”-type.
Test for clf1 in CBA/J
The CBA/J strain, previously used by our laboratory in studies of embryonic traits (Juriloff, 1987), does not express CL(P). The breeding study to test for a hidden presence of the clf1 mutation in CBA/J produced no CL(P) in 210 test-cross embryos, significantly less than the 3–6% expected if the clf1 mutation were present (χ2 = 6.4–13.4; p < 0.025–0.0002), and confirmed that CBA/J has the normal allele at clf1. Thus, for all of the clf1 candidate genes, the CBA/J strain represents the ancestral normal DNA sequence on which the clf1 mutation occurred in the A/∼ strain lineage and comparison of A/WySn with CBA/J will distinguish preexisting polymorphisms from the clf1 mutation.
Wnt3 and Wnt9b Coding Sequence
For Wnt3, the entire A/WySn coding region has the same sequence as C57BL/6J (Refgene NM_009521; UCSC, October 2003). In the 3′UTR, three single-base variants were found in A/WySn compared to C57BL/6J: G→A at bp 1877 of the cDNA, G→A at bp 2115, and C→T at bp 2284. These are the only sequence variants present in the coding region, 5′UTR, or 3′UTR. The CBA/J strain was sequenced across the region containing the three variants and was found to be the same as A/WySn, indicating that these variants are part of the shared ancestral haplotype and are not the clf1 mutation.
For Wnt9b, the entire A/WySn coding region has the same sequence as C57BL/6J (Refgene NM_011719; UCSC, October 2003). In the 3′UTR, three single-base variants were found in A/WySn compared to C57BL/6J: T→C at bp 2023 of the cDNA, A→G at bp 2183, and G→T at bp 2620 (base positions are from NM_011719). These are the only sequence variants present in the coding region, 5′UTR, or 3′UTR. The CBA/J strain was sequenced across the region of the three variants and was found to be the same as A/WySn, indicating that these variants are also part of the shared ancestral haplotype, not the clf1 mutation.
Long PCR to Screen for an Inserted Transposable Element
A total of 27 primer pairs was required to screen a contiguous region of approximately 50 kb of A/WySn genomic DNA in overlapping segments, from within the first intron of Wnt3 through the intergenic sequence, the entire Wnt9b genomic sequence, and 7 kb beyond the 3′UTR of Wnt9b. Of these, 26 indicated no difference in segment size between A/WySn and the C57BL/6J type. One segment, located approximately 4–7 kb beyond the 3′ end of Wnt9b, amplified by primers L59 and L60, produced the expected 2681-bp segment from the control, and a larger, approximately 8000-bp segment from A/WySn. A repeat study on an extended panel showed that all strains (and an individual segregant) known to be homozygous for the clf1 mutation (A/WySn, A/J, A/HeJ, CL/Fr, AXB-23/Pgn, and X115) have the 8000-bp segment, whereas all strains known to lack the clf1 mutation (C57BL/6J, AXB-4/Pgn, LM/Bc, and CBA/J) have the expected 2681-bp segment. Importantly, the CBA/J strain, having the ancestral chromosomal segment on which the clf1 mutation subsequently occurred, does not have the expanded segment, indicating that the alteration is likely to be the lesion that is the clf1 mutation. The size alteration was consistent with the insertion of a transposable element. Subsequent sequencing of the expanded segment in A/WySn demonstrated that the expansion is due to an IAP element inserted 6606 bp 3′ of the Wnt9b 3′UTR (Refseq version) in the same orientation as the Wnt9b gene. The IAP element is 5213 bp, a type 1 delta 1 element (Ishihara et al., 2004), with long terminal repeats (LTRs) of 351 bp that are 99.7% identical, indicating a very recent insertion event.
DISCUSSION
The full list of candidate genes for clf1, defined by normal ED10–11 craniofacial embryonic expression in this study, is as follows: Gosr2, Wnt9b, Wnt3, Nsf, Arf2, Crhr1, Mapt, Cdc27, Myl4, Itgb3, chr11_20.152, 20.154, 20.155, and 20.156. For most, an embryonic craniofacial expression domain was not previously known. Our studies of cDNA from A/WySn transcripts show that none of these genes is deleted or fails to make transcript in clf1 mutant embryos. Moderately large deletions or insertions are also ruled out for the particular exons screened (Table 2).
Based on their expression in cDNA from testis, two genomic segments predicted to be genes by Genscan, chr11_20.118 and 20.161, were confirmed to be genes or parts of genes. They are not expressed in normal embryonic heads, indicating they are not candidates for clf1.
The predicted genes that were not expressed in normal ED10–11 heads were also not expressed in A/WySn embryonic heads (Table 2), indicating that they do not have ectopic expression in A/WySn. Furthermore, a mutation causing ectopic expression or gain-of-function of any gene is unlikely for clf1, because it is recessive (Strachan and Read, 1999).
The available reference mouse genomic DNA sequence is from C57BL/6J and differs from A/WySn at sites that are normal variants. A method to identify the clf1 mutation from among the normal variants in DNA sequence was needed. We demonstrated that another normal strain, CBA/J, shares with the A/∼ strains the same ancestral chromosomal segment (haplotype) that contains all the clf1 candidate genes. This is consistent with their shared ancestry (Wiltshire et al., 2003; Witmer et al., 2003). Our test-cross showed that CBA/J does not have the clf1 mutation. Therefore, we now have in hand two versions of the same haplotype encompassing all of the clf1 candidate genes: one with the clf1 mutation (A/∼ strains) and one without (CBA/J). Consequently, for any variant found, comparison of A/∼ with CBA/J sequence now serves as an essential tool to distinguish, at the DNA level, the clf1 mutation from normal variants.
Part of the haplotype of the chromosomal region containing clf1, distal to the Wnt3 gene (Fig. 3), seems to be present in the LM/Bc strain and its ancestor, SWV/Bc (Harris et al., 1984). The historical relationship of SWV/Bc to other strains is unknown (Juriloff et al., 1991). Neither LM/Bc nor SWV/Bc express CL(P) in embryos (Juriloff and Harris, 1989, 1993). Specific test breeding like that done for CBA/J would be needed to definitively exclude the presence of the clf1 mutation in these strains.
The embryonic phenotype of mutations can be difficult to predict from known gene functions, as demonstrated by the unexpected embryonic craniofacial role of the endothelin-1 gene (Kurihara et al., 1994). Nevertheless, the logical place to start an analysis of candidate genes is with the strongest candidates. From the known or predicted molecular function of the candidate genes (Table 3), the best candidates for the site of the clf1 mutation are the Wnt3 and Wnt9b signaling genes, members of a gene family that influences cranial neural crest cell identity and other aspects of morphogenesis (Garcia-Castro et al., 2002; Katoh, 2002; Lee et al., 2004). The recent description (Niemann et al., 2004) of amelia and CL(P) in a human loss-of-function mutant for WNT3 and observed expression of Wnt9b in the facial prominences (A.C. Lidral, personal communication), further strengthen the Wnt genes as candidates. We found six separate single base substitutions in 3′UTRs in A/WySn relative to C57BL/6J: three in Wnt3 and three in Wnt9b. Each of these sequences was found to be identical between A/WySn and CBA/J, and each base substitution is therefore part of the ancestral haplotype; none is the clf1 mutation. In summary, our sequencing of Wnt9b and Wnt3 cDNA from A/WySn indicates that there is no mutation in the coding sequence of either gene, but there remain other types of genetic alteration affecting gene function that were not ruled out.
The reasons why some common birth defects have a non-Mendelian, complex pattern of heredity are not known. One possibility is that the nature of the DNA alterations themselves differ from the types of mutations that lead to “Mendelian” heredity. Two aspects of the CL(P) trait suggested one of the possibilities: the strong genetic maternal effect on expression rate (Juriloff et al., 2001) and the variable expression within a highly inbred strain (cleft or normal within the A/∼ strains) are hallmarks of some mutations caused by the insertion of transposable elements near genes (Whitelaw and Martin, 2001). Well-studied examples are the Avy and AxinFu mutations (Vasicek et al., 1997; Rakyan et al., 2003; Waterland and Jirtle, 2003), in which ectopic gene expression and chimeric (fusion) transcripts result, respectively, from transcription beginning in the element. These examples demonstrate maternal or parent-of-origin effects, diet effects, and variable phenotype within strains that occur because of heritable variable failure of silencing of the transposon by methylation (Michaud et al., 1994; Rakyan et al., 2003; Waterland and Jirtle, 2003). Many mutations due to the insertion of transposable elements are recessive and involve interference with gene function.
Our screen for an inserted transposable element detected an IAP element at 6.6 kb from the 3′ end of Wnt9b. It is present in all of the A/∼ strains and relatives with clf1, and not present in C57BL/6J, nor in CBA/J, the ancestral haplotype. This is the only variant among the many found so far in A/WySn in the clf1 candidate gene region that is not also present in CBA/J. Given that it is near one of the best candidate genes, is consistent with the non-Mendelian aspects of the occurrence of CL(P) in the A/∼ strains, and is not present in CBA/J, this IAP insertion has a very high probability of being the clf1 mutation. Backwards initiation of transcription (termed “antisense,” “cryptic,” or “bidirectional”) from an IAP promoter, interfering with expression of an adjacent or surrounding gene, has been described for various IAP-induced mutations (Vasicek et al., 1997; Waterland and Jirtle, 2003). One possible mechanism for interference with the Wnt9b gene is the initiation of a transcript “backward” from the IAP promoter into the Wnt9b gene (Fig. 4), leading to the production of an antisense mRNA for part of Wnt9b; interestingly, this mechanism would be compatible with the presence of the normal “sense” mRNA detected in the cDNA studies. A complementation test with a Wnt9b mutation (none have been published) in a breeding design that provides the clf2 and maternal effect factors could confirm that the IAP is the clf1 mutation.
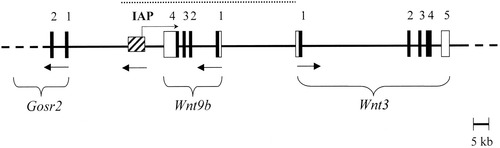
The mouse genomic region containing the Wnt3 and Wnt9b genes, showing the location of the IAP element in the A/∼ strains. The solid horizontal line represents a segment of genomic DNA; solid vertical bars represent exons (numbered); open vertical bars represent UTRs of mRNA. The hatched box represents the IAP. Arrows below the line indicate the direction of transcription. An arrow above the line indicates the hypothetical antisense transcription from the IAP into the Wnt9b gene. The dotted line indicates the region that has been screened for large insertions by long PCR. Exon size is not to scale.
Few, if any, non-clf1 changes are expected in CBA/J relative to A/WySn, if a common ancestor was involved in the initiation of both of the strains. This seems likely because the pattern of single nucleotide polymorphisms (SNPs) among strains indicates that the shared haplotype blocks were identical at the time the breeding of strains was begun (Wiltshire et al., 2003). Given this recent relationship and the small (1.5 Mb) genomic region involved as a target for new mutations and transposon insertions, the likelihood that the IAP found near Wnt9b is not the clf1 mutation seems very low. However, if it proves not to be the mutation, further screening for transposons in and around the best candidate genes, and further complementation tests (as described above) between the existing knockout mutations for the best candidates (e.g., Wnt3) and the A/WySn strain may identify the mutated gene. A brute-force sequencing comparison of the CBA/J and A/WySn strains across the segment containing all candidate genes may be feasible and would identify the mutation.
Why focus on this complex cause of CL(P) in the A/∼ strains, rather than depend on the elucidation of craniofacial developmental genetic signaling pathways to point to the causes of human CL(P)? Human nonsyndromic CL(P) displays, in addition to probable genetic heterogeneity of cause, signs of genetic complexity similar to the A/∼ strains, and to the Avy and AxinFu “metastable epiallele” mutations: maternal effects that may affect methylation (Prescott et al., 2002; Jugessur et al., 2003) and non-Mendelian inheritance patterns. Disruption or modulation of human genes by retrotransposons display similar mechanisms as in the mouse, including transcription from antisense promoters (Nigumann et al., 2002; van de Lagemaat et al., 2003). Not only is it likely that there is a human CL(P)-causing mutation homologous to the clf1 gene, but also that the nature of the mechanisms disrupting gene activity at other CL(P)–causing loci in humans may parallel the non-Mendelian mechanism acting in the A/∼ strains. If so, the availability of the naturally occurring CL(P) of the A/∼ strains for study may be a fortuitous opportunity to illuminate the genetic mechanisms behind the enigma of human CL(P).
Acknowledgements
We thank Sarah M Kennedy and Cindy Chao for molecular technical assistance and Tanya Edwards for taking care of the mice.




