Developmental toxicity induced by triclosan exposure in zebrafish embryos
Abstract
Objectives
The present study aimed to investigate the acute toxicity and the developmental alterations induced by triclosan (TCS) exposure in zebrafish early-life stages using fish embryo acute toxicity tests as a methodological approach.
Material and Methods
Zebrafish embryos were exposed to five concentrations of TCS and the four lethal alterations were daily recorded to determine the toxicological endpoints of acute toxicity. Furthermore, sublethal alterations were recorded to assess the effect of exposure concentrations on zebrafish embryo's development.
Results
The TCS toxicity was determined at 96 h of exposure as lethal concentration 10, lethal concentration 20, lethal concentration 50, lowest observed effects concentration, and no observed effects concentration, reported the following values: 168, 197.2, 267.8, 300, and 200 μg/L. Exposed larvae showed a delay in hatching rate and developed sublethal alterations including reduced blood flow, pericardial oedemata, reduced heartbeat, blood congestion, and craniofacial malformations. The number of zebrafish larvae developing cardiovascular alterations changed according to the tested concentrations and time of evaluation.
Conclusion
The data confirmed the developmental toxicity of TCS on aquatic organisms and the sublethal alterations developed by zebrafish larvae, indicated its cardiotoxicity and neurotoxicity. Moreover, the developmental toxicity was strongly influenced by the concentration tested and the number of survived zebrafish developing this alteration varying according to the time of exposure.
1 INTRODUCTION
Triclosan (5-chloro-2-(2,4-dichloro phenoxy)phenol; TCS) is a nonionic, broad-spectrum antimicrobial used as ingredient in disinfectants, soap, detergent, toothpaste, mouthwash, fabric, deodorant, shampoo, and plastic additives, in addition to innumerable other personal care, veterinary, industrial, and household products (Dann & Hontela, 2011). Humans and animals can be exposed to TCS through dermal, mucosal, and oral routes. The absorption and distribution are rapid and before entry into the bloodstream, TCS is conjugated by the skin. After an oral dose the TCS, is eliminated through urine primarily as sulfate or glucuronide conjugates, (Olaniyan, Mkwetshana, & Okoh, 2016). TCS has been also detected in breast milk, urine, and plasma, showing levels in the blood proportional to the consumer use (Hines et al., 2015). Human epidemiological studies correlated TCS exposure with reproductive and developmental (Etzel et al., 2017; Vélez, Arbuckle, & Fraser, 2015; C. F. Wang & Tian, 2015). Higher TCS urine concentrations in pregnant women were associated with an increase in spontaneous abortion rate (C. F. Wang & Tian, 2015). Moreover, an association between high TCS urine levels and diminished head circumference and body mass index in boys was also observed (Lassen et al., 2016; Li et al., 2015). Enhanced TCS serum levels obtained from human mothers were associated with increased risks of birth defects in newborns (Wei et al., 2017).
The structural similarity of TCS to known estrogenic and androgenic endocrine disruptors chemicals, such as polychlorinated biphenyls, polybrominated diphenyl ethers, and bisphenol A, could be predictive of its endocrine disruption potential (Dann & Hontela, 2011; Sharma, Iqbal, Singh, Kaur, & Faggio, 2021). Several studies have shown that this antimicrobial can influence endocrine function in several species, potentially interfering with thyroid hormone homeostasis, reproductive axis, and also brain development (Pernoncini, Montagnini, de Góes, Garcia, & Gerardin, 2018; Rodríguez & Sanchez, 2010; Tran, Jung, Yoo, Lee, & Jeung, 2020; Vijayakumar et al., 2019).
The antimicrobial TCS is commonly detected in aquatic ecosystems, with concentrations ranging from 0.0006 to 2.3 μg/L in surface waters and from 0.27 to 800 μg/L in the sediments (Agüera, Fernández-Alba, Piedra, Mézcua, & Gómez, 2003; Kolpin et al., 2002; Miller et al., 2008; Nakada et al., 2008). The incomplete removal of TCS during the wastewater treatment process leads to the continual exposure of aquatic biota in receiving waters, and the accumulation of the antimicrobial and its degradation products in tissues of aquatic organisms (Dann & Hontela, 2011). Moreover, there is strong evidence that aquatic species are more sensitive to TCS exposure than mammal vertebrates (Dann & Hontela, 2011). Although TCS is a stable and lipophilic compound, it can be transformed into more toxic and bioaccumulative derivatives once in the aquatic environment, such as 2,8- dichlorodibenzodioxin, other dioxin derivatives and chlorophenols (Anger et al., 2013). The potential toxic effects of TCS on freshwater and marine organisms have been investigated using different experimental models including algae, invertebrates, amphibians, and fish (Khatikarn, Satapornvanit, Price, & Van den Brink, 2018; Palenske, Nallani, & Dzialowski, 2010; Pullaguri, Nema, Bhargava, & Bhargava, 2020; Xin, Huang, An, Raina-Fulton, & Weger, 2019). Among fish, the zebrafish (Danio rerio) represents a valuable model to study both the ecotoxicological and developmental toxicity of TCS. In particular, the zebrafish embryo has achieved high popularity founded on a unique set of properties such as ease of maintenance, small size, high fecundity, rapid development, optical transparency of the embryo, amenability to genetic and chemical screens, and an extensive literature base(Strähle et al., 2012). Moreover, the fish embryo toxicity (FET) test represents an integral and standardized method for hazard identification in ecological risk assessment providing valuable information on the acute toxicity of chemicals (Blahova, Cocilovo, Plhalova, Svobodova, & Faggio, 2020; Braunbeck et al., 2014; Cahova, Blahova, Plhalova, Svobodova, & Faggio, 2021; Fiorino et al., 2018). Thus, the present study aimed to investigate the acute toxicity and the developmental alterations induced by TCS exposure in zebrafish early-life stages using FET tests as methodological approach. Zebrafish embryos were exposed to five concentrations of TCS and the four lethal alterations were daily recorded to determine the toxicological endpoints of acute toxicity (lethal concentration 10 [LC10], lethal concentration 20 [LC20], lethal concentration 50 [LC50], lowest observed effects concentration [LOEC], no observed effects concentration [NOEC]). The implementation of additional endpoints beyond the four core endpoints prescribed by OECD TG 236 adds significant value to the FET as a validated test system (von Hellfeld, Brotzmann, Baumann, Strecker, & Braunbeck, 2020). For these reasons, a detailed evaluation of sublethal alterations, including the evaluation of their time of onset was performed to assess the effect of exposure concentrations on zebrafish embryo's development.
2 MATERIALS AND METHODS
2.1 Zebrafish maintenance and egg collection
Adults AB wild-type zebrafish were obtained from breeding facility at University of Teramo (code 041TE294). Adults were kept in 3.5 L ZebTec tanks (Tecniplast S.p.a., Buguggiate, Italy) in a recirculating aquatic system. Animals were fed twice a day with live food (Artemia salina) and supplemented with Zebrafeed 400–600 (Sparos, Olhão, Portugal). The temperature was maintained at 28°C, pH at 7 ± 0.2, the conductivity at 500 ± 100 μS/cm and dissolved O2 at 6.1 mg/L. The photoperiod was 14 h light/10 h dark. Chemical parameters were kept as follows: ammonia 0.02 mg/L, nitrite 0.02 mg/L, nitrate 21.3 mg/L. The afternoon before spawning, 10 groups of females and males (1:1) were introduced into 1.7 L breeding tanks (beach style design, Tecniplast S.p.a.). Immediately after spawning, fertilized eggs were collected with a sieve, rinsed with deionized water and used in FET tests.
2.2 FET tests
FET tests were performed according to OECD n. 236 (OECD, 2013) on zebrafish fertilized eggs after 1 hour post fertilization (hpf) and within 3 hpf. TCS (CAS number 99–76-3, Pharmaceutical Secondary Standard; Certified Reference Material) was purchased from Sigma-Aldrich, Milan, Italy. Dimethyl sulfoxide (DMSO; >99.9% purity) and 3,4-dichloroaniline (>98% purity) were obtained from Sigma-Aldrich (St. Louis, MO).
TCS was dissolved in DMSO (>99.9% purity) and tested at following concentrations: 50, 100, 200, 300, and 400 μg/L. Concentrations were chosen according to previous data available in the literature (Oliveira, Domingues, Grisolia, & Soares, 2009). Selected embryos were placed individually with 2 ml of test solution in each well of 24-well plates (TPP, Trasadingen, Switzerland). All test plates had been preincubated (saturated) with the test solutions for 24 h. Subsequently, well plates were sealed with self-adhesive foil (SealPlate™ by EXCEL Scientific, Dunn, Asbach, Germany). Twenty embryos per concentration were exposed to the test substance with semi-static renewal technique (renewal every 24 h) and the other four wells served as internal control of the plate. Experiments were repeated three times in several weeks. Negative control solvent control (0.1% DMSO) and positive control (4% 3,4 dichloroaniline) were also tested. Test solutions were daily prepared in dilution water (DW) according to annex 2 of OECD TG 203 OECD, 1992), and before each experiment, the pH of the DW was adjusted at 7.5.
Embryos were exposed for 96 hpf in the incubator at 26 ± 1°C and photoperiod (14 h light:10 dark) conditions. Zebrafish early-life stages were daily observed up to 96 h with the inverted optical microscope (CKX 41; Olympus, Japan) recording the four apical observations as indicators of lethality: coagulation of fertilized eggs, lack of somite formation, lack of detachment of the tail-bud from the yolk sac, and absence of heartbeat. FETs with a minimum mortality rate of 30% in the positive control and a maximum effective rate of 10% in the negative control (DW) at 96 hpf were classified as valid.
In addition to the lethal endpoints specified by OECD TG 236, any other observation was recorded as further sublethal endpoints according to von Hellfeld et al. (2020). Typical examples were reduced heartbeat or reduced blood flow, inhibited or missing pigmentation, delayed or altered development, modified movement(s), distortion of the spine, and formation of various types of oedemata. Moreover, the hatching process was also evaluated at 72 hpf. The developmental stage at the end of the exposure did not fall into the regulatory frameworks dealing with animal experimentation, and all the experiments complied with the EU Directive 2010/63/EU.
2.3 Statistical analysis
Statistical analysis of FET tests results was performed using a Bayesian approach. Beta distribution was calculated to evaluate differences in the percentage of anomalies and hatching rate in different groups (concentrations and time of exposure), comparing the 95% confidence intervals. Significant level was set at p < .05. In addition, toxicological endpoints (LC10, LC20, LC50, LOEC, and NOEC) were calculated using ToxRat software version 3.3 (ToxRat Solutions GmbH, Germany).
3 RESULTS
3.1 Acute toxicity
The toxicity of TCS was determined at 96 h of exposure as LC10, LC20, LC50, LOEC, and NOEC. The values were shown in Table 1. TCS exposure did not increase mortality at 24 and 48 hpf, and zebrafish early-life stages were starting to die at 72 hpf. All zebrafish embryos exposed to 400 μg/L died at the end of the exposure period.
| Time (hpf) | Treated embryos | LC10 (μg/L) | LC20 (μg/L) | LC50 (μg/L) | LOEC (μg/L) | NOEC (μg/L) |
|---|---|---|---|---|---|---|
| 24 | 60 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 48 | 60 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 72 | 60 | 242.3 | 295.1 | 430.2 | 200 | 100 |
| 96 | 60 | 168 | 197.2 | 267.8 | 300 | 200 |
- Abbreviations: FET, fish embryo toxicity; hpf, hour post fertilization; LC, lethal concentration; LOEC, lowest observed effects concentration; n.d., not determined; NOEC, no observed effects concentration; TCS, triclosan.
3.2 Hatching rate
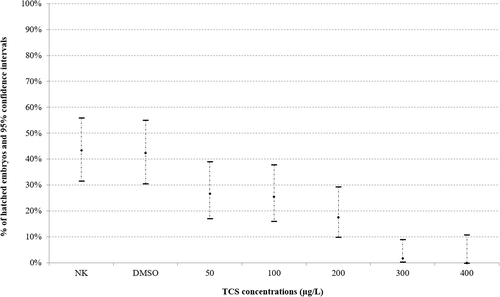
Significant (p < .05) delay (dose-dependent) in hatching rate at 72 hpf among control group and embryos exposed to TCS concentrations was observed (Figure 1).
3.3 Developmental alterations
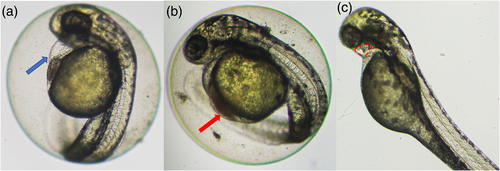
Zebrafish early-life stages exposed to TCS developed sublethal alterations including reduced blood flow, pericardial oedemata, reduced heartbeat, blood congestion, and craniofacial malformations (Figure 2).
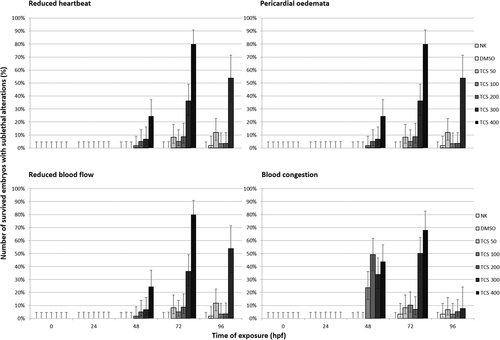
Reduced blood flow, pericardial oedemata, and blood congestion appeared at 48 hpf in zebrafish embryos treated with TCS 100, 200, 300, and 400 μg/L (Figure 3). Reduced heartbeat was also reported at 48 hpf but only in zebrafish embryos exposed to TCS 200 and 400 μg/L. The number of zebrafish embryos that developed these cardiac alterations increased at 72 hpf compared to 48 hpf just for TCS 300 and 400 μg/L. For the other experimental groups (TCS 100 and 200 μg/L) the number of zebrafish early-life stages reporting cardiovascular alterations at 72 hpf, was inferior compared to 48 hpf. At the end of exposure (96 hpf), the number of zebrafish larvae that developed pericardial oedema, reduced blood flow, and reduced heartbeat was less than 10% of survived embryos except for zebrafish larvae exposed to TCS 300 μg/L.
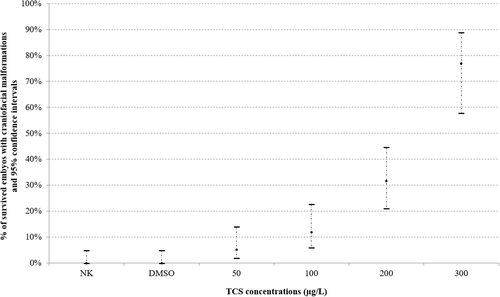
Craniofacial malformations were reported at 96 hpf in all experimental groups and the number of survived embryos with this alteration increased in a dose-dependent manner (Figure 4).
4 DISCUSSION
TCS can bioaccumulate and bioconcentrate within aquatic biota, resulting in concentrations in fish as high as 7.5–10 μg/kg in muscle and leading to potentially harmful effects in the exposed organism (Arnot, Pawlowski, & Champ, 2018). The toxicological properties of TCS in the zebrafish model were investigated by several authors. First, Oliveira et al. (2009) demonstrated as TCS exposure induced acute toxicity in zebrafish early-life stages, with a value of LC50 at 96 hpf of 0.42 mg/L. Recently the LC50 of TCS determined in zebrafish larvae was 0.6 mg/L (Pullaguri et al., 2020). In the present study, the value of LC50 at 96 hpf was 0.27 mg/L and the difference between these results and those of the previous authors could be related to the different experimental procedures and the type of solvent used, as also demonstrated for other personal care products (Merola et al., 2020). Moreover, zebrafish larvae treated with TCS developed delayed hatching, delay on the otolith formation, eye and body depigmentation, spine malformations, pericardial oedema, and undersize (Oliveira et al., 2009).
Among cardiac toxicity, zebrafish larvae exposed to 40 μg/L of TCS showed an increased incidence of pericardial edema and alteration in heart structure. Indeed, cardiac output was reduced following exposure to 400 μg/L of TCS (Saley, Hess, Miller, Howard, & King-Heiden, 2016)). The reduction of cardiac output in exposed embryos could be related to alterations in Ca2+ homeostasis in cardiac cells which can result in a reduction in contraction strength (reducing stroke volume) (Saley et al., 2016). In fact, TCS exposure affects Ca signaling in both cardiac muscle and skeletal myocyte of zebrafish early-life stages (Ma, Liu, & Yu, 2019; Saley et al., 2016).
Moreover, exposure to TCS altered blood flow through the heart in zebrafish embryos, causing an increased incidence of regurgitation between the ventricle and atria. This finding could be related to alteration of TCS exposure on heart valve formation, given the important role of cardiac valves in preventing regurgitation (Saley et al., 2016). This hypothesis was recently supported by the fact that the chronic exposure of 60 hpf zebrafish to TCS and its derivates (TCS-DT) induced cardiotoxicity through the disruption of several signaling pathways, including the cAMP signaling pathway, adrenergic signaling in cardiomyocytes, and pentose and glucuronate interconversions (D. Wang et al., 2020). In particular, the transcription levels of cardiac development-related genes, including ugdh, sox9b, ppp2r3a, and camk2a, were dysregulated following exposure to TCS-DT. These genes play critical roles in lipid balance and cardiac morphogenesis. Among these, ugdh functions in cardiac valve formation, and sox9b in cardiomyocytes for cardiac morphogenesis (D. Wang et al., 2020). Interestingly in the present study, zebrafish early-life stages exposed to TCS developed cardiovascular alterations at 48 hpf, with an increasing number of zebrafish larvae with sublethal alterations at 72 hpf compared to 48 hpf in the experimental group of TCS 300 μg/L and TCS 400 μg/L. However, the number of survived larvae with this sublethal effect was lower than 10% at the end of the exposure period except for zebrafish larvae exposed to TCS 300 μg/L. These results prompt the hypothesis that the development of cardiac alterations following TCS exposure was dependent on a defined range of concentrations and related to a specific developmental time frame of exposure. Cardiovascular alterations are sublethal endpoints with a high potential of regeneration in zebrafish larvae (Faria et al., 2021; Plhalova et al., 2018; von Hellfeld et al., 2020), and the reduction of the number of exposed zebrafish developing these alterations could be related to the activation of repair mechanisms by exposed animals. Moreover, zebrafish larvae exposed to TCS showed acclimation process to the chemical with reduced mortality at 100 μg/L compared to 50 μg/L (Falisse, Voisin, & Silvestre, 2017). The development of an acclimation response depends both on the duration of exposure and on the concentration of the xenobiotic which, combined, triggers priming mechanisms facilitating the organism to address subsequent stress (Shrivastava, Sinha, Datta, Blust, & De Boeck, 2016). Moreover, it has also been suggested that to trigger the acclimation process damages must occur to initiate repair mechanisms conferring increased tolerance without leading to mortality (Zheng et al., 2016). In the present study, the development of acclimation response combined with the higher potential of regeneration of cardiovascular alterations could be involved in the reduction of the number of zebrafish larvae developing these sublethal effects at the end of the exposure period. As previously stated, these mechanisms are dependent on tested concentrations, and exposure to concentrations higher than LC50, such as 300 μg/L, could be responsible for irreversible damage preventing the restore of physiological functions by repair mechanisms. Moreover, the potential restorative effect of TCS on cardiovascular function might be related to the antibacterial properties of TCS against several bacteria which induced infection and diseases in the aquaculture sector (Falisse et al., 2017).
The neurotoxicity of TCS in the zebrafish model was recently investigated by several authors (Ling et al., 2020; Pullaguri et al., 2021; D. Wang, Wang, Huang, & Wang, 2021).
Zebrafish embryos exposed to TCS also showed a delay in hatching rate, according to previous works (Falisse et al., 2017; Oliveira et al., 2009). This alteration, along with craniofacial malformations may be indicative of potential neurotoxicity exerted by TCS exposure in zebrafish larvae. In fact, the hatching process is a combination of biochemical and behavioral mechanisms and neurotoxicant agents could interfere with coiling movements of embryos inside the chorion with consequent inability to hatch (Selderslaghs, Hooyberghs, Blust, & Witters, 2013). Craniofacial malformations are recently designed as a good indicator of changes potentially associated with malformation of the brain and related to developmental neurotoxicity (von Hellfeld et al., 2020). In the present study, the number of survived embryos with craniofacial malformations at 96 hpf increased in a dose-dependent manner. These results are according to those of Kim et al. (2018) in which TCS exposure changes craniofacial morphology and neural structures in exposed zebrafish embryos (Kim et al., 2018).
5 CONCLUSION
The data of the present study confirmed the developmental toxicity of TCS on aquatic organisms. Interestingly, the sublethal alterations developed by exposed zebrafish larvae indicated the cardiotoxicity and the neurotoxicity of the tested compound. Moreover, the developmental toxicity was strongly influenced by the concentration tested and the number of survived zebrafish developing this alteration varying according to the time of exposure. The craniofacial phenotype of exposed larvae should be characterized through the execution of morphometrical analysis. The mechanisms of action involved in the cardiac toxicity of TCS should be addressed and the compensation response potentially involved in the modulation of TCS cardiotoxicity should be investigated.
AUTHOR CONTRIBUTIONS
Carmine Merola: Writing original draft, experiments performance, experimental and formal analyses. Elisabetta Benedetti and Monia Perugini: Conceptualization, supervision and writing review. Giulia Caioni: Investigation, formal analysis. Annamaria Iannetta and Viviana di Vito: Experiments performance and experiments analysis.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




