Atypical cardiac defects in patients with RASopathies: Updated data on CARNET study
Abstract
Background
RASopathies are a set of relatively common autosomal dominant clinically and genetically heterogeneous disorders. Cardiac outcomes in terms of mortality and morbidity for common heart defects (such as pulmonary valve stenosis and hypertrophic cardiomyopathy) have been reported. Nevertheless, also Atypical Cardiac Defects (ACDs) are described. The aim of the present study was to report both prevalence and cardiac outcome of ACDs in patients with RASopathies.
Methods
A retrospective, multicentric observational study (CArdiac Rasopathy NETwork—CARNET study) was carried out. Clinical, surgical, and genetic data of the patients who were followed until December 2019 were collected.
Results
Forty-five patients out of 440 followed in CARNET centers had ACDs. Noonan Syndrome (NS), NS Multiple Lentigines (NSML) and CardioFacioCutaneous Syndrome (CFCS) were present in 36, 5 and 4 patients, respectively. Median age at last follow-up was 20.1 years (range 6.9–47 years). Different ACDs were reported, including mitral and aortic valve dysfunction, ascending and descending aortic arch anomalies, coronary arteries dilation, enlargement of left atrial appendage and isolated pulmonary branches diseases. Five patients (11%) underwent cardiac surgery and one of them underwent a second intervention for mitral valve replacement and severe pericardial effusion. No patients died in our cohort until December 2019.
Conclusions
Patients with RASopathies present a distinct CHD spectrum. Present data suggest that also ACDs must be carefully investigated for their possible impact on the clinical outcome. A careful longitudinal follow up until the individuals reach an adult age is recommended.
1 INTRODUCTION
RASopathies include a heterogeneous group of genetic multisystemic disorders characterized by distinctive facial features, developmental delay, learning difficulties, short stature, congenital heart diseases (CHDs), renal anomalies, lymphatic malformations, and bleeding disorders (Aoki, Niihori, Inoue, & Matsubara, 2016; Rauen, 2013; Romano et al., 2010; Tartaglia & Gelb, 2010). They include different syndromes. The most common disorders are Noonan syndrome (NS; OMIM #163950) CardioFacioCutaneous Syndrome (CFCS; OMIM #115150), Costello syndrome (CS; OMIM #218040), and NS with multiple lentigines (NSML; also known as LEOPARD syndrome; OMIM #151100). These syndromes overlap cardiac and extracardiac features and share germline mutations of the RAS/MAPK pathway.
Heart defects, both CHDs and cardiomyopathies (CMP), were reported in 60–90% of patients affected by RASopathies. Specific types of CHDs and correlation with the specific gene mutations have been described (Calcagni et al., 2017; Calcagni et al., 2018).
Typical CHDs (such as pulmonary valve stenosis—PVS, atrioventricular septal defect—AVSD, atrial septal defect—ASD) have been reported (Digilio et al., 2013; Linglart & Gelb, 2020; Marino, Digilio, Toscano, Giannotti, & Dallapiccola, 1999). Hypertrophic cardiomyopathy (HCM) in the obstructive or nonobstructive form, is the most common CMP associated with this group of disorders (Calcagni et al., 2018). Dilated CMP was reported as well, due to a pathological different mechanism in the RAS/MAPK cascade (Gelb, Roberts, & Tartaglia, 2015).
Recently, we reported a multicentric study on 371 patients followed up in the CARNET (CArdiac Rasopathies NETwork) database, including the interventional and surgical results, genotype–phenotype correlations together with the prognostic assessment (Calcagni et al., 2017a; Calcagni et al., 2017b). Atypical Cardiac Defects (ACDs) were reported also in these patients, but no specific cardiac outcome has been described.
The aim of the present study was to update the CARNET experience and to analyze the prevalence, gene correlations, and outcome in RASopathies' patients presenting with ACDs.
2 METHODS
2.1 Study design and populations
This multicentric retrospective observational study was carried out in patients enrolled from seven centers participating in CARNET (Calcagni, Limongelli, et al., 2017a). All subjects with a molecularly confirmed diagnosis of NS, NSML, CS, or CFCS, with or without cardiac defects were preliminarily included in the study, updated at December 2019.
The following clinical data were collected: date of birth and sex, cardiac details at diagnosis and mutated genes.
Cardiac information included cardiologist's clinical evaluations and reports, electrocardiograms (ECGs), echocardiograms, and discharge letters. When appropriate, cardiac procedures or cause of cardiac or extracardiac deaths were reported. All data was centralized in a unique database.
Once the database was completed, we selected a distinct subgroup of patients for further analysis. These patients included those presenting with ACDs, that is, structural defects different from common defect associated with RASopathy disorders (Digilio et al., 2013; Linglart & Gelb, 2020; Marino et al., 1999), manifesting a low prevalence in this population. Typical cardiac defects in these patients include PVS with usual supravalvular involvement, complete or partial AVCD, ASD, and HCM in the obstructive or nonobstructive forms. Patients presenting with ACDs associated with common cardiac defects, mentioned above were excluded from our analysis. Persistent Foramen Ovalis after 1 year of age, due to its high prevalence in the general population, was not counted as a cardiac defect in this study.
All other defects, different from the common ones, were classified as ACD and considered in the present study.
Molecular diagnosis was performed by Sanger sequencing and targeted resequencing directed to scan the entire coding sequence of CBL, PTPN11, SOS1, KRAS, HRAS, NRAS, SHOC2, RAF1, BRAF, MAP2K1 and MAP2K2, SOS2, LZTR1, RIT1 genes, which at time of our study had been associated with RASopathies (Linglart & Gelb, 2020; Tartaglia & Gelb, 2010; Tidyman & Rauen, 2016a).
Clinical and genetic analyses were performed with the approval of the institutional review boards of the participating institutions.
3 RESULTS
3.1 Study population
Among the 440 molecularly characterized patients followed in CARNET centers, we excluded those who did not present any cardiac defect (83 cases). Among these remaining 357 patients, we then excluded 312 subjects presenting with common CHDs (PVS, ASD, and AVSD) or HCM. The remaining 45 patients were included in this study and analyzed in terms of type of CHD, mutated genes and cardiac outcome. NS, NSML and CFCS were present in 36, 5 and 4 patients, respectively. No CS patient was present in the remaining population. Figure 1 illustrates the cohort of patients and the subgroup with ACDs.
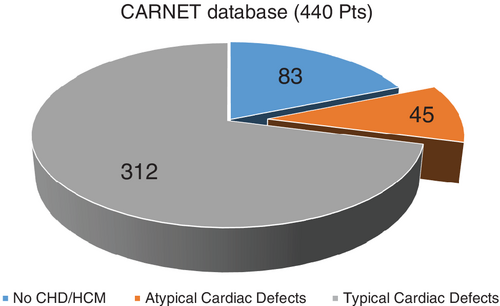
Median age at last follow-up was 20.1 years (range 6.9–47 years). Twenty patients were female (44.4%). Different gene mutations were found in our population, the most common of which affected the PTPN11 gene, followed by SOS1 and BRAF genes. Mutations in other genes of the RAS/MAPK cascade were rarer. Table 1 summarizes demographic, clinical and molecular characteristics of included patients.
| Age at last follow up (median and range) | 20.1 years (6.9–47 years) |
|---|---|
| Female sex | 20 (44.4%) |
| Syndrome (n = 45) | |
| NS | 36 (80.0%) |
| LS | 5 (11.1%) |
| CFC | 4 (8.9%) |
| Mutated genes (n = 45) | |
| PTPN11 | 27 (60.0%) |
| SOS1 | 5 (11.1%) |
| BRAF | 4 (8.9%) |
| SHOC2 | 2 (4.4%) |
| KRAS | 1 (2.2%) |
| NRAS | 1 (2.2%) |
| RAF1 | 1 (2.2%) |
| CBL | 1 (2.2%) |
| LZTR1 | 1 (2.2%) |
| MEK1 | 1 (2.2%) |
| SOS2 | 1 (2.2%) |
3.2 Heart defects
All patients had a single cardiac anomaly or multiple defects. Over half of these patients displayed a valvular disease, with mostly mitral or aortic valve involvement. Mitral valve regurgitation was more common than stenosis, which was recognized in one patient only. Valve insufficiency was related to anatomic dysplasia, occurring in about one third of our patients. Aortic valve regurgitation was also significantly more common than aortic stenosis (about 25% versus 9%). Seven coronary artery (CA) dilations were found, invariably involving left CA. No patients presented with coronary artery stenosis. Structural abnormalities of ascending and descending aorta occurred in five patients, including kinking, aortic coarctation, and aortic root dilation. Three cases had moderate enlargement of left atrial appendage unassociated with other heart defects nor with intracardiac thrombus. Isolated pulmonary branches abnormalities, in terms of dilation or stenosis or crossed pulmonary arteries were regarded as ACDs in the absence of PVS. These anomalies occurred in seven subjects (15.6%).
Table 2 summarizes heart defects and gene mutations found in the different heart disease subgroup.
| Type of heart defects | n (%) | Gene | n | |
|---|---|---|---|---|
| Mitral valve anatomic anomalies (n = 19) | Prolapse | 3 (6.7%) | PTPN11 | 9 |
| Dysplasia | 16 (35.6%) | RAF1 | 1 | |
| SOS1 | 2 | |||
| SHOC2 | 2 | |||
| KRAS | 1 | |||
| BRAF | 2 | |||
| CBL | 1 | |||
| LZTR1 | 1 | |||
| Mitral valve disfunction (n = 12) | Insufficiency | 11 (24.4%) | PTPN11 | 5 |
| Stenosis | 1 (2.2%) | SOS1 | 2 | |
| SHOC2 | 1 | |||
| KRAS | 1 | |||
| BRAF | 1 | |||
| CBL | 1 | |||
| LZTR1 | 1 | |||
| Aortic valve functional anomalies (n = 16) | Insufficiency | 12 (24.4%) | PTPN11 | 10 |
| Stenosis | 4 (8.9%) | RAF1 | 1 | |
| SOS1 | 1 | |||
| BRAF | 1 | |||
| CBL | 1 | |||
| SOS2 | 1 | |||
| LZTR1 | 1 | |||
| Coronary arteries (n = 7) | Dilation | 7 (15.6%) | PTPN11 | 5 |
| SOS2 | 2 | |||
| Aortic arch anomalies (n = 5) | Aortic coartation | 1 (2.2%) | PTPN11 | 3 |
| Kinking | 2 (4.4%) | SOS1 | 1 | |
| Aortic root dilatation | 2 (4.4%) | BRAF | 1 | |
| Left appendage dilation (n = 3) | Dilatation | 3 (6.7%) | PTPN11 | 2 |
| NRAS | 1 | |||
| Isolated pulmonary arteries anomalies (n = 7) | Stenosis | 5 (11.1%) | PTPN11 | 4 |
| Dilatation | 1 (2.2%) | SHOC2 | 1 | |
| Crossing PAs | 1 (2.2%) | RIT1 | 1 | |
| MEK1 | 1 | |||
3.3 Cardiac prognosis
Five of our ACDs patients (5/45:11%) underwent surgical treatment, including one for aortic coarctation corrected by end-to-end anastomosis and one for aortic valve replacement. Mitral Valve repair (plasty) was performed in two patients, either for severe regurgitation or stenosis. In an additional patient, with prolapse and severe regurgitation, aged 5 at the time of first intervention, plasty of the mitral valve was followed by a prosthesis implantation 20 months later. Early post-surgical outcome of this latter patient was characterized by severe pericardial effusion requiring surgical drainage. These five patients were affected by NS with PTPN11 (3 cases), KRAS and SOS1 (one case each) mutated genes. No patient died for cardiac or extracardiac diseases. No other surgical complications were reported, including post-surgical chiylotorax. The remaining patients did not need any surgical operation or percutaneous procedures at the time of the present retrospective analysis, all being on regular clinical and instrumental cardiac follow-up.
4 DISCUSSION
RASopathies are a heterogeneous group of genetic disorders with similar cardiac and extracardiac features.
Typically, mutations in genes involved in the RAS/MAPK cascade have been reported since the PTPN11 gene mutation was discovered in 2001 (Tartaglia et al., 2001). This mutation occurs in about 50% of patients with NS. An additional 30% can be explained by other gene mutations in the RAS/MAPK pathway genes, including SOS1, RAF1, RIT1, KRAS, SHOC2, NRAS, SOS2, BRAF, A2ML1, LZTR1, MYST4, RASA2, RRAS, SPRY1 and SYNGAP1 (Aoki et al., 2013; Tidyman & Rauen, 2016a; Tidyman & Rauen, 2016b; Yamamoto et al., 2015; Yu et al., 2019).
CHDs and CPM are very common features in patients with RASopathies, particularly in NS, in which a prevalence of 50–80% is reported (Linglart & Gelb, 2020).
Classically, PVS, HCM, AVCD, and ASD are considered distinct heart defects in these disorders. Minor defects have been also reported (Linglart & Gelb, 2020; Marino et al., 1999), but a specific correlation between these anomalies and gene mutations and their prognostic assessment are not yet available.
The present study reviews 45 RASopathy patients enrolled from seven centers with high experience in RAS/MAPK disorders. In general, ACDs present in these subjects are prognostically benign, well tolerated and do not required specific treatment. In fact, in our cohort, only five ACD patients needed surgical procedures, mainly due to valvular involvement.
These results are in line with a previous study from CARNET on a large cohort of children and adolescents with RASopathies, suggesting that the need for either cardiac surgery or catheterization, was less than 50% (Calcagni, Limongelli, et al., 2017a). Similar data were reported by other groups (Colquitt & Noonan, 2014; Prendiville et al., 2014). Thus, cardiac treatment could be restricted to a few selected cases, presenting with mitral or aortic valve diseases. While worsening of mitral and aortic valve disease has been reported in RASopathies patients with CHDs or HCM was previously reported (Calcagni, Limongelli, et al., 2017a), the present study argues that these valvular diseases may have a major clinical impact also in the absence of a coexisting typical heart defect. Mitral valve prolapse particularly with valve regurgitation may need surgical treatment in patients with severe valve dysplasia. Nevertheless, in RASopathy patients with significant valvular regurgitation, mitral valve plasty could be ineffective, due to anatomic characteristics of mitral valve, as an abnormal and dysplastic anterior leaflet insertion (Digilio, Marino, Giannotti, & Dallapiccola, 1997; Marino et al., 1995; Marino, Digilio, Gagliardi, Giannotti, & Dallapiccola, 1996). Therefore, isolated mitral or aortic valve disorders in these patients could not be regarded as minor defects.
Surgical treatment is also mandatory for selected cases manifesting other “structural” ACDs, such as aortic coarctation. Preliminary data preceding exhaustive gene screening, disclosed left sided obstructive defects in particular aortic coarctation in NS (Digilio et al., 1997; Digilio et al., 1998).
Coronary artery anomalies in RASopathies are not a rare entity. PTPN11 gene mutations are usually associated with these defects, particularly in NSML patients (Pacileo et al., 2006). Mutations in other genes have been also reported (Calcagni, Baban, De Luca, et al., 2016). Coronary ectasia secondary to HCM is well established, likely as a consequence of increased mass, diastolic dysfunction and obstruction of the outflow tract of the left ventricle (Limongelli et al., 2007). In the present cohort of patients, no individual with coronary artery ectasia was affected by HCM or other common cardiovascular defects. This vascular phenotype could be related to the RAS pathway defect itself, independently from an underlying HCM and/or cardiovascular defects. Cardiologists should be aware of the dilation of the coronary arteries in these patients, for preventing the risk factors of myocardial infarction, such as arterial hypertension and hypercholesterolemia that could accelerate the atherosclerotic coronary artery disease. Once coronary anomaly is recognized by echocardiography, further evaluation with coronary CT angiography or MR angiography should be undertaken, especially after pediatric age, in order to monitor the dilation's progression. Since the long-term outcome of coronary arteries ectasia in patients with RASopathy is unknown and that the dilated coronary arteries predispose to thrombosis, the use of antiplatelet or anticoagulant treatment must be considered also in these patients.
Two of our patients displayed dilation of the ascending aorta. Although rare, aortic root dilation, aortic dissection and giant aneurysms of the Valsalva sinuses, have been described in adult patients with PTPN11 gene mutations. Histological analysis of the surgically removed giant aneurysms has shown deficiency of the medial elastin and nonspecific myxoid degeneration, suggesting a connective tissue disorder-like change in the wall of aorta (Morgan, Coupe, Honey, & Miller, 1989; Power, Lewin, Hannibal, & Glass, 2006; Purnell, Williams, Von Oppell, & Wood, 2005; Shachter, Perloff, & Mulder, 1984). Aortic dilation may be unnoticed before the clinical manifestation of aortic dissection, thus recommending a strict long-term follow-up, notably interval aortic measurements (Power et al., 2006).
We found dilation of the left atrial appendage in three of our patients. Only one adult individual with features of CFCS and NS has been described, presenting with congenital aneurysm of the left atrial appendage (DeSena et al., 2010). Although RASopathy patients are predisposed to hematological disorders, such as abnormal bleeding or bruising caused by coagulation defects, they may also develop thrombi in the enlarged left atrial appendage or atrial fibrillation over the years. Hence, a careful evaluation of the coagulation system is mandatory whenever antiaggregant or anticoagulant therapy is demanded in these patients. In young patients with RASopathies and occurrence of cryptogenic stroke, recognition of an enlarged left atrial appendage may prompt to primary oral anticoagulation therapy.
Although only a few of our patients required surgical treatment, their lesions were benign in terms of morbidity. Furthermore, it is important to highlight these data to confirm the major role of echocardiography both for screening at the time of first diagnosis and during the clinical follow up of individuals with RASopathies, even in absence of CHDs or CMPs. No patients in our cohort died, arguing that despite the common cardiac involvement in these syndromes, their lesions are usually benign in terms of mortality. Nevertheless, accurate follow up based on clinical evaluation, ECG and echocardiogram is highly recommended, as previously suggested (Roberts, Allanson, Tartaglia, & Gelb, 2013; Romano et al., 2010; Wilkinson et al., 2012).
In agreement with published results, PTPN11 was the most commonly mutated gene also in ACDs individuals, followed by SOS1 and BRAF. These results overlap personal and published experience of mutated gene prevalence in patients with typical heart defects. Among the genes more recently associated with RASopathies (Aoki et al., 2013), we found one patient with RIT1 mutation. In agreement with personal and published results, this could suggest a higher penetrance for cardiac disease in these individuals (Calcagni, Baban, Lepri, et al., 2016; Kouz et al., 2016).
5 STUDY LIMITATIONS
The retrospective design of the study represents per se a limitation. Moreover, RASopathy patients in whom no gene mutations were detected, may have biased our results. Finally, another limitation concerns the absence of specific statistical analysis in terms of Kaplan–Mayer for mortality or morbidity. Since no patients died for cardiac nor extracardiac disorders, and because of limited number of patients undergoing reintervention, we were unable to extrapolate these results.
6 CONCLUSIONS
Patients with RASopathies need a lifetime cardiac follow-up, not only for monitoring the most common CHDs and HCM, but also for ACDs. No data regarding dilation CA or left atrial appendage prognosis have prospectively reported in this population. Therefore, these apparently minor cardiac conditions may require cardiac surgery or percutaneous procedures in patients with worsening of valvulopathies. Medical management aimed to prevent complications due to coronary artery dilation and enlarged left atrial appendage might be needed. With a deeper understanding of cardiac characteristics and genotype–phenotype correlations of patients with RASopathies, it will be possible to offer to families appropriate counseling in terms of prognosis and tailored management for optimal patient outcome.
CONFLICT OF INTEREST
The authors report no relationships that could be construed as a conflict of interest.
AUTHOR CONTRIBUTIONS
All the authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
ACKNOWLEDGMENTS
The authors wish to thank the subjects whose participation made this study possible. Furthermore, we wish to thank Italian Ministry of Health (Ricerca Corrente 2019-2020) and EJP-RD (E-Rare).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




