Integrating Direct Experimental Fishing and Environmental DNA Metabarcoding to Assess Fish Biodiversity in the Cibareno River, Indonesia, to Support Fishway Design
Funding: The project funding of the study was supported by the Food and Agriculture Organization and Global Environment Facility project ‘Inland Fisheries Practices in Freshwater Ecosystems of High Conservation Value (IFish)’ (FAO project code: GCP/INS/303/GFF, GEF ID: 5759) and the Australian Centre for International Agricultural Research (Project No. FIS/2018/153).
ABSTRACT
- A fishway is an engineered structure designed to help fish navigate past artificial barriers in rivers such as dams, weirs and regulators. An effective fishway design requires an understanding of fish biodiversity and migration requirements of the local community.
- To support the conceptual design of a fishway at Caringin Weir in the Cibareno River in Indonesia, we conducted a fish biodiversity assessment using experimental fishing and an eDNA metabarcoding survey.
- In early 2022, experimental fishing was conducted at six sampling sites upstream and downstream of the planned weir, and the sites were surveyed seven times during the wet and dry seasons. Environmental DNA sampling was conducted once during the wet season at each of the six sites.
- A total of 72 aquatic species were detected, comprising 40 fish species and seven crustacean species captured during experimental fishing and 36 fish species identified through eDNA metabarcoding. Twelve fish species (18.46%) were detected using both methods. The total number of all species caught and species richness in experimental fishing varied significantly between upstream and downstream areas (p < 0.05), but there were no significant differences between seasons (p > 0.05). There was also a significant difference in the species richness upstream and downstream using eDNA metabarcoding (p < 0.05). There were also numerous diadromous species, further highlighting the need for an effective fishway design to enable these species to complete their migrations.
- Based on the species present, migratory behaviour and economic importance, we prioritised six fish and crustacean species to be considered during fishway design: Anguilla marmorata, Tor tambra, Barbodes binotatus, Sicyopterus sp., Rhyacichthys aspro and Macrobrachium sp.
1 Introduction
Inland waters are among the most vulnerable ecosystems directly impacted by human activities and climate change (Gozlan et al. 2019; Grafton et al. 2012). Globally, biodiversity in freshwater habitats has declined by at least 80% from 1970 to 2014 (Reid et al. 2019). Freshwater fish biodiversity has also become an important issue in Indonesia, where most aquatic freshwater resources are overexploited, and water pollution, ecosystem degradation, introduction of exotic species and habitat fragmentation have been linked to an increase in the number of vulnerable species (Gustiano, Kurniawan, and Haryono 2021; Hubert et al. 2015; Kurniawan et al. 2021). Habitat fragmentation in freshwater landscapes is predominantly caused by the installation of dams, weirs or regulators.
There are a total of 2363 dams, weirs and regulators across Indonesian rivers, with most of these structures located on the islands of Java and Sumatra (DGWR 2023). These structures reduce river connectivity, interrupt fish migrations and threaten fish biodiversity and the associated inland fisheries sector (Baumgartner and Wibowo 2018; Harris et al. 2017). In Indonesia, fish exhibit an enormous diversity in size, shape, biology and reproductive success in their natural habitat. Many species migrate to complete various stages of their life cycle. This includes adults undertaking spawning migrations; juveniles seeking nursery habitat; and all life stages seeking shelter during changing seasons (refugeeing), food foraging and finding a suitable environment for survival (Brönmark et al. 2014; Oliveira et al. 2020; Pollux et al. 2006; Worthington et al. 2022). To enable these migrations to continue in regulated river systems, fishways are often constructed to reinstate fish passage (Baumgartner et al. 2021; Katopodis, Kells, and Acharya 2001; Schletterer, Reind, and Thonhauser 2016).
The Cibareno River in West Java, Indonesia, forms a natural border between Banten and West Java Provinces. The upper reaches are located in the Halimun National Park area of West Java and the river flows approximately 27 km to the Pelabuhan Ratu Bay. The Cibareno River weir was constructed from early 2022 to 2023 to enable farmers to irrigate approximately 2000 ha of land, predominantly made up of rice fields. We conducted the fish survey during the construction of the irrigation weir, at a stage when the weir's foundation had been built to a height of approximately 1 m, out of the planned 3.25 m, extending from the left to the right of the riverbed. This weir construction creates challenges for fish migration, rendering passage feasible primarily during only high tide in the river. Before the river is totally fragmented by the weir, it is of importance to assess the fish biodiversity and composition both upstream and downstream of the Cibareno River. This preweir evaluation is a crucial foundation for subsequent monitoring efforts. In addition, the fragmentation of fish populations may be occurring due to the changes in land use patterns surrounding the Cibareno River. Notable among these alterations are the transformation of forests into rice fields and plantations, the expansion of residential areas and the unregulated gold mining. These activities are inextricably linked to escalating concerns such as the influx of domestic waste, the contamination by pesticides and the discharge of pollutants from gold extraction processes into the river. There is no recent information available to evaluate the river's fish biodiversity. In 2002, there were ~22 species from 10 families recorded caught by an electrofishing method (Rachmatika, Sjafei, and Nurcahyadi 2002). But it is likely that fish biodiversity is much greater given that this river has never been intensively surveyed and the Island of Java is known to have very high fish species diversity with at least 408 species recorded (Wijaya et al. 2014). Furthermore, the Cibareno River flows into the sea, and thus, diadromous species with a requirement to move between freshwater and marine environments are likely to be common. Consequently, due to a highly variable local hydrology, a decision was made to install a vertical-slot fishway on the Caringin Weir to enable fish to complete their migrations.
The design of the fishway is integral to its performance given an inappropriate design will affect fish passage success (Atminarso et al. 2023; Harris et al. 2017; Sanchez-Perez et al. 2022). There are thousands of barriers to fish passage in Indonesia, and only four fishways have been installed to date (Baumgartner and Wibowo 2018). None of these four fishways were designed to meet the migratory needs of the local community. Furthermore, fishway effectiveness is poorly understood with only a single study examining fishway effectiveness at the Perjaya Dam on the Komering River (Atminarso et al. 2023). This study found that the fish community was significantly different upstream and downstream of the Perjaya Dam, indicating that the fishway was not functioning effectively (Atminarso et al. 2023). Therefore, prior to the construction of the Caringin Weir fishway, there was a need to understand whether the fish community was already fragmented by the weir and to determine if the species were dominated by diadromous species given this would influence the fishway design (Rourke et al. 2018; Verhelst et al. 2021). Furthermore, it is also crucial that the fishway can cater to the migratory requirements of the species with high economic importance to enable the local community to derive a living from fishing. Therefore, the fishway needed to be highly functional and ensure the sustainability of the Cibareno River's fish resources.
Fish biodiversity is typically measured through traditional fish sampling methods that use both active and passive fishing gears. Given fish presence and abundance will vary according to the location, time of day and season, it is important to conduct targeted fish surveys at a range of spatial and temporal scales (Baumgartner, Stuart, and Zampatti 2008; DSWEPC 2011). Traditional fish sampling methods are now often complemented by environmental DNA (eDNA) methods to assess fish biodiversity in a range of aquatic environments (Compson et al. 2020; Goutte et al. 2020). eDNA approaches are useful for describing biodiversity because they are non-invasive (Bergman et al. 2016; Dejean et al. 2012; Valentini et al. 2016), cost-effective (Anderson et al. 2018; Biggs et al. 2015; Evans et al. 2017; Miya et al. 2015; Takahara, Minamoto, and Doi 2013) and often more sensitive than conventional methods (Herve et al. 2022; Janosik and Johnston 2015; Sigsgaard et al. 2015). Specifically, eDNA has been shown to be an effective tool for monitoring and conservation (Rourke et al. 2021; Sahu et al. 2023; Zou et al. 2020). There have been few studies using eDNA metabarcoding to detect fish diversity in Indonesian freshwater habitats, but the studies to date indicate great promise for detecting fish species richness in this biodiverse environment (Roesma et al. 2021; Wibowo et al. 2022).
In this study, we combine conventional fishing surveys and an eDNA metabarcoding survey carried out during the construction of the Caringin Weir to (1) update the checklist of the freshwater organisms of the Cibareno River, with a particular emphasis on fish and migratory crustaceans, (2) determine whether the fish and crustacean communities of the Cibareno River have been fragmented by the changes induced by land use and by the constructed weir, (3) determine whether diadromous species have been disproportionately impacted by the weir and (4) make recommendations on the conceptual design of a future fishway in the Cibareno River.
2 Methods
2.1 Study Area
The Cibareno River is one of the eight rivers flowing into the Pelabuhan Ratu Bay. The Cibareno River supports a diverse range of diadromous (catadromous and amphidromous) fish species and heavily harvested species by inland fisheries (Hakim et al. 2015). There is a single weir (Caringin Weir, S 6.8783°, E 106.4200°) that may interfere with fish migration in the Cibareno River. The weir was under construction in early 2022 including during the 7 months of the surveys during which the weir's foundation was elevated by 1 m (the final height will be 3.25 m high) above the riverbed, extending from one bank to the other. To mitigate the impacts of the weir on fish migration, a fishway will be installed to enable fish to move freely between upstream and downstream locations and to ensure river connectivity, particularly for species migrating downstream as larvae (i.e., amphidromy) or adults (i.e., catadromy). Six sampling locations were selected upstream (n = 3) and downstream (n = 3) of the Caringin Weir. Upstream and downstream sampling sites were located at 0, 5 and 10 km from the weir (Figure 1). At the upstream sites, Upstream 1 is surrounded by rice fields, plantations and unregulated gold mining areas on both the left and right sides of the river. Similarly, Upstream 2 includes forest, rice fields, plantations, small-scale gold mining areas, fish ponds and a wood-cutting factory that discharges sawdust into the river. Upstream 3 and Downstream 1 are neighbouring areas but are separated by the Caringin Weir. Upstream 3 is an upstream area of the weir, dominated by rice fields and plantations, whereas Downstream 1, downstream of the weir, is a relatively well-preserved plantation and forest area. Downstream 2 is primarily characterised by rice fields, plantations and residential areas. Downstream 3 is an area adjacent to the estuary (1 km), dominated by rice fields, plantations and settlements and featuring a small-scale sand mine. The coordinates, altitudes, slopes, widths and discharges of the sampling locations along the Cibareno River are detailed in Table 1.
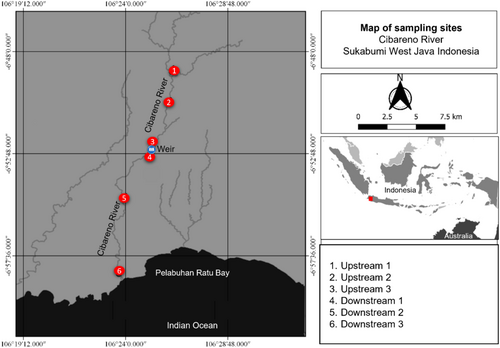
| Coordinate | Sampling site | Altitude (m) | Slope (%) | Width (m) | Discharge (m3/s) |
|---|---|---|---|---|---|
| S 6.8157°, E 106.4361° | Upstream 1 | 499 | 3.07 | 15 | 8.001 |
| S 6.8409°, E 106.4338° | Upstream 2 | 435 | 2.73 | 24 | 10.129 |
| S 06.8808°, E 106.4185° | Upstream 3 | 310 | 3.60 | 22 | 13.247 |
| S 06.8768°, E 106.4200° | Downstream 1 | 278 | 3.49 | 22 | 18.911 |
| S 06.9161°, E 106.3985° | Downstream 2 | 137 | 2.08 | 32 | 17.221 |
| S 06.9743°, E 106.3946° | Downstream 3 | 25 | 0.91 | 36 | 15.469 |
2.2 Experimental Fishing
Over a 7-month period, fish were collected during daytime in the dry season (February–May 2022) and the rainy season (June–September 2022) using a range of fishing gears including cast nets, gillnets, fyke nets, bait traps and bamboo traps.
Experimental fishing with cast nets was carried out in three operational trials, each of which was repeated 30 times of effort (90 times in total). Two sets of gill nets with six different mesh sizes (1.9, 2.5, 3.8, 5.0, 7.6 and 10.2 cm; each mesh panel was 400 cm long by 100 cm high) were set up on opposite sides of the river. Two sets of fyke nets were installed on opposite sides of the river, with one set opposite to the current and the other set in the same direction as the current. Eight sets of Jarvis traps (40 × 20 cm) and bamboo traps (80 × 25 cm) were set at the vegetated areas of the river. All fishing gears including gillnets, fyke nets, Jarvis and bamboo traps were checked after 4 h, conducted once per day. Experimental fishing was conducted on the same day every month from February 2022 to September 2022.
Fish were anaesthetised using 2-phenoxyethanol (0.5 mL/L of water) before being, identified, photographed, measured (fork length or total length) and weighed. When systematic knowledge was insufficient for reliable identification of the species, all adult fish caught were identified to genus level following (Kottelat et al. 1993; Rachmatika, Sjafei, and Nurcahyadi 2002).
2.3 eDNA Collection and Laboratory Methods
eDNA samples were collected from the six sampling locations only in the first survey (February 2022). Triplicate 1-L water samples were collected at the sampling site and stored in an insulated container prior to filtering. Water samples were filtered within 4–6 h of collection using a mixed cellulose ester membrane filter (pore size: 0.45 μm; Whatman) in a sterile filter housing connected to a vacuum pump. Each of the three water samples collected at each site was filtered through the same filter, resulting in six filters per site. The filter paper was cut in half with sterile scissors and tweezers to create two technical replicates. Each filter paper half was placed in a 2-mL cryotube (Cryo Tubes, presterilised) containing 2 mL of DNA shield liquid (ZymoBIOMICS DNA Research) and stored in a plastic cryobox and stored at −20°C (Biologix, 100 wells) (Gelis et al. 2021; Zamani, Zuhdi, and Madduppa 2022).
The metabarcoding work was conducted at the Genetika Science Laboratory (Banten, Indonesia). DNA concentration and quality were determined using NanoDrop spectrophotometers and a Qubit fluorometer. Genomic DNA from each filter paper was extracted using a ZymoBIOMICS DNA Miniprep Kit (Zymo Research, D4300). The MiFish primer pairs (MiFish-U-F, 5′-GCCGGTAAAACTCGTGCCAGC-3′; MiFish-U-R, 5′-CATAGTAGGGTATCTAATCCTA-GTTTG-3′) were used to target a ~172-bp amplicon from a variable region of the 12S rRNA mitochondrial gene (Miya et al. 2015; Taberlet et al. 2018). Following extraction, there were a total of six samples and one PCR blank. The PCRs contained a total volume of 24 μL composed of 12.5 μL of MyTaq Red Mix (Bioline), 1 μL of the 5-μM premixed forward and reverse primers (Macrogen), 3 μL of DNA (10–15 ng/μL) and 7.5 μL of sterile water. Thermal cycling conditions included an initial denaturing step of 95°C for 10 min, 40 cycles of 95°C for 30 s, 60°C for 45 s and 72°C for 30 s and a final extension step of 72°C for 5 min. PCR products were visually examined on a 1.2% agarose gel, stained with GelRed Nucleic Acid Gel Stain using a 100-bp ladder (Invitrogen) to verify that the PCR products were of the expected size.
MinION sequencing libraries were prepared following the native barcoding genomic DNA protocol and consisted of end-prep, barcoding ligation and adapter ligation (Toxqui Rodríguez, Vanhollebeke, and Derycke 2023). Modifications to the protocol included the use of a Bioanalyzer (Agilent) to check the size and to quantify the library at the end of each step (end-prep, barcoding ligation and sequencer adapters) and DNA quantification using the Qubit fluorometer. The libraries were sequenced using a flow cell (which was quality-checked in order to ensure the presence of at least 800 active biological nanopores for each run) and using MinKNOW v20.10.3 to run MinKNOW core v4.1.2 and Guppy 4.0.11 in high-accuracy mode (Wick, Judd, and Holt 2019). The quality of fastq files was visualised using NanoPlot (De Coster et al. 2018). Filtered reads were classified using a Centrifuge classifier (Kim et al. 2016). The consensus of reads was extracted using Medaka v1.5.0 (https://github.com/nanoporetech/medaka). The extracted consensus sequence was aligned against the NCBI database using BLAST (https://blast.ncbi.nlm.nih.gov). The DNA barcoding threshold used to confirm species assignment was defined as a sequence similarity of greater than 97%.
2.4 Fish Migration and Fish Size
To determine which fish migrate from upstream to downstream and vice versa, as well as to assess the size of fish that could pass through Caringin Weir, we categorised fish based on whether they were found exclusively upstream, downstream or in both locations, with the weir serving as the reference point. The specific type of fish migration, namely, catadromous (adults migrate downstream to the sea to spawn and juveniles return upstream), amphidromous (adults live in freshwater, larvae drift downstream to the estuary or sea and juveniles return upstream) or potamodromous (i.e., adults perform migrations in freshwater for reproduction), was determined through a review of relevant literature. Additionally, we employed heat map analysis to visualise the distribution of fish migration at each station, spanning from upstream to downstream. For further analysis, the captured fish were grouped according to their minimum and maximum lengths, serving as a valuable reference for sizing and designing fishways (O'Connor et al. 2022). These findings were then compared to existing literature for a comprehensive assessment.
2.5 Data Analysis
Taxonomic identification data of fish were arranged by order, family, genus and species. The distribution and relative abundance of fish species for each sampling location were visualised on maps using bar charts. A Venn diagram was constructed to illustrate the number of fish species detected upstream and downstream of the weir using experimental fishing and metabarcoding results using an online program (http://bioinformatics.psb.ugent.be/webtools/venn/). Species richness, Shannon diversity and Simpson dominance index (D) analysis were analysed using the Biodiversity Pro 2 package (McAleece et al. 1997). Subsequently, statistical analysis (PERMANOVA) was implemented to evaluate the significance of differences in the distribution of total fish caught, species richness, diversity and dominance indices at each sampling location using the PRIMER v7 package (Clarke and Gorley 2015) for both direct fishing and eDNA survey. This application was also used to generate a heat map to illustrate fish species that were found upstream and downstream of the weir for direct fishing.
3 Results
3.1 Experimental Fishing
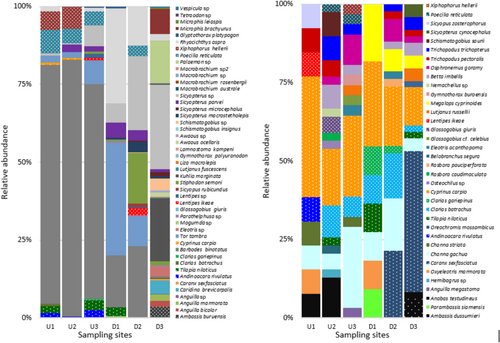
There were 47 aquatic species representing 13 orders caught during experimental fishing. This included 40 fish species from 20 families and seven crustaceans from three families (Table 2). Gobiiformes was the most represented order with 16 species from four families followed by Decapoda with seven species from three families and Anguilliformes with five species from three families, and all the remaining orders had less than four species. Seven fish families were found both upstream and downstream of the weir, 15 families were found only downstream of the weir, and one family was found only upstream of the weir. Cyprinidae was the most common family of fish upstream of the weir (80.27%), followed by Poeciliidae (9.11%), Oxudercidae (4.72%), Cichlidae (3.37%) and the remaining four families (2.53%). The number of fish-based families was greater downstream (22 families) than upstream (eight families) (Table 2). Similarly, downstream sites had 35 more species than upstream sites, which had seven species (Figure 2).
| Order | Family | Genus | Species | Upstream | Downstream | Seasons | ||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | |||||
| Anguilliformes | Anguillidae | 1 | 3 | 0 | 0 | 33 | 2.68 | Wet and dry |
| Muraenidae | 1 | 1 | 0 | 0 | 1 | 0.08 | Dry | |
| Ophichthidae | 1 | 1 | 0 | 0 | 1 | 0.08 | Wet | |
| Centrarchiformes | Kuhliidae | 1 | 1 | 0 | 0 | 155 | 12.57 | Wet and dry |
| Cichliformes | Cichlidae | 2 | 2 | 20 | 3.37 | 6 | 0.49 | Wet and dry |
| Cypriniformes | Cyprinidae | 3 | 3 | 476 | 80.27 | 196 | 15.90 | Wet and dry |
| Cyprinodontiformes | Poeciliidae | 2 | 2 | 54 | 9.11 | 11 | 0.89 | Wet and dry |
| Gobiiformes | Eleotridae | 2 | 2 | 0 | 0 | 24 | 1.95 | Wet and dry |
| Gobiidae | 4 | 5 | 7 | 1.18 | 70 | 5.68 | Wet and dry | |
| Oxudercidae | 3 | 8 | 28 | 4.72 | 386 | 31.31 | Wet and dry | |
| Rhyacichthyidae | 1 | 1 | 5 | 0.84 | 108 | 8.76 | Wet and dry | |
| Mugiliformes | Mugilidae | 1 | 1 | 0 | 0 | 1 | 0.08 | Wet |
| Perciformes | Ambassidae | 1 | 1 | 0 | 0 | 27 | 2.19 | Wet |
| Carangidae | 1 | 1 | 0 | 0 | 1 | 0.08 | Wet | |
| Lutjanidae | 1 | 1 | 0 | 0 | 1 | 0.08 | Wet | |
| Scorpaeniformes | Tetrarogidae | 1 | 1 | 0 | 0 | 3 | 0.24 | Wet and dry |
| Siluriformes | Clariidae | 1 | 2 | 2 | 0.34 | 0 | 0 | Wet |
| Sisoridae | 1 | 1 | 1 | 0.17 | 1 | 0.08 | Dry | |
| Syngnathiformes | Syngnathidae | 1 | 2 | 0 | 0 | 63 | 5.11 | Wet and dry |
| Tetraodontiformes | Tetraodontidae | 1 | 1 | 0 | 0 | 1 | 0.08 | Wet |
| Decapoda | Atyidae | 1 | 1 | 0 | 0 | 32 | 2.60 | Wet and dry |
| Gecarcinucidae | 1 | 1 | 0 | 0 | 4 | 0.32 | Wet | |
| Palaemonidae | 2 | 5 | 0 | 0 | 108 | 8.76 | Wet and dry | |
| Total | 34 | 47 | 593 | 100 | 1233 | 100 | ||
The number of fish species and species richness caught were significantly different between upstream and downstream sites (p < 0.05), but there was no difference between seasons (p > 0.05) (Tables 3 and 4). In the downstream area, 19 fish and crustacean species were not represented in the other five sampling sites, namely, Ambassis buruensis, Anguilla bicolor, Anguilla sp., Caridina brevicarpalis, Caranx sexfasciatus, Eleotris sp., Glossogobius giuris, Kuhlia marginata, Lutjanus fuscescens, Liza macrolepis, Awaous ocellaris, Schismatogobius insignus, Schismatogobius sp., Macrobrachium australe, Macrobrachium rosenbergii, Palaemon sp., Microphis brachyurus, Tetraodon sp. and Vespicula sp. Only nine of the 41 fish species were identified both upstream and downstream of the weir, including Barbodes binotatus, Glyptothorax platypogon, Poecilia reticulata, Rhyacichthys aspro, Sicyopterus parvei, Sicyopterus sp., Tilapia niloticus and Tor tambra (Figure 3). We also found six species, namely, Andinoacara rivulatus, Clarias batrachus, Clarias gariepinus, Cyprinus carpio, Lentipes ikeae and Xiphophorus hellerii represented only in upstream areas.
| Source | df | SS | MS | Pseudo-F | p (perm) | Perms |
|---|---|---|---|---|---|---|
| Location | 1 | 22,749 | 22,749 | 12.29 | 0.0001 | 9939 |
| Season | 1 | 1318.9 | 1318.9 | 0.71 | 0.6168 | 9938 |
| Location vs. season | 1 | 1695.6 | 1695.6 | 0.92 | 0.4546 | 9926 |
| Residual | 38 | 70,346 | 1851.2 | |||
| Total | 41 | 99,111 |
- Note: Significant value (α = 0.05); p values are indicated in bold font.
- Abbreviations: df, degree of freedom; MS, mean squares; Perms, number of permutations; SS, sum of squares.
| Source | df | SS | MS | Pseudo-F | p (perm) | Perms |
|---|---|---|---|---|---|---|
| Source | 1 | 3447.4 | 3447.4 | 8.4 | 0.0003 | 9924 |
| Location | 1 | 88.2 | 88.2 | 0.2 | 0.9273 | 9951 |
| Season | 1 | 118.5 | 118.5 | 0.3 | 0.8169 | 9943 |
| Location vs. season | 38 | 15,662 | 412.2 | |||
| Residual | 41 | 19,844 |
- Note: Significant value (α = 0.05); p values are indicated in bold font.
- Abbreviations: df, degree of freedom; MS, mean squares; Perms, number of permutations; SS, sum of squares.
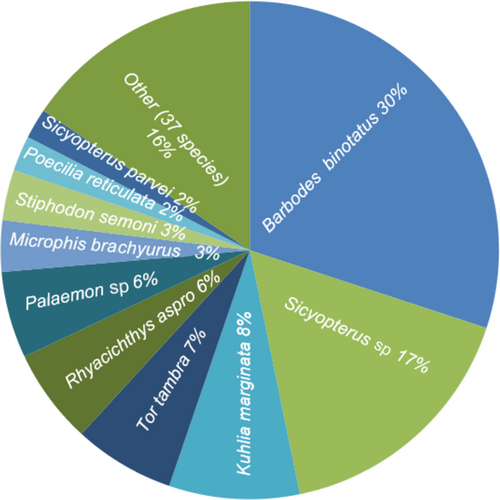
The relative abundance of fish per species varied across the six sampling sites, with B. binotatus, for example, having a relatively high abundance ranging from 0.52% at Downstream 3 to 82.46% at Upstream 2 (Figure 3a). Furthermore, when fish were ranked by abundance (Figure 2), two species dominated all sites: B. binotatus (30% of all specimens) and Sicyopterus sp. (17% of all specimens).
3.2 eDNA Survey
The one-off eDNA survey detected 36 species from nine orders and 20 families (Table 4). Gobiiformes was the most abundant order, with nine species from four families followed by Cypriniformes with five species from three families and Perciformes with four species from three families, with the remaining orders having less than four species. There were two new orders discovered: Anabantiformes (four species from three families) and Elopiformes (one species from one family). We detected nine families both upstream and downstream of the weir, whereas three families were detected only upstream, and five families were detected only downstream. The largest number of raw reads of the fish families in the upstream area was Cyprinidae (23.86%) followed by Osphronemidae (17.50%), Channidae (14.77%) and Anabantidae (6.82%), and the remaining was 13 families (37.50%). The number of fish families was greater upstream (17 families) than downstream (15 families) (Table 5).
| Taxonomic level collected by eDNA | DNA raw reads | ||||||
|---|---|---|---|---|---|---|---|
| Order | Family | Genus | Species | Upstream | Downstream | ||
| Number of reads | % | Number of reads | % | ||||
| Anabantiformes | Anabantidae | 1 | 1 | 6 | 6.90 | 1 | 0.94 |
| Channidae | 1 | 2 | 15 | 17.24 | 12 | 11.32 | |
| Osphronemidae | 3 | 4 | 15 | 17.24 | 12 | 11.32 | |
| Anguilliformes | Anguillidae | 1 | 1 | 1 | 1.15 | 0 | 0.00 |
| Muraenidae | 1 | 1 | 1 | 1.15 | 0 | 0.00 | |
| Cichliformes | Cichlidae | 2 | 3 | 3 | 3.45 | 2 | 1.89 |
| Cypriniformes | Cyprinidae | 2 | 2 | 21 | 24.14 | 17 | 16.04 |
| Danionidae | 1 | 2 | 1 | 1.15 | 1 | 0.94 | |
| Nemacheilidae | 1 | 1 | 1 | 1.15 | 0 | 0.00 | |
| Cyprinodontiformes | Poeciliidae | 2 | 2 | 5 | 5.75 | 2 | 1.89 |
| Elopiformes | Megalopidae | 1 | 1 | 0 | 0.00 | 7 | 6.60 |
| Gobiiformes | Butidae | 1 | 1 | 1 | 1.15 | 1 | 0.94 |
| Eleotridae | 2 | 2 | 1 | 1.15 | 2 | 1.89 | |
| Gobiidae | 2 | 3 | 4 | 4.60 | 0 | 0.00 | |
| Oxudercidae | 3 | 3 | 4 | 4.60 | 2 | 1.89 | |
| Perciformes | Ambassidae | 2 | 2 | 0 | 0.00 | 4 | 3.77 |
| Carangidae | 1 | 1 | 0 | 0.00 | 31 | 29.25 | |
| Lutjanidae | 1 | 1 | 1 | 1.15 | 2 | 1.89 | |
| Siluriformes | Bagridae | 1 | 1 | 1 | 1.15 | 0 | 0.00 |
| Clariidae | 1 | 2 | 6 | 6.90 | 10 | 9.43 | |
| Total | 30 | 36 | 87 | 100 | 106 | 100 | |
3.3 Integrated Findings
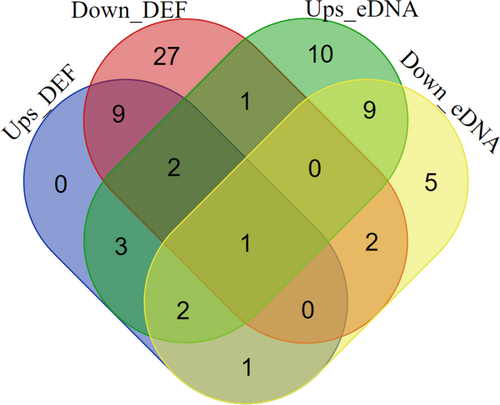
The result of the fish biodiversity assessment integrating experimental fishing and eDNA surveys is shown in Table 6 and Figure 3. In total, 72 fish and crustacean species were detected in the Cibareno River including 65 fish species from 26 families and seven crustacean species from three families. We identified 40 fish species and seven crustaceans using direct fishing and detected 36 fish species using eDNA. A total of 12 species (18.46%) detected in the eDNA survey were also found in direct fishing (12 species from the present study), whereas the remaining 19 species have not been previously recorded from the Cibareno River. In addition, the eDNA results showed that a further 12 fish species were found upstream and downstream of the weir, namely, Anabas testudineus, Oxyeleotris marmorata, Channa gachua, Oreochromis niloticus, C. batrachus, C. carpio, Eleotris acanthopoma, Lutjanus russellii, Betta imbellis, Osphronemus goramy, Trichopodus pectoralis and Trichopodus trichopterus (Table 5). The number of fish species overlapping from the experimental fishing and eDNA metabarcoding survey upstream and downstream of Caringin Weir can be found in Figure 4.
| No. | Family | Species | Identification method | Fish collection | Remark |
|---|---|---|---|---|---|
| A | Fish | ||||
| 1 | Ambassidae | Ambassis buruensis | Morphological identification | Present study | Native |
| 2 | Ambassis dussumieri | eDNA/blast | Present study | Native | |
| 3 | Parambassis siamensis | eDNA/blast | Present study | Native | |
| 4 | Anabantidae | Anabas testudineus | eDNA/blast | Present study | Native |
| 5 | Anguillidae | Anguilla bicolor | Morphological identification | Present study | Native |
| 6 | Anguilla marmorata | Morphological identification | Present study | Native | |
| 7 | Anguilla sp. | Morphological identification | Present study | Native | |
| 8 | Anguilla megastoma | eDNA/blast | Present study | Native | |
| 9 | Bagridae | Hemibagrus sp. | eDNA/blast | Present study | Native |
| 10 | Butidae | Oxyeleotris marmorata | eDNA/blast | Present study | Native |
| 11 | Carangidae | Caranx sexfasciatus | Morphological identification and eDNA/blast | Present study | Native |
| 12 | Channidae | Channa gachua | Morphological identification and eDNA/blast | Previous study (Rachmatika, Sjafei, and Nurcahyadi 2002) and eDNA/blast | Native |
| 13 | Channa striata | eDNA/blast | Present study | Native | |
| 14 | Cichlidae | Andinoacara rivulatus | Morphological identification and eDNA/blast | Present study | Exotic |
| 15 | Oreochromis mossambicus | Morphological identification and eDNA/blast | Previous study (Rachmatika, Sjafei, and Nurcahyadi 2002) and eDNA/blast | Exotic | |
| 16 | Tilapia niloticus | Morphological identification and eDNA/blast | Present study | Exotic | |
| 17 | Clariidae | Clarias batrachus | Morphological identification and eDNA/blast | Present study | Native |
| 18 | Clarias gariepinus | Morphological identification and eDNA/blast | Present study | Exotic | |
| 19 | Cyprinidae | Cyprinus carpio | Morphological identification and eDNA/blast | Present study | Exotic |
| 20 | Tor tambra | Morphological identification | Present study | Native | |
| 21 | Osteochilus sp. | eDNA/blast | Present study | Native | |
| 22 | Danionidae | Rasbora caudimaculata | eDNA/blast | Present study | Native |
| 23 | Rasbora pauciperforata | eDNA/blast | Present study | Native | |
| 24 | Eleotridae | Eleotris sp. | Morphological identification | Present study | Native |
| 25 | Eleotris acanthopoma | eDNA/blast | Present study | Native | |
| 26 | Mogurnda sp. | Morphological identification | Present study | Native | |
| 27 | Belobranchus segura | eDNA/blast | Present study | Native | |
| 28 | Gobiidae | Glossogobius giuris | Morphological identification and eDNA/blast | Present study | Native |
| 29 | Lentipes ikeae | Morphological identification and eDNA/blast | Present study | Native | |
| 30 | Lentipes sp. | Morphological identification | Present study | Native | |
| 31 | Sicyopus rubicundus | Morphological identification | Present study | Native | |
| 32 | Sicyopus zosterophorus | Morphological identification and eDNA/blast | Present study | Native | |
| 33 | Stiphodon semoni | Morphological identification | Present study | Native | |
| 34 | Glossogobius cf. celebius | eDNA/blast | Present study | Native | |
| 35 | Kuhliidae | Kuhlia marginata | Morphological identification | Present study | Native |
| 36 | Lutjanidae | Lutjanus fuscescens | Morphological identification | Present study | Native |
| 37 | Lutjanus russellii | eDNA/blast | Present study | Native | |
| 38 | Megalopidae | Megalops cyprinoides | Morphological identification and eDNA/blast | Previous study (Rachmatika, Sjafei, and Nurcahyadi 2002) and eDNA/blast | Native |
| 39 | Mugilidae | Liza macrolepis | Morphological identification | Present study | Native |
| 40 | Muraenidae | Gymnothorax polyuranodon | Morphological identification | Present study | Native |
| 41 | Gymnothorax buroensis | eDNA/blast | Present study | ||
| 42 | Oxudercidae | Awaous ocellaris | Morphological identification | Present study | Native |
| 43 | Awaous sp. | Morphological identification | Present study | Native | |
| 44 | Schismatogobius insignus | Morphological identification | Present study | Native | |
| 45 | Schismatogobius saurii | eDNA/blast | Present study | Native | |
| 46 | Schismatogobius sp. | Morphological identification | Present study | Native | |
| 47 | Sicyopterus microcephalus | Morphological identification | Present study | Native | |
| 48 | Sicyopterus macrostetholepis | Morphological identification | Present study | Native | |
| 49 | Sicyopterus parvei | Morphological identification | Present study | Native | |
| 50 | Sicyopterus cynocephalus | Morphological identification and eDNA/blast | Present study, previous study (Rachmatika, Sjafei, and Nurcahyadi 2002) and eDNA/blast | Native | |
| 51 | Sicyopterus sp. | Morphological identification | Present study | Native | |
| 52 | Nemacheilidae | Nemacheilus sp. | eDNA/blast | Present study | Native |
| 53 | Osphronemidae | Betta imbellis | eDNA/blast | Present study | Native |
| 54 | Osphronemus goramy | eDNA/blast | Present study | Native | |
| 55 | Trichopodus pectoralis | eDNA/blast | Present study | Native | |
| 56 | Trichopodus trichopterus | Morphological identification and eDNA/blast | Previous study (Rachmatika, Sjafei, and Nurcahyadi 2002) and eDNA/blast | Native | |
| 57 | Ophichthidae | Lamnostoma kampeni | Morphological identification | Present study | Native |
| 58 | Poeciliidae | Poecilia reticulate | Morphological identification and eDNA/blast | Present study | Exotic |
| 59 | Xiphophorus hellerii | Morphological identification and eDNA/blast | Present study | Exotic | |
| 60 | Rhyacichthyidae | Rhyacichthys aspro | Morphological identification | Present study | Native |
| 61 | Sisoridae | Glyptothorax platypogon | Morphological identification | Present study | Native |
| 62 | Syngnathidae | Microphis brachyurus | Morphological identification | Present study | Native |
| 63 | Microphis leiaspis | Morphological identification | Present study | Native | |
| 64 | Tetraodontidae | Tetraodon sp. | Morphological identification | Present study | Native |
| 65 | Tetrarogidae | Vespicula sp. | Morphological identification | Present study | Native |
| B | Crustacean | ||||
| 66 | Palaemonidae | Macrobrachium australe | Morphological identification | Present study | Native |
| 67 | Macrobrachium rosenbergii | Morphological identification | Present study | Native | |
| 68 | Macrobrachium sp. 1 | Morphological identification | Present study | Native | |
| 69 | Macrobrachium sp. 2 | Morphological identification | Present study | Native | |
| 70 | Palaemon sp. | Morphological identification | Present study | Native | |
| 71 | Atyidae | Caridina brevicarpalis | Morphological identification | Present study | Native |
| 72 | Gecarcinucidae | Parathelphusa sp. | Morphological identification | Present study | Native |
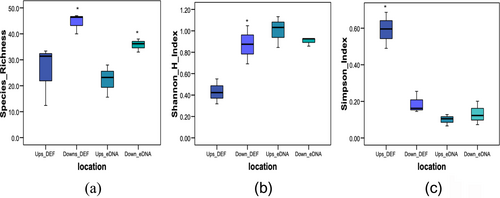
Estimates of species richness differed significantly between upstream and downstream sites of the river in both direct fishing and eDNA surveys (p < 0.05) (Figure 5a). The Shannon–Wiener index (H′) and Simpson dominance index were significantly different upstream and downstream in experimental fishing (p < 0.05), whereas there were no significant differences in the Shannon–Wiener index (H′) and Simpson dominance index in the eDNA survey. The diversity index H′ of direct fishing ranged from 0.32 to 1.05 categorised as low to moderate level; similarly, the eDNA survey ranged from 0.85 to 1.13, indicating low to moderate criteria. The dominance index (D) of direct fishing ranged from 0.15 to 0.69 categorised dominance, whereas the eDNA survey ranged from 0.07 to 0.13. The diversity index (H′) index in both direct fishing and the eDNA survey is negatively correlated to the dominance index (D) (r = −0.94, p < 0.05) (Figure 5b,c). The downstream area has significantly more diverse fish communities than the upstream location in both direct fishing and eDNA survey.
3.4 Fish Migration and Size
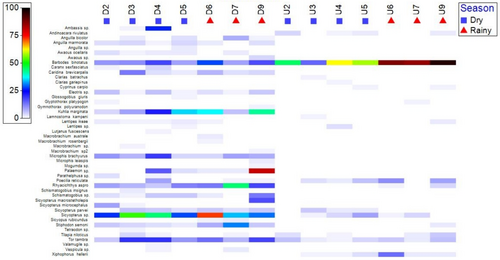
The fish species present at the Cibareno River migrate upstream and downstream at a variety of life stages, including juveniles and adults. We collected catadromous and amphidromous species, which were commonly associated with freshwater–marine migration patterns. Three species categorised as catadromous species were identified, namely, A. bicolor, Anguilla marmorata, and Anguilla sp. (Table 6). The freshwater eel A. megastoma was also detected in the eDNA survey. Based on the experimental fishing, adult fish at the Cibareno River could vary in size from 10 to >600 mm long. We also found that many fish species in the Cibareno River were gobies (Gobiidae = 7 species and Oxudercidae = 11 species), which are likely amphidromous. Gobies migrating upstream and downstream had a minimum size of 22 mm and a maximum size of 148 mm (Table 7). Besides the catadromous and amphidromous species, there were migratory freshwater species that are also presented in Figure 6, where seven fish can be found upstream and downstream, namely, T. tambra, B. binotatus, A. rivulatus, T. niloticus, P. reticulata, R. aspro and G. platypogon. These species may migrate from upstream to downstream for spawning, feeding and refuge. The largest size in this group is observed for T. tambra (256 mm). The maximum size of T. tambra can be even larger than observed here as we observed that T. tambra cultivated at the research station for freshwater aquaculture in Bogor can reach 500–700 mm long (5 kg). Although most fishes were collected in the downstream area, which is closed to the estuary system, many small-bodied fish are likely to migrate up into the freshwater environments; therefore, juvenile and small fish species (10–100 mm long) also migrate upstream and downstream and need to use the fishway at various times. These small fish form the basis of the food chain for larger fish species and also make up an important component of the subsistence livelihood–based species essential for food and nutritional security for local communities. Therefore, all sizes of fish that we collected (i.e., 10–600 mm long) and crustaceans will need to be considered in the fishway design.
| No. | Family | Scientific name | Upstream of the weir | Downstream of the weir | Fish number | Min. length (mm) | Max. length (mm) |
|---|---|---|---|---|---|---|---|
| A | Fish | ||||||
| 1 | Ambassidae | Ambassis buruensis | − | + | 27 | 25 | 81 |
| 2 | Anguillidae | Anguilla bicolor | − | + | 14 | 227 | 600 |
| 3 | Anguilla marmorata | − | + | 11 | 224 | 451 | |
| 4 | Anguilla sp. | − | + | 2 | 235 | 286 | |
| 5 | Carangidae | Caranx sexfasciatus | − | + | 5 | 60 | 102 |
| 6 | Cichlidae | Andinoacara rivulatus | + | + | 9 | 55 | 148 |
| 7 | Tilapia niloticus | + | + | 17 | 55 | 136 | |
| 8 | Clariidae | Clarias batrachus | + | − | 1 | 190 | 190 |
| 9 | Clarias gariepinus | + | − | 1 | 271 | 271 | |
| 10 | Cyprinidae | Barbodes binotatus | + | + | 550 | 20 | 120 |
| 11 | Cyprinus carpio | + | − | 2 | 145 | 300 | |
| 12 | Tor tambra | + | + | 120 | 47 | 285 | |
| 13 | Eleotridae | Eleotris sp. | − | + | 22 | 23 | 111 |
| 14 | Mogurnda sp. | − | + | 1 | 85 | 85 | |
| 15 | Gobiidae | Glossogobius giuris | − | + | 1 | 101 | 101 |
| 16 | Lentipes ikeae | + | + | 9 | 35 | 47 | |
| 17 | Lentipes sp. | + | + | 4 | 43 | 47 | |
| 18 | Sicyopus rubicundus | − | + | 3 | 43 | 48 | |
| 19 | Sicyopus zosterophorus | − | + | 1 | 0 | 0 | |
| 20 | Stiphodon semoni | − | + | 60 | 22 | 51 | |
| 21 | Kuhliidae | Kuhlia marginata | − | + | 155 | 10 | 146 |
| 22 | Lutjanidae | Lutjanus fuscescens | − | + | 1 | 93 | 93 |
| 23 | Mugilidae | Valamugil sp. | − | + | 1 | 106 | 106 |
| 24 | Muraenidae | Gymnothorax polyuranodon | − | + | 1 | 480 | 480 |
| 25 | Oxudercidae | Awaous ocellaris | − | + | 10 | 70 | 92 |
| 26 | Awaous sp. | − | + | 4 | 75 | 95 | |
| 27 | Schismatogobius insignus | − | + | 2 | 63 | 90 | |
| 28 | Schismatogobius sp. | − | + | 16 | 74 | 130 | |
| 29 | Sicyopterus microcephalus | − | + | 8 | 106 | 106 | |
| 30 | Sicyopterus parvei | + | + | 36 | 60 | 138 | |
| 31 | Sicyopterus sp. | + | + | 306 | 24 | 147 | |
| 32 | Sicyopterus macrostetholepis | − | + | 17 | 40 | 115 | |
| 33 | Ophichthidae | Lamnostoma kampeni | − | + | 1 | 30 | 30 |
| 34 | Poeciliidae | Poecilia reticulata | + | + | 42 | 15 | 47 |
| 35 | Xiphophorus hellerii | + | − | 23 | 25 | 68 | |
| 36 | Rhyacichthyidae | Rhyacichthys aspro | + | + | 113 | 40 | 145 |
| 37 | Sisoridae | Glyptothorax platypogon | + | + | 2 | 54 | 76 |
| 38 | Syngnathidae | Microphis brachyurus | − | + | 61 | 55 | 155 |
| 39 | Microphis leiaspis | − | + | 2 | 151 | 151 | |
| 40 | Tetraodontidae | Tetraodon sp. | − | + | 1 | 85 | 85 |
| 41 | Tetrarogidae | Vespicula sp. | − | + | 3 | 48 | 105 |
| B | Crustaceans | ||||||
| 1 | Atyidae | Caridina brevicarpalis | − | + | 28 | 20 | 40 |
| 2 | Palaemonidae | Macrobrachium australe | − | + | 3 | 70 | 92 |
| 3 | Macrobrachium rosenbergii | − | + | 1 | 106 | 106 | |
| 4 | Macrobrachium sp. | − | + | 2 | 50 | 70 | |
| 5 | Palaemon sp. | − | + | 103 | 17 | 36 | |
| 6 | Gecarcinucidae | Parathelphusa sp. | − | + | 4 | 30 | 75 |
| Total number of fish | 1830 | ||||||
4 Discussion
The findings of this study offer a significant update on the biodiversity of fish in the Cibareno River. The comprehensive use of diverse fishing gears and eDNA surveys has yielded more robust results compared to prior studies that relied solely on an electrofishing method conducted by Rachmatika, Sjafei, and Nurcahyadi (2002). Monitoring fish biodiversity based on direct fishing and eDNA metabarcoding revealed a significant difference in species richness upstream and downstream of the Caringin Weir, indicating that the weir is limiting fish passage.
Using the combined methods, we collected a greater variety of fish species (72 species) than was observed 20 years ago by Rachmatika, Sjafei, and Nurcahyadi (2002) (22 species). This includes 14 species that have never been recorded from the Cibareno River while being known from other areas in Java islands (Dahruddin et al. 2017). However, we did not detect five species previously recorded in the river. The failure to detect these five species could be due to them no longer being present, insufficient spatial or temporal sampling and sample replication (Altermatt et al. 2023), unsuitable primer pairs (Blackman et al. 2024) or a lack of a reference database (Takahashi et al. 2023). The primary challenges in eDNA monitoring in Indonesia revolve around the limitations of the reference library within the Indonesian public database. Furthermore, the unavailability of multiple mitochondrial and nuclear markers, which are instrumental in identifying the most effective targets for species identification, exacerbates these issues (Lecaudey et al. 2019; Wibowo et al. 2022). Nevertheless, our integrated methods provide a better understanding of the fish community in the Cibareno River than either method on its own. The eDNA metabarcoding approach detected either more fish taxa (~47%) or the same species (~42%) in 89% of the studied sites compared to traditional methods, suggesting the combined methods can identify more species than each method on its own (Valentini et al. 2016). The combined data set revealed the detection of 30 species not captured by gillnets in the previous 3 years (Gillet et al. 2018). A similar study in Boardman Lake in Michigan, United States, using traditional fishing methods captured 12 species of fish, but 40 taxa were detected using eDNA metabarcoding, which included all of the species observed using traditional fishing methods (Gehri et al. 2021).
Land use in the vicinity of the Cibareno River predominantly comprises forestry, rice fields and plantations. However, various human activities occur at each surveyed point. The potential for habitat fragmentation in the Cibareno River is not solely attributed to the initiation of river damming in early 2022. A significant contributing factor is the presence of unregulated gold mining operations in the areas of Upstream 1 and 2, which have persisted for the past decade. These mining activities discharge their washing waste into the river, leading to pollution that deters fish from this region. The measurements of mercury (Hg), lead (Pb) and cadmium (Cd) in the upstream Cibareno River were < 0.020, 0.237 and < 0.001 mg/L, respectively, signifying that the river was lightly polluted (Vamellia 2014). However, at a gold mine site with a holding pond that released waste into the Cikidang River, one of the Cibareno River's tributaries, Hg concentrations in fish reached 1.66 ppm, whereas spinach and soil contained 4.61 and 0.0127 ppm, respectively (Pamungkas, Thayib, and Inswiasri 2015). Further statistical analysis has revealed that the two surveyed points exhibit similar fish diversity, which significantly differs from other points. This suggests that only specific fish species can thrive in areas where gold washing waste is regularly discharged. The disparity in fish diversity between sites Upstream 3 and Downstream 1, which are separated by the construction of the Caringin Weir, is anticipated to manifest in early 2022 once the river is dammed. Fish within the Cibareno River may encounter difficulties passing through the newly constructed 1-m-high barrier. Survey Downstream 2, located in a rice field area, is hampered by residents disposing of pesticides and conducting domestic activities in the river, potentially limiting the aquatic life. In contrast, Survey Point 6, adjacent to the estuary, stands out as the area with the richest variety of fish species. This is attributed to the estuary's unique characteristics, fostering a habitat where marine and freshwater ecosystems intermingle, resulting in a diverse and flourishing fish population (de Moura, Vieira, and Garcia 2012; Gillanders et al. 2022).
The current fish biodiversity assessment of the Cibareno River provides an excellent understanding of the importance of river network connectivity in maintaining fish biodiversity. Historically, there were no barriers constructed in the river, and overall fish biodiversity remained well preserved at the Cibareno River, indicated by the relatively unchanged number of species compared to the last two decades (2002–2022). In the Citarum River, West Java, a large dam, the Djuanda Reservoir, was constructed in 1968 for hydropower purposes. Prior to the construction of the dam, 31 species were detected in the reservoir. But in 2008, after 40 years of operation, the number of species had declined to 18 (Kartamihardja 2008). Understanding the connection between fish diversity and river network connectivity is crucial to species survival, ecosystem integrity and human well-being (Shao et al. 2019).
The presence of catadromous and amphidromous species in this study indicates the crucial need to maintain the connectivity of the Cibareno River to enable species to complete the crucial migratory components of their life cycle. Although the overall species diversity detected in the current study was high, there were clearly more species detected downstream of the weir, suggesting some species were not able to migrate past the weir. Therefore, managing waterway connectivity for migratory species is crucial for their conservation and warrants the careful consideration of fish passage development in dam infrastructures (Franklin and Gee 2019). We also detected 21 fish species found in both upstream and downstream areas of the river, emphasising the importance of river connectivity in preserving fish biodiversity. These findings can help to guide water resource authorities in sustainably managing the river for irrigation and constructing fishways in weirs/dams to facilitate species migration. Additionally, ongoing research should explore the interconnectedness among riverine habitats to better understand how spatial mechanisms shape riverine fish assemblages (Fullerton et al. 2010).
The Cibareno River hosts many species with complex migratory habits. Often, both adults and juveniles are involved in migration. Therefore, for the purposes of the fishway concept design, we assume that both adults and juveniles will require passage (i.e., 10–600 mm long). The main objectives of fish passage development are to facilitate the migration of multispecies, both upstream and downstream, thereby contributing to the preservation of fish biodiversity (O'Connor et al. 2022). The development of selective passage is founded on interspecific variations in physical capabilities, body shape, sensory capacities, behaviour and movement patterns. These distinctions have been studied in an effort to create selective passage (Rahel and McLaughlin 2018). The selection of targeted species for fish passage design primarily hinges on factors such as migration pattern, fish size, population abundance and economic consideration.
The target fish species of the Cibareno River for fishway design are A. marmorata, T. tambra, B. binotatus, Sicyopterus sp. and R. aspro plus crustaceans. The fishway design needs to accommodate the size of T. tambra and A. marmorata, which are the two largest fish in the river. The larger fish will need appropriate depth and space, which should be considered in determining the size and depth of the fishway pools. Species like mahseer (Tor genera) typically engage in an upstream spawning migration at the onset of the rainy season as they search clear water areas for spawning and their larvae drift downstream after hatching (Amanda et al. 2023; Jaafar et al. 2021). Elvers (juvenile eels) migrate upstream during the dry season and early rainy season (Wahju et al. 2021; Wibowo et al. 2021). The smaller fish such as B. binotatus, larvae and juveniles, require low water velocity and reduced turbulence when navigating the fishway. Therefore, the channel, baffles and gradient/slope of the passage can be appropriately designed to accommodate the migration of these smaller fish. The artificial rock substrate on the channel bottom of the fishway must be designed to accommodate the high abundance of demersal fish from the Gobiidae family such as Sicyopterus sp. and R. aspro, which inhabit the rock areas and may also improve crustacean passage. Utilising less invasive culvert remediation techniques to accommodate small-bodied fish species can contribute to their sustained survival in the region for the future (Knapp et al. 2019).
5 Conclusion
Fish biodiversity assessment surveys are an essential phase in fishway design. They provide critical insights into fish biodiversity, abundance and migration characteristics, which are invaluable for developing an appropriate conceptual model fishway. The distribution of fish in both downstream and upstream areas serves as a guide and aids in studying changes in biodiversity, including planning for artificial barriers such as dams or other physical structures. Out of a total of 47 aquatic species caught through experimental fishing in the Cibareno River, 13 inhabited both upstream and downstream sites, indicating their potential for migration in both areas. Among this group, the two most dominant species are T. tambra and B. binotatus, consistently found in nearly every sampling event. On the other hand, some species are exclusively found either downstream or upstream. The findings from this study highlight the significance of combining experimental fishing and eDNA surveys, providing vital information to support the planning of weir construction for the design of fishways. The design and implementation of a fishway will potentially improve the natural migration patterns of several fish species.
Author Contributions
Kurniawan Kurniawan: conceptualisation, methodology, data curation, investigation, formal analysis, software, visualisation, writing – original draft preparation, writing – review and editing. Arif Wibowo: conceptualisation, data curation, investigation, funding acquisition, software, validation, supervision, writing – original draft preparation, writing–review and editing. Vitas Atmadi Prakoso: methodology, data curation, investigation, funding acquisition, project administration, software, validation, writing – review and editing. Fathur Rochman: data curation, investigation, methodology. Deni Irawan: methodology, technical assistance, data acquisition. Dwi Atminarso: conceptualisation, methodology, software, formal analysis. Andhika Prima Prasetyo: methodology, writing–review and editing. Tri Deniansen: methodology, technical assistance, data acquisition. Rendy Ginanjar: format manuscript, writing – review and editing. Mochammad Zamroni: format manuscript, writing – review and editing. Aliati Iswantari: methodology, data curation, investigation, writing – review and editing. Sapto Andriyono: methodology, visualisation, writing – review and editing. Indah Lestari Surbani: funding acquisition, project administration, data acquisition. Imron Rosadi: funding acquisition, project administration, data acquisition. Yohanes Yudha P. Jaya: funding acquisition, project administration, data acquisition. Sudarsono Sudarsono: funding acquisition, project administration, supervision. Satoshi Nagai: software, formal analysis, validation. Meaghan L. Rourke: writing–review and editing. Nicolas Hubert: writing – review and editing. Ivor Stuart: writing – review and editing. Lee J. Baumgartner: conceptualisation, supervision, writing – review and editing.
Acknowledgements
The authors are grateful to the FAO Indonesia (IFish) and the Australian Centre for International Agricultural Research (ACIAR) teams for this wonderful opportunity and collaboration. The authors gratefully acknowledge Research Center for Fisheries, the Ministry of Marine Affairs and Fisheries, the Government Office of Fisheries, Government Office of Water Resource Management, West Java and UPTD Water Resource Management of Sukabumi, West Java. Finally, we would like to thank Fery, Darman and the undergraduate students of the Aquatic Resource Management, IPB University, who significantly contributed to the fieldwork. Open access publishing facilitated by Charles Sturt University, as part of the Wiley - Charles Sturt University agreement via the Council of Australian University Librarians.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data used in this manuscript are organised by the first author. All sequences were submitted to https://www.ncbi.nlm.nih.gov/genbank/ (PP352528–PP352565).




