Landscape factors predict local extirpation in an imperilled minnow species, the bridle shiner (Notropis bifrenatus)
Abstract
- Local extirpation events are often poorly documented and the causes are not well understood. A case in point is the bridle shiner (Notropis bifrenatus), a cyprinid that is declining and carries various conservation listings over most of its range.
- Recent research suggested that the apparent decline of the bridle shiner in the state of Connecticut may be in part an artefact of changes in survey methods: seining has been replaced by electrofishing, which is less efficient in bridle shiner habitat. The present study included a re-evaluation of the distribution of the species in light of this recent finding.
- Historical sites in Connecticut were seined, and it was found that nine populations once thought to be extirpated were in fact extant. Nonetheless, bridle shiner occurrences have declined by 60% over 50 years. Potential correlates of extirpation, such as landscape and habitat fragmentation metrics, were identified using geospatial tools. Through an information-theoretic approach, models were constructed to investigate the relative contribution of land use and habitat fragmentation to the extirpation of the bridle shiner.
- Land use had greater support as a correlate of the decline, and the current bridle shiner distribution in Connecticut could be explained by areas of high forest cover and low impervious cover. It is hypothesized that the adverse impacts of land use on the bridle shiner are attributable to its ecological specialization on submerged vegetation, notwithstanding its otherwise broad physiochemical tolerance.
- These results provide needed context on the decline of this species and provide potential avenues for conservation actions. This research emphasizes the impacts that land alterations can have on freshwater species and the importance of accurately characterizing extirpation events.
1 INTRODUCTION
Freshwater ecosystems are among the most seriously threatened habitats on Earth (Dudgeon et al., 2006), with predicted species loss rates for North American freshwater organisms that parallel or exceed the losses in tropical rainforests (Burkhead, 2012). Extinction rates are higher in aquatic ecosystems than in the surrounding terrestrial areas (Ricciardi & Rasmussen, 1999), perhaps because they are more susceptible to the multiple stressors that accumulate in watercourses. Human impacts such as habitat loss, degradation, and fragmentation, and changes in land-use patterns, are a few of the factors that interact to influence species abundance and distribution, and have increased in prevalence over recent decades (Paul & Meyer, 2001; Walsh et al., 2005). Development continues at a rapid pace throughout the USA, imposing direct and indirect effects of development on the physical (Booth, Hartley, & Jackson, 2002), chemical (Dietz & Clausen, 2008), and biological characteristics of streams (Stanfield & Kilgour, 2006). Urban run-off carries excessive nutrients and sediments, toxic contaminants, pathogens, and debris; impervious surfaces lead to increased variation in stream flow, increased stream temperatures, and destabilization of the channel (Booth et al., 2002). As a consequence, stream ecosystem integrity is negatively correlated with the extent of urbanization in its surrounding catchment (Miltner, White, & Yoder, 2004).
Habitat fragmentation is an important stressor in aquatic systems. Barriers that fragment habitat reduce the viability of species that have evolved life-history strategies in response to natural flow regimes (Bunn & Arthington, 2002; Poff et al., 1997), and migrate among habitats for reproduction, feeding, and other activities (Schlosser, 1991). Furthermore, barriers that fragment habitat reduce the probability of replenishment by migrants from other populations (Olden, Jackson, & Peres-Nato, 2001). Connectivity can reduce the risk of localized extirpation events for a species by increasing the chance that a site will be recolonized (Hanski, 1991).
Identifying threats is particularly critical for species that occur in only a few locations (Master, 1991). Species that are rare in terms of limited range, frequency of occurrence, or local abundance often have a greater likelihood of extinction than more common species. A commonly held view is that the fewer occurrences a species has or the more fragmented its distribution is, the more vulnerable that species will be to localized extirpation and extinction (Fagan, 2002). Unfortunately, the causes of the decline of listed species are often unknown (Worthington, Brewer, Grabowski, & Mueller, 2013), and are rarely determined by a single factor (Jackson, Peres-Neto, & Olden, 2001).
Since 1900 nearly 57 species and subspecies of North American freshwater fishes have become extinct (Burkhead, 2012), and nearly 40% of North American freshwater and diadromous fishes are imperilled (Jelks et al., 2008). Many imperilled freshwater fishes in North America are in the family Cyprinidae, in part because of their small body size and limited dispersal ability (Angermeier, 1995; Winemiller, 2005). Biodiversity loss in cyprinids has been pronounced, highlighting the need for increased monitoring to document species occurrence and detect population changes in this family (Whittier, Halliwell, & Paulsen, 1997). Imperilled cyprinids are frequently threatened by total habitat destruction (hydrological alteration, siltation, and pollution) and invasive species (Angermeier, 1995; Jelks et al., 2008). A case in point is the bridle shiner, Notropis bifrenatus, which has undergone a drastic decline in the north-eastern USA and south-eastern Canada (NatureServe, 2014). The bridle shiner occurs in low-velocity areas and stands of aquatic macrophytes (Jensen & Vokoun, 2013) in a variety of habitats with clear water, including artificial ponds, beaver impoundments, swamps, and the low-gradient pool reaches of streams (Sabo, 2000). Range wide, the bridle shiner is listed as being of ‘least concern’, with noted decreasing population trends (NatureServe, 2014), and carries various conservation listings throughout its range (Sabo, 2000). For instance, the species has been extirpated in parts of the USA (e.g. Maryland; Kilian, Raesly, Stranko, Becker, & Decker, 2011), and is listed as being of ‘special concern’ throughout Canada (Committee on the Status of Endangered Wildlife in Canada (COSEWIC), 2014). Little research has investigated the causes of the decline, but there is speculation that it may result from habitat degradation or changes in predator assemblages over time (Sabo, 2000; Whittier et al., 1997).
The present study focused on bridle shiner declines in Connecticut, USA, where these fish were listed as a Species of Special Concern in 2010. A comparison of fish surveys conducted by Whitworth, Berrien, and Keller (1976) in the 1960s, and by what is now known as the Connecticut Department of Energy and Environmental Protection (CT DEEP) in the 1990s, showed a marked reduction in the range of bridle shiner in Connecticut (Jacobs & O'Donnell, 2009), from 112 occupied sites to only 36. Of the 40 lakes hosting bridle shiner in the 1960s, only 19 lakes still supported the species three decades later. Even more dramatic was the decline in stream occurrences, from 72 to 17 locations. Similar trends are reported in other eastern states of the USA (NatureServe, 2007).
The study objective included two hypotheses to identify patterns of extirpation and tested the relative effects of correlates of contemporary land cover and land use known to change habitat conditions, and habitat fragmentation correlates that could lead to isolation from other potential sources of recolonization. This approach provides needed context on declines in this species and potential avenues for conservation actions.
2 METHODS
2.1 Fish sampling
The study area included all known historical locations of bridle shiner in Connecticut (Figure 1), including both streams and lakes. These locations were identified from surveys conducted by the Connecticut Department of Energy & Environmental Protection in the 1960s using seines, and in the 1990s survey using electrofishing gear. There was concern that some of the apparent decline might be an artefact of the sampling methods, because the earlier surveys in the 1960s had exclusively used seines whereas the later surveys in the 1990s used electrofishing gear. Bridle shiner occur in highly structured habitats such as maple swamps, which can be difficult to sample, and thus might be avoided by backpack or tow-barge electrofishers. Jensen and Vokoun (2013) demonstrated that seine sampling was effective for detecting bridle shiner in these habitats. To test this potential bias between the two gears directly, a formal gear comparison was conducted in streams known to be recently occupied by bridle shiner (Pregler, Vokoun, Jensen, & Hagstrom, 2015). Electrofishing was found to be half as efficient as seine fishing in detecting bridle shiner. There was a need to determine whether the inefficiency of electrofishing could account for the apparent decline of this species. Thus, it was necessary first to seine all sites with historical records for bridle shiner to generate better occurrence data and potentially reveal still-extant populations that may have been imperfectly detected by previous electrofishing surveys.
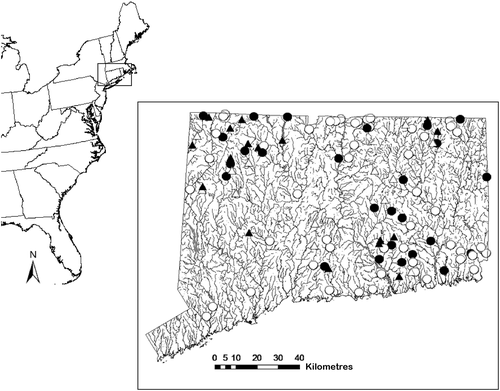
Of the 76 sites with apparently extirpated populations, 63 sites (53 stream sites and 10 lake sites that were occupied in the 1960s but not in the 1990s) were re-sampled during summer 2013 with a seine. Thirteen (18% of those extirpated) sites with apparently extirpated populations could not be located or accessed for a variety of reasons, ranging from insufficient landmarks recorded in the original 1960s field notes, to rapid urbanization and land-use change. These sites were omitted from the analyses. To be consistent with the sampling methods of Pregler et al. (2015) in the gear comparison study to detect bridle shiner, a 3.175-mm mesh, 5-m-wide bag seine was used to sample vegetated areas of lake littoral zones and slow-flowing reaches of streams and small impoundments. A seine was used because this sampling gear had a much higher probability of detecting bridle shiner compared with electrofishing (0.77 versus 0.36; Pregler et al., 2015). All seine hauls were approximately 35 m in length. The sampling effort ranged from three to 12 seine hauls at each site, depending on the amount of aquatic vegetation available. The capture was processed at the end of each haul. The 2013 sampling season comprised months when detection rates are highest, from mid-July until the end of August (Jensen & Vokoun, 2013).
2.2 Analysis
Two competing hypotheses were compared for the decreasing number of bridle shiner occurrences in Connecticut. The first hypothesis is that the decline has been caused by changes in land use. It was not possible to account for land-use change directly, given that land-cover data were not available for Connecticut before 1985. Thus, a correlative analysis of contemporary land-use patterns was performed. This analysis was conducted to investigate whether or not there was a correlation between contemporary land-use patterns and the current distribution of bridle shiner. The second hypothesis is that the decline is a consequence of habitat fragmentation caused by dams, the position of a population in the catchment, and the distance to the nearest historical site (population isolation).
To quantify variables for the land-use hypothesis environmental data were incorporated, which were available online (Table 1) in geospatial data layers in a Geographic Information System (GIS): ArcMap10.1. Land cover (compiled from the 2011 dataset; Homer et al., 2015) within a 250-m riparian buffer surrounding the upstream catchment of a site was quantified as percentage cover in two categories: the total proportion of impervious cover as a metric of development and forest cover. Land-cover covariates were standardized by their mean and standard deviation before analysis.
| Model covariate | Description | Source |
|---|---|---|
| Land cover | National land cover database. Resolution 30 m. | Homer et al. (2015) |
| Population isolation | National hydrography dataset | McKay, Bondelid, and Dewald (2012) |
| Stream order | Modified Strahler stream order | NHD (2014) |
| Number of dams | GIS point shapefile | CT DEEP (2014) |
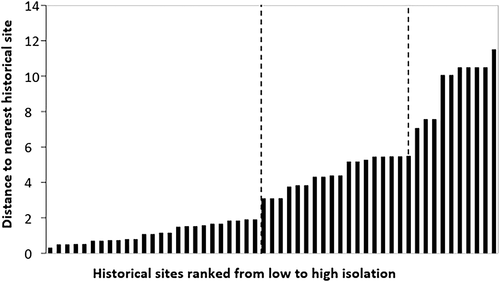
To quantify variables for the habitat fragmentation hypothesis, three relevant metrics were calculated. Position within a catchment for lake and stream sites was determined from the National Hydrography Dataset (Table 1) and represented by Strahler stream order (Strahler, 1957), encoded as a categorical variable (headwaters, i.e. stream orders of 1 or 2, or downstream, i.e. stream order of 3 or higher). Lakes were assigned catchment position based on the order of the stream on which it was located. Population isolation was coded as a categorical variable, determined by measuring the waterway distance from one site to the nearest site (for both streams and lakes) where there was a population record in the historical surveys. Population isolation categories 1–3 were determined by natural breaks in the distribution of distances (Figure 2). Sites that were completely isolated and had no nearest historical site within a catchment were assigned isolation category 4. Dams were counted if they were situated within 2 km of a site and in the same catchment.
Variables were tested in sets of models derived from two hypotheses in order to understand what explained bridle shiner occurrences. Occurrence was modelled as detection/non-detection data in logistic regression rather than abundance because previous sampling events used different types of sampling gear. A total of 99 sites were included in the analysis, including 63 sites at which populations were presumed to be extirpated that were resampled in 2013, and 36 sites at which bridle shiner are extant according to CT DEEP sampling records. Candidate models for both hypotheses included a categorical variable separating sites into lake and stream water bodies. Candidate models otherwise incorporated only variables associated with a single hypothesis, and then candidate models from both hypotheses were evaluated together to assess which hypothesis was more predictive of bridle shiner occurrences. Analyses were performed using logistic regression and models were evaluated using Akaike's Information Criterion adjusted for small sample size (AICc; Burnham & Anderson, 2002). Models were considered to be competing if found within two AICc units of one another, or if they carried more than 10% of model weight (wt). Variance inflation factors were calculated to determine whether multicollinearity was present in the models (Zuur, Ieno, & Elphick, 2010).
As fish occurrences are often controlled by a combination of hydrogeomorphic characteristics of streams and the landscape, a post-hoc model set was evaluated combining the variables from the most supported land-use and fragmentation models. The area under a receiver operating characteristic curve (AUROC) was used to assess the goodness of fit of the most supported model (Hanley & McNeil, 1982). When interpreting AUROC values, values of 0.5 indicate that the model performs no better than random; the closer the value is to 1, the higher the predictive capability of the model (Pearce & Ferrier, 2000; Zipkin, Grant, & Fagan, 2012).
3 RESULTS
The 2013 bridle shiner survey showed that 14% of the sites previously thought to be extirpated (nine sites out of 63) contained bridle shiner populations. Seven of these rediscovered extant populations were found in lentic portions of streams and two were found in lakes. Five of the seven stream populations were in small impoundments and two were in swamp-like backwaters. With this updated distribution information, a 60% decline was evident since the 1960s with an observed change from 112 occupied sites to 45. Given that 13 sites with apparently extirpated populations were unable to be sampled, a change from 99 sites to 45 (55% decline) is also presented, as those sites may have had bridle shiner present. Associated aquatic vegetation included submerged weeds such as milfoils (Myriophyllum) and pondweeds (Potamogeton), emergent weeds such as smartweeds (Polygonum), and filamentous algae. Bridle shiner co-occurred with a variety of fish species, including predators such as largemouth bass (Micropterus salmoides) and pickerels (Esox spp.).
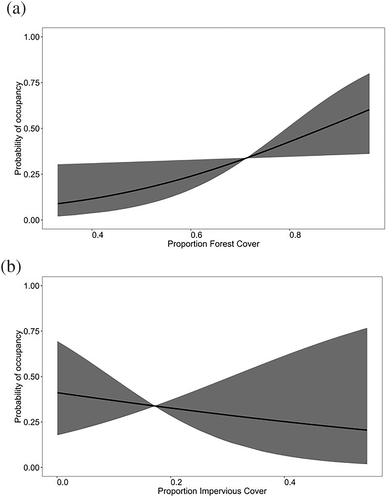
With the updated distribution information for bridle shiner, the statistical analysis ultimately contained 45 sites with extant populations and 54 extirpated populations. These 54 populations were presumed to be extirpated as there were no bridle shiner detected at these locations in the 2013 survey. Logistic regression modelling showed that land cover was more strongly predictive of bridle shiner occurrences than habitat fragmentation (Table 2). Models including land-cover parameters carried 89.1% of the total weight, whereas models including habitat fragmentation parameters carried 8.1% of the total weight. The most supported model combined forest cover and water-body type and carried 54.5% of the total model weight. At bridle shiner sites, impervious cover ranged from 0.1 to 55.0%, and forest cover ranged from 33.0 to 96.0%. When comparing land cover among extant versus extirpated sites, impervious cover ranged from 1.7 to 48.1% (mean = 14.7%) for extant populations, and from 0.1 to 54.5% (mean = 19.23%) for extirpated sites, whereas forest cover ranged from 46.9 to 96.1% (mean = 75.45%) for extant populations and from 33.0 to 90.5% (mean = 67.78%) for extirpated sites. When total model weights were calculated for model parameters, forest cover appeared to be more important compared with impervious cover, with model weights of 73.2 and 34.3%, respectively. Model-averaged effect sizes for both forest cover (effect size = 0.710; Table 3) and impervious cover (effect size = −0.260) also showed that forest had a larger effect on the distribution of bridle shiner. The increase in forest cover from 33% to nearly 96% increased the probability of occurrence by more than half, from 8% to about 60% (Figure 3). Lake sites were more likely than stream sites to have bridle shiner (model-averaged effect size = 1.670). Of the variables representing the habitat fragmentation hypothesis, the single most supported covariate was catchment position, and models including catchment position had 50% of the total weight in comparison with other models within the habitat fragmentation model group. The most supported model in Table 2 had an AUROC value of 0.75.
| Hypothesis | Model | AICc | ∆AICc | wi | df | AUROC |
|---|---|---|---|---|---|---|
| 1 | Forest + water-body type | 124.9 | 0.0 | 0.545 | 3 | 0.75 |
| 1 | Forest + impervious + water-body type | 127.0 | 2.2 | 0.184 | 4 | 0.75 |
| 1 | Impervious + water-body type | 127.3 | 2.5 | 0.159 | 3 | 0.74 |
| Water-body type | 131.4 | 6.6 | 0.020 | 2 | 0.64 | |
| 2 | Catchment position + water-body type | 131.5 | 6.6 | 0.019 | 6 | 0.71 |
| 2 | Dams + water-body type | 131.8 | 6.9 | 0.017 | 3 | 0.67 |
| 1 | Catchment position + dams + population isolation + water-body type | 132.2 | 7.3 | 0.013 | 8 | 0.74 |
| 2 | Population isolation + water-body type | 132.4 | 7.6 | 0.012 | 3 | 0.65 |
| 2 | Dams + population isolation + water-body type | 132.9 | 8 | 0.010 | 4 | 0.67 |
| 2 | Catchment position | 133.7 | 8.9 | 0.006 | 5 | 0.67 |
| 2 | Catchment position + dams + population isolation | 134.7 | 9.9 | 0.004 | 7 | 0.72 |
| 1 | Forest | 134.9 | 10.1 | 0.003 | 2 | 0.63 |
| 1 | Forest + impervious | 136.3 | 11.5 | <0.001 | 2 | 0.64 |
| 1 | Impervious | 138.0 | 13.1 | <0.001 | 2 | 0.61 |
| 2 | Dams | 140.3 | 15.4 | <0.001 | 2 | 0.52 |
| 2 | Dams + population isolation | 141.1 | 16.3 | <0.001 | 3 | 0.57 |
| Parameter | Estimate | SE | P | vif |
|---|---|---|---|---|
| Intercept | −0.670 | 0.400 | 0.007 | |
| Land-cover type | ||||
| Forest | 0.710 | 0.330 | 0.006 | 1.151 |
| Water-body type | ||||
| Stream | N/A | N/A | N/A | N/A |
| Lake | 1.670 | 0.54 | 0.001 | 1.151 |
- N/A: no estimate is available as the category is used as a reference in coefficient determinations.
After combining the variables from the most supported models of the land-use and fragmentation hypotheses, the most supported model included forest cover, catchment position, and water-body type (Table 4), carrying ~49.3% of the total model weight. Four out of the five top models carried more than 10% of the model weight (49.3, 18.1, 15.5, and 15.4% respectively), so these were then multi-model averaged (Table 5). The larger catchment position of a site negatively affected occurrence: the contrast with a catchment position of 1 was strongest in a catchment position of 3 (effect size = −2.22). The addition of catchment position to the previously most supported model also improved the AUROC value to 0.78, which suggests a good level of model fit (Pearce & Ferrier, 2000).
| Model | AICc | ∆AICc | wi | df | AUROC |
|---|---|---|---|---|---|
| Catchment position + forest + water-body type | 124.7 | 0 | 0.493 | 7 | 0.78 |
| Catchment position + impervious + water-body type | 126.7 | 2.0 | 0.181 | 7 | 0.79 |
| Forest + impervious + water-body type | 127.0 | 2.3 | 0.155 | 4 | 0.75 |
| Catchment position + forest + impervious + water-body type | 127.0 | 2.3 | 0.154 | 8 | 0.78 |
| Catchment position + water-body type | 131.5 | 6.8 | 0.017 | 6 | 0.72 |
| Parameter | Estimate | SE | P | vif |
|---|---|---|---|---|
| Intercept | −0.210 | 0.550 | 0.826 | |
| Land-cover type | ||||
| Forest | 0.750 | 0.38 | 0.005 | 1.446 |
| Catchment position | 1.310 | |||
| 1 | N/A | N/A | N/A | |
| 2 | −0.380 | 0.590 | 0.506 | |
| 3 | −2.220 | 0.920 | 0.016 | |
| 4 | −0.370 | 1.310 | 0.835 | |
| 5 | −16.070 | 1455.4 | 0.991 | |
| Water-body type | 1.363 | |||
| Stream | N/A | N/A | N/A | |
| Lake | 1.390 | 0.590 | 0.018 |
- N/A: no estimate is available as the category is used as a reference in coefficient determinations.
4 DISCUSSION
Overall, bridle shiner populations have undergone a substantial decline in Connecticut. Although seining has uncovered nine additional bridle shiner locations, bringing the known occurrence total to 45 populations in Connecticut, there has nonetheless been a 60% decrease in occurrences over the past 50 years. Bridle shiner are more likely to persist in areas of high forest cover and low impervious cover, and in the headwaters of catchments. These findings emphasize the potential impacts that land-use alterations can have on freshwater species decline and highlights the importance of accurately characterizing species distributions in order to understand extirpation events.
The rediscovery of bridle shiner in some sites where they were thought to be extirpated may be attributed to gear effectiveness and/or habitat preferences. In streams, bridle shiner are found in impoundments or backwaters with submerged aquatic vegetation and silt substrate. Records of surveys in the 1960s did not include habitat details, so it is not possible to assess whether the electrofishing surveys in the 1990s sampled the same habitats. Given the difficulty in sampling such swampy, heavily vegetated areas, we suspect that the 1990s surveys may have avoided sampling in prime bridle shiner habitat where alternative habitat was available. Survey design must take careful consideration of habitats that are under-sampled but potentially important. For instance, Mueller, Pander, Knott, and Geist (2017) found that 68% of species found in their study area could be detected in off-channel habitats, and a number of species were found exclusively in off-channel habitats. Perhaps another explanation for why bridle shiner were detected in the 2013 surveys could be that individuals dispersed into these habitats from nearby locations; however, given that this species is small bodied with limited dispersal capabilities, and occurs in such patchy habitat, it seems more likely that these fish may have been imperfectly detected through sampling bias.
Extirpation events are rarely observed, and although it is not possible to determine the timing of bridle shiner extirpations, the rate of extirpation can be estimated from patterns of species occurrence. Comparing occurrences between the surveys in the 1960s and those in the 1990s, unadjusted for detection probabilities, yields an extirpation rate of 2.70 bridle shiner sites lost per year. An improved estimate can be developed using detection probabilities for the sampling gears used in these surveys (Pregler et al., 2015). Applying detection probabilities for sampling with backpack shockers or a seine (0.36 and 0.77, respectively) to the surveys from the 1960s, 1990s, and 2013 (147, 70, and 59 adjusted occurrences, respectively), reduces the extirpation rate to 1.72 bridle shiner sites lost per year. It is important to understand the rate of extirpations because current rates of population extirpation have been documented to be at least three orders of magnitude higher than species extinction rates (Schindler et al., 2010).
Numerous studies comparing historical with contemporary fish collection data have documented extensive changes in abundance, species richness, and community structure in relation to changes in land use over time (Gido, Dodds, & Eberle, 2010; Matthews, Marsh-Matthews, Cashner, & Gelwick, 2013; Sutherland, Meyer, & Gardiner, 2002; Walters, Freeman, Leigh, Freeman, & Pringle, 2005; Weaver & Garman, 1994). As habitats become homogenized, research has shown a correlative relationship where cyprinids tend to be replaced by more cosmopolitan taxa such as centrarchids (Johnston & Maceina, 2009). The stocking of non-native predators has also been shown as a potential correlate of decline for cyprinids (Whittier et al., 1997). Landscape alteration contributes to declines in other species: in brook trout (Salvelinus fontinalis), forest clearing and agriculture has degraded stream conditions, and in turn has decreased fish distribution and abundance (Stranko et al., 2008). We suggest, however, that the mechanisms of decline are different in bridle shiner than in classically intolerant species such as brook trout. Bridle shiner are found in swampy, low-flow habitats that are susceptible to varying dissolved oxygen levels and fluctuating temperatures (Sabo, Bryan, Kelson, & Rutherford, 1999), which suggests that they may be tolerant of such conditions, but they do require submerged vegetation for reproduction and predator avoidance (Sabo, 2000). Ecological specialization has been identified as a trait linked to increased risk of extirpations and extinction in freshwater fishes (Angermeier, 1995). Changes in land use, and the associated decline in forest and increase in impervious cover, could act negatively on submerged vegetation. Streams in developed landscapes have a flashier flow regime, increased scour, and are more susceptible to vegetation loss (Meyer, Paul, & Taulbee, 2005; Paul & Meyer, 2001; Sutherland et al., 2002). Sites further downstream in the catchment (e.g. of stream order 3 or higher) were found to be more adversely affected relative to headwater locations, perhaps because floodplain development is more likely in these areas. Floodplain development has been implicated in the decline of bridle shiner in Pennsylvania (Fairchild, Horwitz, Nieman, Boyer, & Knorr, 1998). Developed land cover in riparian corridors in Connecticut increased by 2%, from 14.5 to 16.8%, over a recent 20-year period (Nelson et al., 2009; Wilson & Arnold, 2011). This level of development reduces biodiversity: the occurrence probabilities of some fish species approached 0% where there is 2–4% impervious cover (Wenger, Peterson, Freeman, Freeman, & Homans, 2008).
Given these results, the stream populations of bridle shiner may be at the greatest risk. The remaining stream populations are in small impoundments or backwaters. Although impoundments can disrupt the natural flow regime and erode critical habitats for fluvial fish species (Freeman & Marcinek, 2006; Poff et al., 1997), they apparently provide critical habitat for the remaining stream populations of bridle shiner. The restoration of rivers that have been extensively altered should take into account the potential that some otherwise marginal habitat may represent an important refuge for some species of concern (Lyon, Stuart, Ramsey, & O'Mahoney, 2010; Sedell, Reeves, Hauer, Stanford, & Hawkins, 1990).
Lentic sites are less dynamic than lotic habitats (Ward, Tockner, Arscott, & Claret, 2002) and may have more stable vegetated habitat. This analysis indicated that stream habitats have a negative effect on occurrence compared with lakes; however, developed lake shorelines tend to have an increased frequency of winter drawdowns and herbicide treatments, which can limit the availability of submerged vegetation (Scheuerell & Schindler, 2004; Wilcox & Nichols, 2008). A single year drawdown may reduce habitat for reproduction and increase vulnerability to predation (Sutela, Vehanen, & Rask, 2011), imposing a significant longer-term impact on the abundance, as has been found in other cyprinids (Yamamoto, Kohmatsu, & Yuma, 2006). Over time, these human habitat modifications may have severely reduced the opportunities for any future recolonization.
The present study resulted in improved distribution information for bridle shiner populations in Connecticut, and we advocate the continued monitoring of these populations. Furthermore, although the results provide correlative information to explain bridle shiner declines, more research is needed to understand the underlying mechanisms of decline. Recent physiological studies have shown that increased turbidity affected bridle shiner performance and behaviour (Gray, Bieber, McDonnell, Chapman, & Mandrak, 2014). Further research may also be needed to assess the critical limits of hypoxia and temperature tolerances. When physiological information is incorporated into ecological models, it can improve the predictions of species-specific responses to environmental change (Cooke et al., 2013).
Given that future population growth and urbanization will continue (Martinuzzi et al., 2014), conservation actions need to be taken to ensure the persistence of species like bridle shiner, perhaps in the form of translocations. These types of conservation actions have been successful for imperilled fishes such as the Oregon chub (Oregonichthys crameri), a small floodplain minnow endemic to the Willamette River in Oregon that inhabits off-channel floodplain habitats with abundant aquatic vegetation (Scheerer, 2002). Like the bridle shiner, the range of this species was found to be severely restricted in comparison with its historical range. Introductions and translocations into restored or suitable habitats where the species previously occurred can be effective methods for conserving threatened and endangered fishes (Weeks et al., 2011), particularly those with limited dispersal or gene flow (Hayes, Krahl, Werneke, & Armbruster, 2016). Genetic data should be used to guide the movement of fishes, given the potential for local adaptation and depleted genetic diversity in small isolated populations (Laikre, Schwartz, Waples, Ryman,, & GeM Working Group, 2010). Furthermore, future restoration projects should incorporate a monitoring component to track population recovery (Laikre et al., 2010).
Knowledge of unbiased estimates of species distribution is critical for the effective conservation of imperilled fish species and the determination of conservation listings. Furthermore, providing detailed data on the mechanisms of declines aids legislation for the protection of species’ habitats and populations (Cooke et al., 2013). In Connecticut, present legislation defines a species of concern as any native non-harvested species documented to have a naturally restricted range or habitat within the state. However this legislation does not provide guidelines or restrictions on what can or cannot be done in regard to the fish population or its habitat, whereas in Canada, legislation for bridle shiner carries both federal and provincial legal protection, where it is prohibited to cause harmful alterations or destruction of fish habitats (Boucher, Berubé, Boyko, & Bourgeois, 2011). Legislation in Connecticut could be improved by providing more specific guidelines that aid the management of this species. The findings from this study highlights areas that might be suitable for future conservation and reintroductions, and thus can better inform land-use policy decisions in Connecticut.
These results allow a better understanding of the distribution and correlates of decline for this species of concern in Connecticut, and generates information to guide future studies, not only in this part of its range but also range-wide, and conservation decision-making processes to maximize its protection. Species conservation cannot wait for all of the information needed from monitoring and experimentation, and adaptive management actions should be taken to prevent further decline and mitigate impacts (McFadden, Hiller, & Tyre, 2010) using known information about the species until more data can become available. Future efforts to improve the resilience of bridle shiner conservation across its range could be improved through a range-wide investigation of bridle shiner decline. As financial resources for conservation are often limited, and accurately documenting population trends is a major challenge to fishery managers (Pregler et al., in press), range-wide monitoring efforts could be facilitated by collaborations among managers and researchers across geographical boundaries (e.g. state and country borders).
This study adds to the evidence linking land use to species distributions and provides insights for the conservation of other imperilled species and remaining data gaps. Specifically, we advocate more detailed life-history studies for freshwater fishes to aid species distribution and trend analyses. Recently, efforts have been taken towards compiling fish trait data to describe the characteristics of a species that are linked with its fitness and performance (e.g. trophic ecology, life history, and physiological tolerances; Frimpong & Angermeier, 2009; Mims, Olden, Shattuck, & Poff, 2010), in order to facilitate conservation and management for North American freshwater fish species; however, this trait information is often incomplete or is lacking for many species. Thus, some attributes that might be useful in explaining extirpation, such as physiological tolerances or reproductive ecology, are missing from analyses (Frimpong & Angermeier, 2009). Where underlying mechanisms of decline are poorly understood, additional studies have advocated the preservation of the ecological integrity of entire catchments as far as possible (Angermeier & Karr, 1994; Fausch, Torgensen, Baxter, & Li, 2002; Frissell & Bayles, 1996).
ACKNOWLEDGEMENTS
The project was supported by funding from the Connecticut Department of Energy and Environmental Protection (CT DEEP) Endangered Species/Wildlife Income Tax Check off Fund (CGS Sec. 22a-27I). We also thank CT DEEP for supplying historical location data for bridle shiner, and Matt Traceski and other volunteers for aiding sampling efforts. We are especially grateful to the Connecticut landowners that let us access sites adjacent to their properties. We also thank Chris Elphick and Yoichiro Kanno for providing helpful feedback on this article.




