An integrated approach for cetacean knowledge and conservation in the central Mediterranean Sea using research and social media data sources
Abstract
- Sources of data other than those derived from conventional research protocols may contribute valuable information to fill gaps in knowledge about cetacean occurrences and diversity in a given area and help address conservation issues.
- The performance of a method to examine cetacean communities based on presence records systematically derived from shared photographs and videos posted by boaters and maritime operators on social media (e.g. YouTube and Facebook) combined with patchy distributed visual/acoustic data collected by researchers has been evaluated.
- Records (N = 1,274) gathered over a 10-year period (2008–2017) have been used to obtain insights into species' presence and habitat selection in a scattered study area of the central Mediterranean Sea (Italy). The effectiveness of the method, practical and theoretical advantages, limitations, and challenges of using data originated from social media for research and conservation purposes are discussed.
- Seven out of the eight cetacean species regularly residing in the Mediterranean have been reported in the area, with different relative densities. Maximum entropy modelling techniques have been applied to the datasets derived from (a) social media, (b) research surveys, and (c) the combination of the two, using six fixed variables as proxies for cetacean presence. Distance from the coast and depth emerged as the main variables predicting encounters, with specificities related to the ecology of the species.
- The approach was reliable enough to obtain broad-scale, baseline information on cetacean communities in the region, on the basis of which initial conservation recommendations and future research programmes can be proposed.
- With the increasing need for studying whale and dolphin population ecology coming from national/international directives, support from citizens to aid research may act as a practical, inexpensive solution to gathering extensive spatial–temporal data for regional-scale monitoring and for the development of management priorities.
1 INTRODUCTION
Cetaceans are an integral part of the pelagic and coastal fauna of the Mediterranean Sea. They are major consumers at most trophic levels, with key influences on marine community structure and services (Estes, Heithaus, McCauley, Rasher, & Worm, 2016). They are vulnerable to short-term natural and anthropogenic threats caused by activities on land and at sea, but also to the long-term chronic and cumulative effects of various stressors (Pace, Tizzi, & Mussi, 2015). Knowledge about their distribution and abundance in the Mediterranean basin is still limited with heterogeneous data (Mannocci et al., 2018), and many ecological and conservation questions remain unanswered. Studying cetaceans presents a number of challenges and is severely limited by both logistical and financial constraints. Data collection across different locations and habitats over a number of years, or even decades, requires a large workforce and substantial budget, consequently constraining opportunities for monitoring and research, and resulting in important data gaps (Braulik et al., 2018; Lodi & Tardin, 2018).
It has been recently suggested that other sources of data than those derived from conventional research protocols could help address major knowledge gaps and conservation challenges (Caitlin-Groves, 2012; Di Minin, Tenkanen, & Toivonen, 2015; Goffredo et al., 2010; Klemann-Junior, Villegas Vallejos, Scherer-Neto, & Vitule, 2017; McKinley et al., 2017). For example, incidental sightings by sea users (e.g. recreational sailors and fishermen, professional fishermen) may be a cost-effective method to obtain valuable scientific background information in uninvestigated regions or to acquire data over a wide geographic area (Embling, Walters, & Dolman, 2015; Robinson et al., 2013). With the advent of the Web 2.0 world, and thanks to advances in portable electronic devices (smartphones) and applications during the last decade, people are able to record data, images, and locations about species sightings in the wild at any time and share them on various social media platforms. This unwitting ‘citizen sensor network’ (Caitlin-Groves, 2012; Goodchild, 2007) allows non-scientists to considerably contribute to research, whether they intend to or not (Bonney et al., 2009).
The opportunity of exploiting social media posts as a way to scan and retrieve different information collected and transmitted by ‘connected’ citizens, and the use of such information (e.g. text, pictures, or videos) is developing fast (Di Minin et al., 2015; Dylewski, Mikula, Tryjanowski, Morelli, & Yosef, 2017; Mikula & Tryjanowski, 2016). Both conservation science (Barve, 2014; Daume, Albert, & Von Gadow, 2014; Di Minin et al., 2015; Papworth et al., 2015; Richards & Friess, 2015; Roberge, 2014; Saito et al., 2015) and scientific research (e.g. Daume, 2016; Dyderski et al., 2016; Giovos, Ganias, Garagouni, & Gonzalvo, 2016; Leighton, Hugo, Roulin, & Amar, 2016; Mikula & Tryjanowski, 2016; Mori et al., 2017) are increasingly using this novel approach to extract information from various types of social media platforms, such as Facebook (FB), YouTube (YT), Twitter, and Instagram, to provide new insights into the study of certain species or help identify knowledge gaps in their ecology and/or conservation (Dylewski et al., 2017).
In social media, each post contains information about when the content was created or shared; when geotagged, the videos, pictures, and text have geographic coordinates, or place name, showing the location where they were taken or posted from. Thus, social media content bears great potential for monitoring target species at greater spatial scales and temporal resolution than many other available data allow (Longley, Adnan, & Lansley, 2015; McKinley et al., 2017). Of the various types, online video-sharing applications have been shown to have high interactive/usage level in the social media scenario (Khan, 2017; Ricke, 2014). YT is the most well-known video-hosting service, with more than a billion users consisting of nearly 33% of the Internet populace (YT, 2016). Through greater use of smartphones with video recording capabilities, YT represents a tool that facilitates rapid accumulation of data from shared recordings, including those depicting animal sightings and behaviour (Dylewski et al., 2017; Yosef & McPherson, 2016). FB is another commonly used social platform to release texts, videos, and photographs that makes up about 18% of global social media activity (Chaffey, 2016). By tracking photographs and videos posted on platforms such as YT and FB from boaters, maritime tourists, divers, sea lovers, and professionals, potentially valuable information on cetacean species can be extracted (Giovos et al., 2016), providing much larger datasets than could be collected by traditional research alone. Although this approach can provide a substantial array of scientific and conservation benefits, limitations such as data fragmentation and over-reporting in high-use areas (Bird et al., 2014) should be properly evaluated and potential results interpreted with caution (Hann, Stelle, Szabo, & Torres, 2018).
In this paper, the performance of a method to detect and examine cetacean communities based on presence records systematically derived from shared photos and videos on YT and FB combined with patchy distributed visual/acoustic systematic and non-systematic data collected by researchers and stranding data, is introduced and evaluated. Records gathered over a 10-year period (2008–2017) off the Lazio region (central Mediterranean Sea, Italy) as a paradigmatic site are used to assess the effectiveness of the method, and indicate practical and theoretical advantages, opportunities, limitations, and challenges of using data originated from social media for both research and conservation purposes.
2 METHODS
2.1 Study area
The study area is located in the Tyrrhenian Sea (Italy), a zone of the central Mediterranean featuring one of the most complex marine structures in the seas surrounding the Italian peninsula. The study area covers about 39,000 km2 (Figure 1) and was specifically selected to include a variety of environmental features (e.g. bathymetries) and a range of different habitats (seagrass meadows; hard-bottom communities with coastal banks, cliffs, and caves; seamounts; sand and mud). In the northern section, it includes the islands of Giglio and Giannutri (Tuscany Archipelago), and the islands of Ponza, Palmarola, and Ventotene (Pontine Archipelago) are in the southern portion.
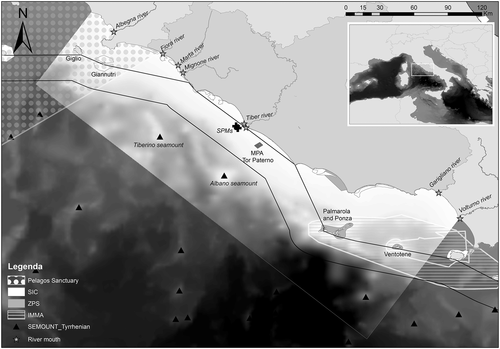
Two marine protected areas (MPAs) are found in the region: the MPA Islands of Ventotene and S. Stefano and the MPA Tor Paterno bank, the only Italian MPA completely submerged. Giglio and Giannutri islands are part of the Tuscany Archipelago National Park and are included in the Pelagos Sanctuary for Mediterranean Marine Mammals, an international area classified as a Specially Protected Area of Mediterranean Importance, subject to an agreement between Italy, Monaco, and France for the protection of marine mammals. In addition, a number of Sites of Community Importance and Special Protection Areas under the Natura 2000 European network are included in the study area (see the Italian Ministry of Environment list by region, http://www.minambiente.it/pagina/sic-zsc-e-zps-italia), and more recently, the marine region including the Pontine Archipelago was acknowledged by the International Union for Conservation of Nature (IUCN) Marine Mammal Protected Areas Task Force (MMPATF) as an Important Marine Mammal Area (IMMA; discrete portions of habitat, important to marine mammal species that have the potential to be delineated and managed for conservation) for the sperm whale (Physeter macrocephalus) in the Mediterranean (IUCN-MMPATF, 2017).
The study area includes seven river estuaries, from north to south: Albegna, Fiora, Marta, Mignone, Tiber, Garigliano, and Volturno. The Tiber is the major source of organic material in the Rome coastal area and nearby regions, and is also one of the main contributors of heavy metals in the Mediterranean Sea (Inghilesi et al., 2012; Montuori, Aurino, Garzonio, Nardone, & Triassi, 2016). At about 3 nmi off the two Tiber mouths, there is a terminal comprising two single-point moorings handling crude oil and petroleum products. Navigation, anchoring, diving, and fishing activities are banned within a radius of 0.75 nmi from each single-point mooring. These types of structures are reported to attract some dolphin species (Triossi, Willis, & Pace, 2013).
In the offshore zone of the study area, there are two seamounts, Tiberino and Albano; the former rises from the sea bed at 600 m to a depth of 250 m, the latter from 800 m depth to 300 m (Würtz & Rovere, 2015). It is known that seamounts attract pelagic top predators, particularly sea turtles and cetaceans (Fiori, Paoli, Alessi, Mandich, & Vassallo, 2016).
2.2 Data sources
Data on the occurrence of cetaceans in the study area over a 10-year period (January 2008 to December 2017) were obtained from three different sources: (1) social media and other sighting records collected by citizens during recreation activities or work; (2) published and unpublished data collected by researchers during scientific surveys; and (3) stranding records from the official (governmental) national stranding network and from other data sources (social media, local newspaper websites, further stranding database). All data were organized by season, defined as summer (July to September), autumn (October to December), winter (January to March), and spring (April to June).
For social media data, YT and FB were scanned for all available cetacean visual information (video footage and/or photographs). Both searches were restricted only to videos/photographs shared in personal accounts and not in official YT and/or FB channels from research projects, institutes, or non-governmental organizations, in order to ensure independency in the sample collections (as in Giovos et al., 2016). Three keyword categories were used for searches (for a full list of keywords, see Supporting Information, Table S1): locations (e.g. Ostia, Ponza island), cetaceans (dolphins, whales in Italian language, i.e. delfini, balene), and name of the species regularly present in the Mediterranean Sea (both common in Italian language and scientific). All keywords were extracted using a case-insensitive search technique, and variations of the same word (e.g. ‘dolphin’, ‘dolphins’) were compiled as the same keyword. This process, which was repeated iteratively until no new video/photograph was encountered, giving confidence that all available information was identified, resulted in 889 records.
To maximize the efficiency and quality of data retrieval, storage, and analysis, the way in which data were registered was standardized. To avoid biases, records were collected independently by two experienced scientists and the reliability coefficient (Cronbach's alpha; Cronbach, 1951) calculated. The coefficient was very high (0.96) for both YT and FB. The list of records was then filtered to guarantee reliability, reduce variability, and eliminate outliers or more trivial errors. As in Giovos et al. (2016), double entries, different fragments of the same footage, and different pictures of the same sighting were used only once in the final analysis in order to ensure that each dolphin encounter was represented exclusively by a unique video or set of photographs. Moreover, videos with more than one sighting were also excluded, resulting in 557 records being retained for the analysis (Table 1).
| Data | Method | Provider | Source/project | Period | No. of records |
|---|---|---|---|---|---|
| Social media | — | Citizens | YouTube (video) | 2008–2017 | 220 |
| Social media | — | Citizens | Facebook (video) | 2008–2017 | 190 |
| Social media | — | Citizens | Facebook (photograph) | 2008–2017 | 147 |
| Social media/Direct reporting | — | Citizens | Call/mail to researchers | 2008–2017 | 40 |
| Research surveys (visual) | Ferry-based fixed line transects | ISPRA | Fixed line transects Mediterranean monitoring network project (Arcangeli, Marini, & Crosti, 2012) | 2008–2017 | 326 |
| Research surveys (visual and acoustic) | Systematic boat-based line transects and non-systematic (‘haphazard’; sensu Corkeron et al., 2011) boat based in closing mode (cetaceans were approached after detection) | Sapienza University of Rome | Costa Concordia project | 2012–2016 | 55 |
| Research surveys (visual) | Non-systematic boat-based surveys | Sapienza University of Rome | Fishery projects | 2008–2012 | 35 |
| Research surveys (visual and acoustic) | Systematic boat-based line transects and non-systematic (‘haphazard’; sensu Corkeron et al., 2011) boat based in closing mode (cetaceans were approached after detection) | Sapienza University of Rome | CETYS/TASM projects | 2017 | 5 |
| Research surveys (visual) | Systematic aerial line transects | Tethys Research Institute; Observatoire PELAGIS University La Rochelle—CNRS | Lanfredi & Notarbartolo di Sciara, 2011; Van Canneyt, 2016 | 2009–2012 | 37 |
| Research surveys (visual) | Systematic boat-based line transects | IFAW, Song of the Whale Team; Tethys Research Institute; Italo-Tunisian Cetacean Research Project | Boisseau, 2014; Lanfredi, & Notarbartolo di Sciara, 2014; Tringali, 2015 | 2012–2014 | 19 |
| Research surveys (visual) | Boat-based line transects | Tuscia University | Seabirds/dolphins project | 2013–2014 | 9 |
| Strandings | — | Italian stranding network | Stranding National Data Bank (Pavan, Bernuzzi, Cozzi, & Podestà, 2013). Data downloaded from http://mammiferimarini.unipv.it [March 26, 2018]. | 2008–2017 | 164 |
| Strandings | — | Various | YouTube, Facebook, local newspaper websites, call/mail to researchers | 2008–2017 | 19 |
| Strandings | — | Centro Studi Cetacei |
GeoCetus Data downloaded from http://geocetus.spaziogis.it/index.php on March 26, 2018 |
2008–2017 | 8 |
| Total | 1,274 |
The minimum requirements for a species occurrence record (the type of data in this study) include taxonomic identification, data collection time (date or season), and geographical coordinates or location of the sighting. For species identification, only the photographs and video footage in which the diagnostic features of the taxon (i.e. body size and shape, coloration pattern in the dorso-lateral area of the animal's body) were clearly visible were selected. In most cases, this was a relatively easy and straightforward process, and the reliability coefficient between the two experienced researchers was 99%. On a few occasions, when the species could not be reliably determined or confirmed (e.g. animals far away, dorso-lateral area not clearly visible), videos/photographs were recorded as ‘undetermined’. Then, geo-referenced/location data available from each video/photograph included in the analyses were identified to determine the locations in the study area (see Supporting Information, Table S1). When the geographical coordinates were unavailable, or locations were not assessable from clear landmarks or other precise descriptions, the originator of the data was contacted to obtain further details on the location of the sighting. For each of the 557 records, the following variables were identified or estimated and extracted by researchers: number of individuals observed, behaviour, presence of immature individuals, interaction with the observation boat, presence of other vessels in the vicinity, presence of professional fishing boats (i.e. trawlers or artisanal boats), and concurrent recreational fishing by observers. Any other information, such as unusual events (e.g. deformities or mutilations, nurturant behaviour) from social media videos was also collected and reported in this study (see Supporting Information). Finally, to ensure data quality, the accuracy of the data extracted from each social media video/photograph was checked by a third researcher after inclusion in the database to verify and validate the data. Along with data collected by citizens and posted on YT and FB, 40 verified and validated cetacean records directly reported to researchers by citizens were considered and included in the social media dataset (Table 1).
As for research data, cetacean sighting records in the study area were collected from different monitoring/research surveys covering various coastal and offshore areas, cetacean species, time frames, and methods (Table 1). The following data sources were used: (a) survey data (n = 326 records) collected from ferries by ISPRA within the Fixed Line Transect Mediterranean Monitoring Network project; (b) boat-based survey data (n = 100 records) collected from different research campaigns by Sapienza University of Rome; (c) boat-based and aerial survey data (n = 56 records) compiled in OBIS SEAMAP (http://seamap.env.duke.edu/; Halpin et al., 2009); (d) boat-based survey data (n = 9 records) collected from a research campaign by Tuscia University.
Finally, cetacean stranding records (n = 191) were extracted from the National Data Bank (n = 164 http://mammiferimarini.unipv.it), which is a real-time updated database by the Italian Stranding Network supported by the Italian Ministry of Environment and managed by the Natural History Museum of Milan and the University of Pavia (Pavan et al., 2013), and from other sources (n = 19 from local newspapers, social media, and personal information; n = 8 from GeoCetus http://geocetus.spaziogis.it/index.php).
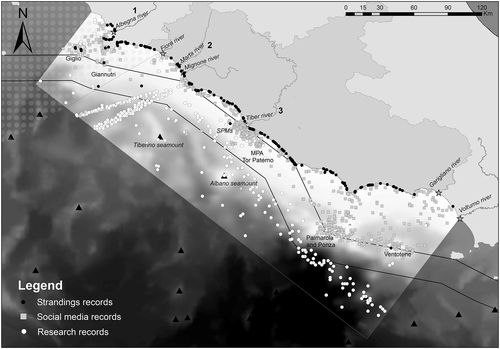
The distribution of all records from social media, research surveys, and stranding datasets is reported in Figure 2. Social media accounts have a more coastal diffusion than the research sources, with an overlap between research effort and social media data in three areas within the study region: (1) Albegna river estuary and Giglio island; (2) Mignone/Marta/Fiora river estuaries; and (3) Tiber river estuary and Tor Paterno MPA.
2.3 Data analysis: Group size
‘Best estimate’ group sizes were used for social media and research datasets. ANOVA was applied to test differences in species group size between the two datasets. Linear models were used to examine changes each season for species for each dataset. When the assumptions of linear models were violated, generalized linear models (GLMs) were fitted using a Poisson distribution if there was evidence of over-dispersion (as in Dwyer, Clement, Pawley, & Stockin, 2016). Analyses were carried out in Past version 3.20 (https://folk.uio.no/ohammer/past/; Hammer, Harper, & Ryan, 2001).
2.4 Data analysis: Spatial relative densities
Kernel density estimates (KDEs) were used as a measure of the species relative densities (i.e. ‘use of space’, as in Hauser, 2006). KDEs were calculated based on a combined dataset of research and social media records in ArcGIS version 10.2.2 (Environmental Systems Research Institute, Redlands, CA) setting cell size of 100 m square and a search radius of 20,000 m. The same scaling factor across the study area was used for each species, allowing comparison of relative density values. KDEs were calculated only for species with more than 15 records. The resulting KDE maps were visually assessed in order to recognize any high-density areas (i.e. hotspots; Clement, 2005).
2.5 Data analysis: Habitat suitability modelling
A characteristic of social media data is the absence of a designed sampling scheme on a geographic scale, so the observation density over both space and time is more representative of observer concentration than of the targeted data points themselves (Garcia-Soto et al., 2017). The absence of correction for heterogeneous observations may lead to important and significant biases and hence to spurious conclusions on spatial patterns of species distribution (e.g. a seasonal increase in the number of observers may lead to the conclusion that there is a seasonality in the presence of species). It has been suggested that a convenient way to address such issues involves pooling opportunistic, heterogeneously distributed presence-only data with controlled data (e.g. scientific survey data based on a predefined protocol) when available (Pagel et al., 2014) and use the latter in a global model to calibrate the purely opportunistic data. The maximum entropy method (MaxEnt version 3.3.3, http://www.cs.princeton.edu/~schapire/maxent/), which generates pseudo-absences (‘background points’) to fill the gaps and hence enable the analysis of presence-only data (Kramer-Schadt et al., 2013; Renner et al., 2015), was applied to model the relationships between environmental predictors and the occurrence records of different cetacean species in the study area, using research and social media datasets separately and then as a combined dataset.
Six fixed variables were selected to generate models: depth, slope, Euclidean distance from the shoreline, Euclidean distance from estuaries, Euclidean distance from seamounts, and Euclidean distance from main harbours; these were then used as proxies of factors that could affect the cetacean presence and distribution (Bombosch et al., 2014; Breen, Brown, Reid, & Rogan, 2016; Correia, Tepsich, Rosso, Caldeira, & Sousa-Pinto, 2015; Gómez & Cassini, 2015; Pace et al., 2018). The environmental variables were obtained from geographic information system raster layers.
Since MaxEnt accounts for sampling biases via correction features that consider sampling effort (in which the spatial bias in the sightings data is transferred to the background data by approximating areas where the probability of detection is non-zero; Phillips et al., 2009), the bias file feature to input a layer representing the area of the sampling effort for each dataset (research and social media) was used, as recommended by Stolan and Nielsen (2015), validated by Syfert, Smith, and Coomes (2013), and applied by Pace et al. (2018). Sampling bias was regulated by adding a specific bias originated from occurrence data and applying it as a template for the extraction of background points in effort areas (Bombosch et al., 2014; Elith, Kearney, & Phillips, 2010; Fourcade, Engler, Rödder, & Secondi, 2014; Pace et al., 2018). The number of background points was set separately for each dataset to generate the same background density in all models (research, social media, and combined). Then, each background and presence location was associated with the series of environmental variables, and MaxEnt was set to eliminate duplicates at the same location to reduce pseudo-replications and spatial autocorrelation of samples (Hammond, O'Keefe, Aldrich, & Loeb, 2016). For each dataset (research, social media, and combined), distinct MaxEnt models were run by stratifying data per different species, using default regularization parameters with maximum iterations up to 500 to reach convergence. MaxEnt was controlled using only linear, quadratic, and product feature classes, restricting it to produce relatively simple models to minimize the likelihood of overfitting the data (Merckx, Steyaert, Vanreusel, Vincx, & Vanaverbeke, 2011; Merow, Smith, & Silander, 2013; Syfert et al., 2013). A minimum of 15 presence points per species was used (Pearson, Raxworthy, Nakamura, & Peterson, 2007), limiting MaxEnt predictions to four species for the social media dataset (fin and sperm whales, bottlenose and striped dolphins) and five species for the research dataset (the aforementioned four and Cuvier's beaked whale).
The descriptive power of each model was assessed by calculating the area under the receiver operating characteristic curve (AUC; Thorne et al., 2012). This metric determines model discriminatory power by comparing model sensitivity (i.e. true positives) against model specificity (i.e. false positives). The AUC values range from 0 to 1; when AUC value is 0.5, it means that model predictions are not better than random; values below 0.5 are worse than random, and higher values denote improving precision. The relative contribution of individual environmental variables to each of the resulting models was estimated by jackknife analysis, which then measured the percentage contribution and permutation importance for each variable (Baldwin, 2009). Jackknife analysis included creating a model excluding one variable, followed by the generation of a model using only the omitted individual variable (Moura, Sillero, & Rodrigues, 2012), thus providing an indication of how well the model performed when an environmental variable was omitted and additionally how each variable contributed to the model individually (Bombosh et al., 2014).
Using ArcGIS, spatial prediction maps of habitat suitability based on MaxEnt outputs were generated, which depict habitat suitability across the region investigated with values ranging from 0 (very unsuitable habitats) to 1 (very suitable habitats).
3 RESULTS
3.1 Social media (citizens') dataset
The searches in social media platforms produced 557 cetacean group encounters (annual mean = 60 ± SD 24 groups), with 410 unique videos (220 in YT and 190 in FB) and 147 records of FB photographs. The duration of all videos totalled 12 hr 38 min, with an average of 2 min 20 s and 1 min 16 s per footage in YT and FB respectively. The number of records found in YT and FB was different over the years, with a clear increasing trend in FB (see Supporting Information, Figure S1).
Species were positively identified in 92.5% (n = 515) of the social media records. It was not possible to recognize the species in 4% of YT videos (n = 9), in 8% of FB videos (n = 15), and in 12% of FB pictures (n = 18), but species were accurately determined in the 40 sightings collected by citizens and directly reported to researchers. The list of the observed species and their occurrence by year and season for all citizen accounts (n = 597; 557 found in social media platforms and 40 collected as direct reporting) is presented in Table 2.
| Social media/citizens' dataset | Research dataset | Strandings dataset | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ‘08 | ‘09 | ‘10 | ‘11 | ‘12 | ‘13 | ‘14 | ‘15 | ‘16 | ‘17 | O | ‘08 | ‘09 | ‘10 | ‘11 | ‘12 | ‘13 | ‘14 | ‘15 | ‘16 | ‘17 | O | ‘08 | ‘09 | ‘10 | ‘11 | ‘12 | ‘13 | ‘14 | ‘15 | ‘16 | ‘17 | O | ||
| Fin whale | Sp | — | — | 1 | — | — | — | 2 | — | 1 | — | 4 | — | 4 | 12 | — | — | — | 5 | 1 | — | — | 22 | — | — | — | — | — | — | — | — | — | — | — |
| Su | 1 | 2 | — | 3 | 1 | — | 1 | — | 1 | — | 9 | 5 | 2 | 13 | 5 | — | 3 | 2 | — | — | 1 | 31 | — | — | — | — | — | 1 | — | — | — | — | 1 | |
| A | — | — | — | — | — | 1 | — | — | — | 3 | 4 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | |
| W | — | — | — | 1 | — | — | — | — | — | — | 1 | — | — | — | — | — | — | 2 | — | — | — | 2 | 1 | — | — | — | — | — | — | — | — | — | 1 | |
| O | 1 | 2 | 1 | 4 | 1 | 1 | 3 | — | 2 | 3 | 18 | 5 | 6 | 25 | 5 | — | 3 | 9 | 1 | — | 1 | 55 | 1 | — | — | — | — | 1 | — | — | — | — | 2 | |
| Sperm whale | Sp | — | — | 1 | — | — | — | — | 2 | — | — | 3 | — | 1 | — | — | — | 1 | — | — | — | — | 2 | 1 | — | — | — | — | — | — | — | — | — | 1 |
| Su | — | — | — | — | 1 | 1 | — | 1 | 1 | 6 | 10 | — | — | 4 | 1 | — | — | 1 | — | — | — | 6 | — | — | — | — | — | — | — | — | 2 | — | 2 | |
| A | — | 1 | — | — | — | — | — | — | — | — | 1 | — | — | 2 | — | — | — | — | — | 1 | — | 3 | — | — | — | — | — | — | — | — | — | — | — | |
| W | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 1 | — | — | — | — | — | — | — | 1 | |
| O | — | 1 | 1 | — | 1 | 1 | — | 3 | 1 | 6 | 14 | — | 1 | 6 | 1 | — | 1 | 1 | — | 1 | — | 11 | 1 | — | 1 | — | — | — | — | — | 2 | — | 4 | |
| Cuvier's beaked whale | Sp | — | — | — | — | — | — | — | — | — | — | — | — | 2 | — | 3 | — | — | — | — | — | — | 5 | — | — | — | — | — | — | — | — | — | — | — |
| Su | — | — | — | — | — | — | — | — | — | — | — | 2 | — | 3 | 3 | — | — | 1 | — | 1 | — | 10 | — | — | — | — | — | — | — | — | — | — | — | |
| A | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | |
| W | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | |
| O | — | — | — | — | — | — | — | — | — | — | — | 2 | 2 | 3 | 6 | — | — | 1 | — | 1 | — | 15 | — | — | — | — | — | — | — | — | — | — | — | |
| Killer whale | Sp | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Su | — | — | — | — | — | — | — | — | 1 | — | 1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | |
| A | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | |
| W | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | |
| O | — | — | — | — | — | — | — | — | 1 | — | 1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | |
| Risso's dolphin | Sp | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Su | 1 | — | 1 | — | — | 1 | — | — | — | — | 3 | — | — | 1 | — | — | — | — | — | — | — | 1 | — | — | — | — | — | — | — | — | — | — | — | |
| A | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | |
| W | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 1 | — | — | — | — | 1 | |
| O | 1 | — | 1 | — | — | 1 | — | — | — | — | 3 | — | — | 1 | — | — | — | — | — | — | — | 1 | — | — | — | — | — | 1 | — | — | — | — | 1 | |
| Rough—toothed dolphin | Sp | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Su | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 1 | — | — | — | — | — | — | 1 | — | — | — | — | — | — | — | — | — | — | — | |
| A | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | |
| W | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | |
| O | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 1 | — | — | — | — | — | — | 1 | — | — | — | — | — | — | — | — | — | — | — | |
| Bottlenose dolphin | Sp | 2 | 4 | 9 | 5 | 9 | 10 | 11 | 17 | 16 | 13 | 96 | — | 1 | — | 3 | 4 | 1 | 3 | 2 | 1 | — | 15 | — | — | 1 | 1 | — | 2 | — | — | 3 | 3 | 10 |
| Su | 3 | 8 | 9 | 14 | 7 | 23 | 15 | 27 | 42 | 29 | 177 | 2 | 5 | 6 | 13 | 9 | 14 | 2 | 5 | — | 1 | 57 | 1 | — | — | 3 | — | — | 1 | 3 | 1 | 2 | 11 | |
| A | 3 | 2 | 8 | 7 | 3 | 11 | 6 | 10 | 4 | 16 | 70 | — | — | — | 1 | 6 | 4 | 3 | 3 | — | 2 | 19 | — | — | — | — | 1 | — | — | — | 3 | — | 4 | |
| W | 2 | 2 | 7 | 6 | 3 | 4 | 7 | 4 | 9 | 5 | 49 | — | — | — | — | 3 | 3 | — | — | — | — | 6 | — | — | 1 | — | — | 2 | 1 | 1 | 2 | — | 7 | |
| O | 10 | 16 | 33 | 32 | 22 | 48 | 39 | 58 | 71 | 63 | 392 | 2 | 6 | 6 | 17 | 22 | 22 | 8 | 10 | 1 | 3 | 97 | 1 | — | 2 | 4 | 1 | 4 | 2 | 4 | 9 | 5 | 32 | |
| Common dolphin | Sp | — | — | 1 | — | — | 1 | — | — | — | — | 2 | — | — | — | — | — | — | 2 | — | — | — | 2 | — | — | — | — | — | — | — | — | — | — | — |
| Su | — | 1 | — | — | — | 1 | 1 | — | — | — | 3 | 2 | — | — | 4 | — | — | — | — | — | — | 6 | — | — | — | — | — | — | — | — | — | — | — | |
| A | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | |
| W | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | |
| O | — | 1 | 1 | — | — | 2 | 1 | — | — | — | 5 | 2 | — | — | 4 | — | — | 2 | — | — | — | 8 | — | — | — | — | — | — | — | — | — | — | — | |
| Striped dolphin | Sp | 2 | 3 | 4 | 1 | 11 | 4 | — | 2 | 5 | 5 | 37 | — | — | 10 | 2 | 1 | 5 | 5 | 3 | — | — | 26 | 2 | 1 | 2 | 1 | 3 | 1 | 3 | 1 | — | 1 | 15 |
| Su | 2 | 5 | 9 | 9 | 7 | 8 | 3 | 4 | 6 | 10 | 63 | 7 | 5 | 38 | 25 | — | 10 | 6 | 7 | — | — | 98 | — | 1 | 2 | 1 | — | 1 | — | — | 1 | 4 | 10 | |
| A | 3 | 4 | — | 3 | 1 | 2 | 1 | 1 | 1 | 2 | 18 | — | — | 2 | — | — | 4 | 4 | — | — | 1 | 11 | 1 | 3 | 2 | — | 3 | 1 | 1 | 1 | 2 | 5 | 19 | |
| W | — | 1 | — | 1 | 2 | — | — | 1 | — | 1 | 6 | — | — | — | — | — | 1 | 7 | 2 | — | — | 10 | 5 | 3 | 4 | 1 | 6 | 36 | 1 | 1 | 9 | 8 | 74 | |
| O | 7 | 13 | 13 | 14 | 21 | 14 | 4 | 8 | 12 | 18 | 124 | 7 | 5 | 50 | 27 | 1 | 20 | 22 | 12 | — | 1 | 145 | 8 | 8 | 10 | 3 | 12 | 39 | 5 | 3 | 12 | 18 | 118 | |
| Undetermined dolphins | Sp | — | — | 2 | — | 1 | 2 | 1 | 1 | 2 | 3 | 12 | — | 4 | 4 | 4 | 1 | 1 | 2 | 1 | 5 | — | 22 | — | — | 1 | — | 2 | — | — | — | 3 | 3 | 9 |
| Su | 1 | 1 | — | 2 | 3 | 2 | — | 3 | 5 | 3 | 20 | 2 | 8 | 38 | 20 | — | 5 | 1 | 1 | — | — | 75 | — | — | — | 1 | — | 1 | — | — | 1 | 1 | 4 | |
| A | — | 1 | — | 2 | — | 2 | — | — | — | — | 5 | — | — | 4 | — | 21 | 1 | — | 1 | — | 1 | 28 | 1 | 1 | 1 | — | 2 | 1 | — | — | — | 3 | 9 | |
| W | — | — | — | — | — | 1 | 1 | 2 | — | 1 | 5 | — | — | — | — | — | 3 | 4 | 1 | 1 | — | 9 | — | — | — | — | — | 6 | 4 | — | 1 | 1 | 12 | |
| O | 1 | 2 | 2 | 4 | 4 | 7 | 2 | 6 | 7 | 7 | 42 | 2 | 12 | 46 | 24 | 22 | 10 | 7 | 4 | 6 | 1 | 134 | 1 | 1 | 2 | 1 | 4 | 8 | 4 | — | 5 | 8 | 34 | |
- A: autumn; O: overall; Sp: spring; Su: summer; W: winter.
Two species of dolphins were encountered regularly: 65.6% of sightings were of bottlenose dolphin Tursiops truncatus (n = 392) and 20.6% were striped dolphins Stenella coeruleoalba (n = 124). Other species encountered were 3% fin whales Balaenoptera physalus (n = 18), 2.3% sperm whales Physeter macrocephalus (n = 14), 0.8% common dolphins Delphinus delphis (n = 5), 0.5% Risso's dolphins Grampus griseus (n = 3), and 0.2% killer whale Orcinus orca (n = 1). In 7% of the observations (n = 42) it was not possible to determine the species. Citizens' sightings have been principally reported in the summer (47.7%), followed by spring (25.9%), autumn (16.4%), and winter (10.2%). Three species were sighted in all seasons (bottlenose dolphin, striped dolphin, and fin whale).
Bottlenose and striped dolphin groups were frequently observed with immature individuals (37.5% of sightings, n = 147 and 27% of sightings, n = 34, respectively) all year round (at least one group with immatures per month). Immatures were principally observed in groups of bottlenose dolphin in summer (40% of the seasonal sightings; n = 71) and autumn (50% of the seasonal sightings; n = 35), from August to October; however, they were also spotted in 33% of the winter encounters (n = 16) and 26% of the spring ones (n = 25). Likewise, immature striped dolphins were spotted in groups during the summer (30% of the seasonal sightings; n = 19), but they were also observed in 33% (n = 2), 24% (n = 9), and 22% (n = 4) of the winter, spring, and autumn encounters respectively.
Records derived from social media also provided information on spinal deformities, mutilations, and nurturant behaviour in the bottlenose dolphin (see Supporting Information, Figure S2).
3.2 Research dataset
Research surveys (vessel n = 122, ferry n = 326, and aerial n = 37 based) accounted for a mean of 49 ± SD 38 annual cetacean group encounters. Differences in the mean annual number of sightings between social media and research datasets were not statistically significant (two-way ANOVA: F = 0.48, df = 19, P > 0.5).
Nine species (Table 2) were recorded, of which 33.4% of sightings were of striped dolphins (n = 162), 20% bottlenose dolphin (n = 97), 11.3% fin whales (n = 55), 3.1% Cuvier's beaked whales Ziphius cavirostris (n = 15), 2.5% sperm whales (n = 12), 1.6% common dolphins (n = 8), 0.2% Risso's dolphins (n = 1), and 0.2% rough-toothed dolphin Steno bredanensis (n = 1); 27.6% records were undetermined species (n = 134). Four sightings were mixed groups of striped and common dolphins, one of bottlenose and striped dolphins, and one of striped dolphin and sperm whale.
Encounters were principally collected in the summer (59.4%), followed by spring (20.8%), autumn (14%), and winter (5.8%). Two species were observed in all seasons (bottlenose and striped dolphin).
Very little and scattered evidence of the presence of immature individuals was available in this dataset, so this information is not presented here.
3.3 Stranding dataset
One hundred and ninety-one stranded animals in the study area were recorded between 2008 and 2017 (Table 2). The species was determined in 156 cases (82%). Five different species were represented in the dataset, of which ~78.5% comprised two species (striped and bottlenose dolphins). The stranding events mainly occurred in winter (45%) and were located all along the ~500 km long coast of the study area (Figure 2).
3.4 Group size
Only species with more than one sighting were included. Differences in overall mean group size between social media and research datasets were statistically significant for fin whale and striped and common dolphins (Table 3).
| Social media dataset | Research dataset | P (between datasets) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | SD | Range | n | Mean | Median | SD | Range | n | |||
| Fin whale | Spring | 1.50 | 1 | 1 | 1–3 | 4 | 1.22 | 1 | 0.53 | 1–3 | 22 | |
| Summer | 1.78 | 1 | 1.20 | 1–4 | 9 | 1.22 | 1 | 0.49 | 1–3 | 31 | ||
| Autumn | 2.25 | 2 | 1.50 | 1–4 | 4 | 1 | 1 | 0 | 1 | 2 | ||
| Winter | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | ||
| Overall | 1.77 | 1 | 1.16 | 1–3 | 18 | 1.21 | 1 | 0.50 | 1–3 | 55 | <0.05 | |
| Sperm whale | Spring | 1 | 1 | 0 | 1 | 3 | 1 | 1 | 0 | 1 | 2 | |
| Summer | 1.90 | 1 | 2.51 | 1–9 | 10 | 2.43 | 1 | 2.57 | 1–8 | 7 | ||
| Autumn | 1 | 1 | 0 | 1 | 1 | 2.00 | 1 | 1.41 | 1–3 | 2 | ||
| Winter | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Overall | 1.65 | 1 | 2.13 | 1–9 | 14 | 2.20 | 1 | 4.04 | 1–8 | 11 | 0.774 | |
| Cuvier's beaked whale | Spring | 0 | 0 | 0 | 0 | 0 | 1.80 | 0.83 | 1.40 | 1–3 | 5 | |
| Summer | 0 | 0 | 0 | 0 | 0 | 1.40 | 2 | 1 | 1–3 | 10 | ||
| Autumn | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Winter | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Overall | 0 | 0 | 0 | 0 | 0 | 1.53 | 1 | 0.74 | 1–3 | 15 | n.a. | |
| Risso's dolphin | Spring | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Summer | 4.33 | 5 | 3.05 | 1–7 | 3 | 0 | 0 | 0 | 0 | 0 | ||
| Autumn | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Winter | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Overall | 4.33 | 5 | 3.05 | 1–7 | 3 | 0 | 0 | 0 | 0 | 0 | n.a. | |
| Bottlenose dolphin | Spring | 5.61 | 5 | 4.69 | 1–28 | 96 | 7.30 | 7 | 6.13 | 1–20 | 13 | |
| Summer | 7.05 | 6 | 5.25 | 1–30 | 177 | 6.85 | 5 | 5.18 | 1–20 | 54 | ||
| Autumn | 8.32 | 7 | 5.58 | 1–25 | 70 | 9.00 | 7 | 7.06 | 1–20 | 18 | ||
| Winter | 6.27 | 6 | 4.48 | 1–17 | 49 | 2.20 | 1 | 2.17 | 1–6 | 6 | ||
| Overall | 6.83 | 6 | 5.14 | 1–30 | 392 | 7.06 | 6 | 5.71 | 1–20 | 91 | 0.1532 | |
| Common dolphin | Spring | 3.50 | 3.5 | 0.70 | 3–4 | 2 | 12.00 | 12 | 5.65 | 8–16 | 2 | |
| Summer | 5.33 | 5 | 2.51 | 3–8 | 3 | 9.66 | 10.5 | 5.35 | 1–17 | 6 | ||
| Autumn | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Winter | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Overall | 4.60 | 4 | 2.07 | 3–8 | 5 | 10.00 | 10 | 5.07 | 1–17 | 8 | <0.05 | |
| Striped dolphin | Spring | 5.65 | 4 | 4.55 | 2–21 | 37 | 18.62 | 4.5 | 6.62 | 1–120 | 26 | |
| Summer | 7.61 | 6 | 4.94 | 1–22 | 63 | 10.74 | 6 | 1.67 | 1–100 | 98 | ||
| Autumn | 5.89 | 4.5 | 4.30 | 2–20 | 18 | 5.91 | 4 | 1.60 | 1–20 | 11 | ||
| Winter | 4.83 | 5.5 | 2.40 | 1–7 | 6 | 3.30 | 2 | 0.86 | 1–10 | 10 | ||
| Overall | 6.62 | 5 | 4.70 | 1–22 | 124 | 11.27 | 5 | 20 | 1–120 | 145 | <0.001 | |
In the social media dataset, there was evidence of a seasonal difference in group size for fin whale (Poisson GLM; P < 0.05), with significantly higher group sizes in autumn than in spring, and for sperm whale (Poisson GLM; P < 0.05), with significantly higher values in summer than in spring (Table 3).
As for seasonal differences in the research dataset, significantly lower average group sizes were recorded in bottlenose dolphin (Poisson GLM; P < 0.05) during winter than in other seasons, and in striped dolphin (negative binomial GLM; P < 0.001) during winter compared with spring and summer (Table 3).
3.5 Spatial relative density
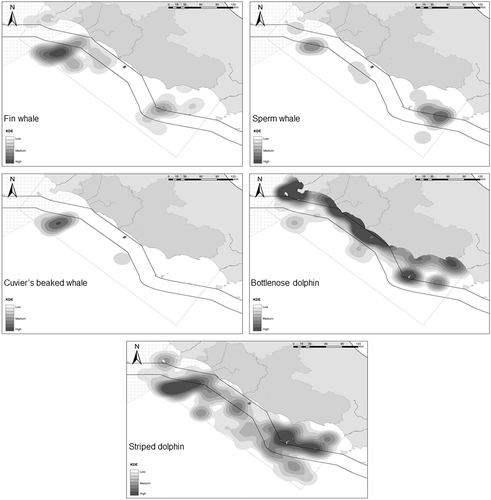
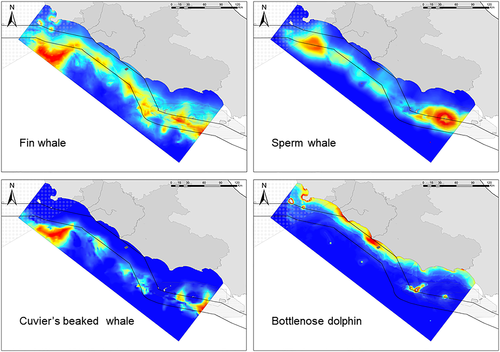
KDEs were calculated for the species with at least 15 records using the combined dataset (research and social media; Figure 3), whereas records of the rarer species were just plotted on a map (Figure 4). Overall, fin whales were more spatially concentrated in a wide northern area, with the highest relative density in the pelagic waters just outside of the Italian territorial seas, and a medium relative density zone was located in the south, around Ponza island (Figure 3). Sperm whale records were mainly grouped off the coasts of the island of Ventotene, and a medium relative density zone was recorded in the north in an area partially overlapping fin whale distribution (Figure 3). Cuvier's beaked whales were sighted infrequently in the study area but were concentrated principally in the north in the same region as fin whales and, to a certain extent, sperm whales (Figure 3). Bottlenose dolphin sightings were clustered more evenly in coastal areas, including Giglio and Ponza islands. Striped dolphin records were unevenly distributed across the study area and did not show any patterns, and the ‘hotspots’ were most likely driven by the methodology, as a higher number of coastal records were likely related to the number of sightings from citizens rather than reliable higher relative densities. This fact has motivated and supported the choice of not modelling habitat suitability for this species.
3.6 Habitat suitability
To determine the MaxEnt distributions, after the removal of duplicates, a total of 163 presence records out of 486 for the research dataset, 383 presence records out of 597 for the social media dataset, and 533 points out of 1,086 for the combined dataset were used.
All MaxEnt models obtained AUC ≥ 0.77 (Table 4), which suggests very good predictive power of the fitted model compared with the value (0.5) expected from a random prediction (Lobo, Jiménez-Valverde, & Real, 2008).
| Research dataset | Social media dataset | Combined dataset | |
|---|---|---|---|
| Balaenoptera physalus | 0.936 | 0.770 | 0.830 |
| Physeter macrocephalus | 0.858 | 0.892 | 0.923 |
| Ziphius cavirostris | 0.975 | — | — |
| Tursiops truncatus | 0.943 | 0.905 | 0.922 |
The relative contribution of environmental variables based on both the numerical measures of variable importance (percentage contribution and permutation importance) and the graphical results of jackknife tests is shown in Table 5. Overall, ecological relationships between species presence and environmental conditions showed different predictions across the datasets. In fin whale, the distance from the nearest coast had the greatest explanatory power for both research and combined datasets, and slope (permutation importance) and distance from the river estuaries (percentage of contribution) was greatest for the social media dataset. Models built on the combined dataset demonstrated that fin whales preferred relatively greater distances from the coast in the north and shorter distances in the south (Pontine Archipelago) of the study area with a depth range of 200–1,000 m and some topographic complexity (top of seamounts). Distance from the nearest coast emerged as a relevant variable for sperm whales as well. It had the greatest explanatory power for both social media and combined datasets and for distance from the seamounts for the research dataset. Models developed using the combined dataset revealed that the highest logistic probability for finding sperm whales was at relatively greater distances from the coast in the north and shorter distances in the south (Pontine Archipelago) of the study area at around 800 m in depth. Depth best predicted encounters with Cuvier's beaked whales (research dataset only), with a high probability of prediction at 800–1,000 m depth far from the coast. For bottlenose dolphin, depth emerged as the variable with the greatest explanatory power for both social media and combined datasets combined with distance from estuaries for the research dataset. Overall (combined dataset), bottlenose dolphins favoured shallow waters less than 100 m deep near to the coast.
| Species | Variable | Research dataset | Social media dataset | Combined datasets | |||
|---|---|---|---|---|---|---|---|
| Contribution (%) | Permutation importance | Contribution (%) | Permutation importance | Contribution (%) | Permutation importance | ||
| Balaenoptera physalus | Distance from coast | 47.11 | 52.77 | 12.57 | 28.81 | 41.03 | 44.08 |
| Distance from rivers | 1.08 | 6.49 | 45.23 | 5.27 | 0.70 | 4.59 | |
| Distance from harbours | 8.30 | 23.49 | 31.70 | 19.02 | 10.26 | 12.32 | |
| Distance from seamounts | 21.41 | 13.92 | 0.00 | 0.00 | 18.05 | 16.47 | |
| Depth | 16.05 | 1.94 | 0.00 | 0.00 | 20.20 | 22.33 | |
| Slope | 6.06 | 1.39 | 10.51 | 46.90 | 9.76 | 0.22 | |
| Physeter macrocephalus | Distance from coast | 17.63 | 19.11 | 39.35 | 41.29 | 32.13 | 51.54 |
| Distance from rivers | 0.00 | 0.00 | 14.48 | 15.31 | 11.17 | 16.05 | |
| Distance from harbours | 0.00 | 0.00 | 11.88 | 0.00 | 16.93 | 15.24 | |
| Distance from seamounts | 52.05 | 55.98 | 4.59 | 5.77 | 11.26 | 9.04 | |
| Depth | 23.80 | 13.58 | 29.17 | 37.51 | 28.26 | 7.74 | |
| Slope | 6.53 | 11.33 | 0.52 | 0.12 | 0.25 | 0.39 | |
| Ziphius cavirostris | Distance from coast | 27.02 | 23.50 | ||||
| Distance from rivers | 0.00 | 0.00 | |||||
| Distance from harbours | 4.08 | 14.60 | |||||
| Distance from seamounts | 6.90 | 2.56 | |||||
| Depth | 55.97 | 45.79 | |||||
| Slope | 6.03 | 13.55 | |||||
| Tursiops truncatus | Distance from coast | 8.26 | 3.11 | 8.64 | 5.06 | 9.86 | 10.50 |
| Distance from rivers | 48.38 | 42.74 | 6.29 | 4.55 | 4.99 | 1.95 | |
| Distance from harbours | 14.61 | 18.74 | 2.79 | 4.35 | 2.60 | 1.42 | |
| Distance from seamounts | 8.67 | 16.50 | 6.67 | 3.70 | 6.48 | 5.49 | |
| Depth | 18.12 | 17.26 | 71.65 | 78.93 | 74.75 | 79.31 | |
| Slope | 1.95 | 1.66 | 3.96 | 3.41 | 1.32 | 1.34 | |
An extended area in the northern part of the study region outside Italian territorial waters was highly suitable for fin whale, as well as a smaller area between Ponza and Ventotene islands and south-east Ventotene with a predicted wide-ranging pattern of suitability in the area between these two areas (Figure 5). A less evident but similar pattern emerged for sperm whale. The best predicted conditions were in the waters surrounding Ventotene island and between Ponza and Ventotene and in a northern zone bordering the highly suitable area for fin whales. This area in the northern part of the study area emerged as extremely suitable for Cuvier's beaked whales as well, showing a major overlap with fin whales and, to a lesser extent, with sperm whales (Figure 5). Another area for beaked whales emerged in the south far from Ventotene island. The areas of highest suitability for bottlenose dolphin were principally located near estuaries (Tiber in particular) and in the waters surrounding all the islands (Figure 5).
4 DISCUSSION
Social media is becoming a rich source of data on species occurrence and, therefore, is a new and promising way to assess cetacean distribution, seasonality, or habitat use, particularly in unknown or scarcely investigated areas. The collection of sightings from citizens is a useful first activity for researchers entering a new study region to identify where to focus future intensive research (Alessi, Bruccoleri, & Cafaro, 2019), but the combination of research data and social media information to study cetaceans in a pilot area, like the one presented here, may add value to this new approach.
The proposed scheme uses two global data sources, namely FB and YT, which are freely accessible and available online, making the method attractive for large-scale assessments. With increasing requests to study whale and dolphin population ecology coming from national and international directives, support from citizens to aid research may act as a practical, inexpensive solution to gathering extensive spatial and temporal data for regional-scale population monitoring and for the development of management priorities (Braulik et al., 2018; Hann et al., 2018). The information obtained from social media includes potential limitations, biases, and errors that can reduce its scientific benefits, and these need to be accounted for when utilizing such a methodology. For example, a lack of spatial and temporal effort data complicated the analysis of space use patterns to some extent, and encounters are likely to be skewed in favour of more widely distributed species.
The area in the Tyrrhenian Sea investigated here was used as a case study to test an integrated approach using research and social media data to provide the first assessment on cetacean species in a sea sector where survey effort and scientific information are mostly scattered. Nevertheless, few or no data are available from both sources for some portions of the region yet, further highlighting the need to investigate gap areas to confirm the inferences of this study.
As far as the accuracy and quality of the reported observations by citizens is concerned, the analysis showed that most cetacean records are reliable, with particular regard to more coastal presences, distribution, and species identification. Experts recorded similar observations, which complemented information in more pelagic waters. A further data check can be made by comparing records with independent observations of stranding events, despite the possibility of carcasses ending up stranding at a point distant from where the animal died, making inferences problematic. Regardless, stranding records often compare well with sightings records (Maldini, Mazzuca, & Atkinson, 2005; Peltier et al., 2012), and results obtained here in terms of species diversity and relative abundance seem to confirm this model.
Possible biases in the social media dataset have to be considered, too. For example, most of the citizens' accounts found in social media platforms were principally associated with human-populated coastal areas, travel routes, and common holiday destinations (e.g. Ponza, Ventotene, and Giglio islands), with a degree of variability connected to the presence of harbours and to seasons (summer is more suitable for recreational activities at sea, thus increasing the potential for cetacean encounters). Moreover, the difference found between research and social media datasets for fin whales and striped and common dolphins mean group size may be due to the fact that videos and pictures could represent only a small part of the observed groups, resulting in an incorrect group size estimation. Additionally, the more coastal nature of the citizens' observations may reflect the habit of striped and common dolphins to form smaller groups than in pelagic areas, and the opportunistic aggregation of fin whales to exploit more coastal, ephemeral food patches (Notarbartolo di Sciara, Castellote, Druon, & Panigada, 2016). The proximity to the coast of the citizens' data and the ecological characteristics of the striped dolphin, such as the plastic and opportunistic behaviour and the absence of a clear preference for any specific physiographic features with an almost homogeneous distribution over different habitats (Arcangeli et al., 2012; Arcangeli, Campana, & Bologna, 2017; Azzellino, Gaspari, Airoldi, & Nani, 2008), could have also affected the relative density estimates, leading to unreliable preconditions for the MaxEnt modelling exercise.
In any case, choosing a consistent analysis method with the appropriate adjustments, such as MaxEnt in this case, could be helpful to minimize the differences in the combination of research, social media, and stranding records, allowing sound information on cetacean relative abundance, distribution, and habitat selection to be analysed.
In the following subsections, the results for each single species are discussed in light of evaluating the reliability of the approach applied here.
4.1 Fin whales
Several studies have demonstrated the association of fin whales with a range of physical aspects of the marine environment, including depth, bottom slope, and distance from shore, and using them as presence indicators or predictors (Arcangeli et al., 2012; Azzellino et al., 2008; Cañadas, Sagarminaga, & Garcia-Tiscar, 2002; Hastie, Swift, Slesser, Thompson, & Turrell, 2005; Ingram, Walshe, Johnston, & Rogan, 2007; MacLeod, Weir, Pierpoint, & Harland, 2007; Ready et al., 2010; Redfern et al., 2006; Yen, Sydeman, & Hyrenbach, 2004). In this study, distance from the nearest coast was the environmental variable with the greatest explanatory power for fin whales, with a preference for relatively greater distances from the coast in the north and shorter in the south (Pontine Archipelago) with a depth range of 200–1,000 m and some topographic complexity (top of seamounts). Fin whales in the Mediterranean are most common in deep waters (400–2,500 m), but they can occur in slope and shelf waters as well, depending on the distribution of their prey (Gannier, Drouot, & Goold, 2002; Laran & Gannier, 2008; Notarbartolo di Sciara, Zanardelli, Jahoda, Panigada, & Airoldi, 2003; Pace, Miragliuolo, & Mussi, 2012; Panigada, Notarbartolo di Sciara, & Zanardelli, 2006; Panigada et al., 2017). Fin whale summer distribution and its interannual variability are closely linked to spatial and temporal interactions with zooplankton concentrations, demonstrating large-scale fidelity corresponding to the prey spatial and temporal predictable distribution, and mesoscale fidelity with higher density in the areas where northern krill (Meganyctiphanes norvegica) tend to concentrate (Cotté, Guinet, Taupier-Letage, Mate, & Petiau, 2009; Littaye, Gannier, Laran, & Wilson, 2004). Previous studies reported the presence of the species in the study area since the beginning of the 1990s, with an increased occurrence after 20 years (Arcangeli et al., 2012; Arcangeli et al., 2014). It was recently suggested that the central Tyrrhenian Sea is an opportunistic feeding ground while transiting to the Ligurian Sea in summer (Arcangeli et al., 2014; Panigada et al., 2017; Santoro et al., 2015), with a marked change in the use of the area from the early 1990s, when it was primarily considered as a transit zone (Marini et al., 1996; Nascetti & Notarbartolo di Sciara, 1996). More fin whales have been observed spending longer periods in the central Tyrrhenian Sea for feeding purposes instead of just moving through this area, possibly due to the presence of two gyres (Barale, Jaquet, & Ndiaye, 2008; Vetrano, Napolitano, Iacono, Schroeder, & Gasparini, 2010), which enhance the productivity in the region. One of these two cyclonic eddies is located close to the most suitable area for fin whales recognized in this study, reinforcing the prediction of local favourable conditions for the species' occurrence. A second hotspot of fin whale relative abundance was highlighted here in the waters surrounding the Pontine Archipelago, and some other zones between the two were also suitable, defining other potential regions of importance for the species and depicting a sort of ‘corridor’ between the most suitable areas in the north and in the south. Fin whales are considered to be nomadic opportunists that travel between highly concentrated feeding areas (Notarbartolo di Sciara et al., 2016), and these results seem to be in accordance with this model. It is worth noting that even the two stranding records of fin whales correspond with the area where higher densities were found.
4.2 Sperm whales
Distance from the nearest coast emerged as a relevant environmental variable for sperm whales, as well as the distance from the seamounts. Higher relative abundance was found at 800 m depth in the waters surrounding Ventotene island, between Ponza and Ventotene, and in a northern zone bordering the highly suitable area for fin whales. This seems consistent with previous studies reporting that sperm whales select for two main habitats: one where complex topographical features (escarpments, canyons, seamounts) characterize the sea floor and one where downwelling or upwelling water movements are associated with frontal zones in higher seas (Aïssi, Fiori, & Alessi, 2012; Arcangeli et al., 2017; Carpinelli et al., 2014; Fiori, Giancardo, Aïssi, Alessi, & Vassallo, 2014; Gannier & Praca, 2007; Mussi, Miragliuolo, Zucchini, & Pace, 2014; Pace, 2016; Pace et al., 2018). In this way, sperm whales would be able to capitalize on the food resources (cephalopods) available in both areas by shifting between different prey targets (Gannier et al., 2002). The lower sperm whale relative abundance in the northern part of the study area than in the southern part (Pontine Archipelago) also seems to be consistent with recent published results showing variable encounters in the north (Arcangeli et al., 2017) and regular occurrences near Ponza and Ventotene islands (Mussi et al., 2014; Pace, 2016; Pace et al., 2018; Pace, Miragliuolo, Mariani, Vivaldi, & Mussi, 2014). This is also in line with sperm whale strandings, all located in the southern part of the area where deep waters are closer to the coast.
4.3 Bottlenose dolphins
Bottlenose dolphin was the most frequently recorded species in the study area. Depth and distance from the river estuaries emerged as the variables with the greatest explanatory power. Overall, bottlenose dolphins favoured shallow waters about 0–100 m deep, near the shore and close to river mouths with sandy–muddy substrates and gentle slopes. This is consistent with the species' general distribution in the Mediterranean Sea (e.g. Gnone et al., 2011; Marini et al., 2015), where shallow water preference of the bottlenose dolphin could be related to its feeding habits of preying mostly on benthic and demersal fish (e.g. Blanco, Salomon, & Raga, 2001; Orsi Relini, Cappello, & Poggi, 1994). In addition, the results of this study are in accordance with the only published account on bottlenose dolphins in the study area (Cafaro et al., 2016), which reported near-shore foraging at the mouths of two river basins (Fiora and Mignone), and with preliminary, unpublished data collected in 2017–2018 by the University of Rome near the Tiber river mouths and Tor Paterno MPA. These findings are also in agreement with other studies that documented high numbers of bottlenose dolphins at the mouths of rivers in the Mediterranean, such as the Magra river in the Ligurian Sea (Alessi & Fiori, 2014), and outside the basin (e.g. the Shannon estuary on the Ireland's west coast; Ingram & Rogan, 2002). Rivers may play an important role, at a local scale, in affecting water temperature, salinity, sediment distribution, and nutrient loads, producing algal blooms and subsequent secondary production processes, which can in turn sustain species at higher trophic levels (Cafaro et al., 2016). According to the peak in immature/calf presence in August–October and to stranding data (mostly spring/summer), a significant fraction of bottlenose dolphins in the study area would dwell in coastal waters for calving and thus would mainly forage on coastal demersal and benthic prey (mainly hake) during the warmer season, whereas in the coldest season, when calves are older, they would switch towards deeper waters where they would have to forage on different species. This hypothesis seems to be supported by pilot observations of feeding bottlenose dolphin groups at the Albano seamount in autumn/winter (University of Rome and Pavia 2017–2018 visual and acoustic surveys; unpublished data), a pelagic area proposed as an attractive site for cetacean species in the Tyrrhenian Sea (Fiori et al., 2016).
The regular coastal presence of bottlenose dolphins at the Tiber river mouths poses relevant conservation issues, since the River Tiber is the most polluted river among the 20 longest rivers in Italy (Crosti, Arcangeli, Campana, Paraboschi, & González-Fernández, 2018; Legambiente, 2006), with high heavy metal concentrations (Inghilesi et al., 2012; Montuori, Aurino, Garzonio, Nardone, & Triassi, 2016), organophosphate pesticides pollution (Montuori, Aurino, Garzonio, Sarnacchiaro, et al., 2016), and solid waste reported around its two mouths.
Areas with higher relative abundance estimates and identified as suitable habitat for bottlenose dolphins are likely to be influenced by other anthropogenic factors as well. In particular, bottlenose dolphins were observed both by citizens and researchers to regularly follow trawlers or move near different fishing gears, resulting in the overlap between recreational, artisanal, and professional fishing operations and the species; this well-known interaction behaviour, already reported in numerous coastal areas in the Mediterranean Sea and worldwide, where animals are attracted towards easily accessible and concentrated food sources (Bonizzoni et al., 2014; Fertl & Leatherwood, 1997; Gonzalvo, Giovos, & Moutopoulos, 2015; Lauriano, Fortuna, Moltedo, & Notarbartolo di Sciara, 2004; Pace, Pulcini, & Triossi, 2003, 2012; Pennino, Rotta, Pierce, & Bellido, 2015; Pulcini et al., 2014), was then documented for the first time in the study area, conferring further support to the reliability of the approach applied.
Bottlenose dolphin strandings were principally recorded near the mouths of the rivers, but they also occurred over other coastal portions of the study area.
4.4 Cuvier's beaked whales
Depth best predicted encounters with Cuvier's beaked whales, with a high probability at 800–1,000 m depth, far from the coast. This coincides with a number of descriptions of the habitat of this species in the Mediterranean, which report a clear habitat preference for areas at least 1,000 m deep, and complex bottom topographies related to phenomena (upwelling, increased primary production, and aggregation of zooplankton) that could play a role for beaked whales’ main prey species (cephalopods) (Azzellino et al., 2008; Cañadas et al., 2018; Podestà et al., 2006, 2016). Several studies demonstrated the importance of the central Tyrrhenian Sea for the species, showing long-term site fidelity and changes in habitat selection over time (Arcangeli et al., 2012, 2017; Arcangeli, Campana, Marini, & MacLeod, 2016; Cañadas et al., 2018). One of the hypotheses for these changes is related to the possible negative influence of maritime traffic (Campana et al., 2015). Our findings, both in terms of relative abundance and habitat suitability predictions, seem to support this theory, as the species was never recorded in one suitable area recognized by the modelling in the south (near Ventotene island), a highly touristic region heavily affected by boat traffic. The absence of Cuvier's beaked whales in this part of the study area was also reported in a recent Mediterranean assessment (Cañadas et al., 2018). In accordance with the low density of the species, no strandings were registered in the study area.
4.5 Other species
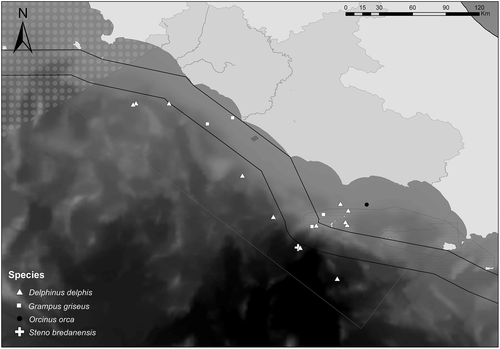
Other species were relatively rare in the study area, in line with previous research results, and confirmed by the absence of stranding records, except for one species. The common dolphin is in steep decline throughout the Mediterranean (Pace et al., 2016), occurs in few delimitated areas, and is present in low numbers in Italian waters (Arcangeli et al., 2017; Pace et al., 2015, 2016). Mixed groups of common dolphins with other cetacean species were recorded here in both social media and research datasets, reinforcing similar observations already reported in the Mediterranean (Arcangeli et al., 2017; Frantzis & Herzing, 2002; Pace et al., 2015). This kind of association generally involved a few common dolphin individuals within a group of striped dolphins, supporting the hypothesis that the relative low abundance of common dolphin restrains the possibility of forming single-species groups (Arcangeli et al., 2017; Frantzis & Herzing, 2002).
Risso's dolphin was observed three times, and only one stranding occurred over the 10-year period. On average, Risso's dolphin in the Mediterranean appears to be a low-density species, showing a preference for deep waters over steep slopes and submarine canyons (Azzellino et al., 2008, 2012, 2016; Casacci & Gannier, 2000; David & Di-Meglio, 2012; Gannier, 2005; Mariani et al., 2016; Moulins, Rosso, Ballardini, & Würtz, 2008; Pace, Miragliuolo, & Mussi, 2012; Praca & Gannier, 2008). This kind of habitat was less represented in our study area, and results seem to be in accordance with previous studies in the central Tyrrhenian Sea (Arcangeli et al., 2017). One of the regularly encountered Mediterranean species, the pilot whale (Globicephala melas), was never observed in this study. This is consistent with previous reports, with no records of the species in the central Tyrrhenian Sea since the 1990s (Arcangeli et al., 2012, 2017), and there were a few sightings of a small group south-west of Ventotene island at a depth of over 400 m until 2006 (Pace, Miragliuolo, & Mussi, 2012).
Finally, rough-toothed dolphins and killer whales were confirmed as sporadic species within the Mediterranean (they are reported as ‘visitors’; Notarbartolo di Sciara, 2016).
4.6 Management implications
This study showed how information gathered from citizens can support scientific research acting as a feasible solution to improve spatial and temporal coverage for cetacean monitoring and research, even across scattered survey areas. Producing robust baseline data on cetacean communities at a wide spatial scale is a critical first step to recognizing threats and prioritizing species/sites that may necessitate conservation actions (Pace, Mussi, Gordon, & Würtz, 2014). This kind of information is of fundamental interest for the development of management priorities (Braulik et al., 2018; Hann et al., 2018), whose aim should be to preserve the most favourable areas for cetaceans and to enable these species to be included in global and regional initiatives, such as IMMAs and MPAs. For example, the central Tyrrhenian Sea was identified as a candidate IMMA in the Mediterranean (https://www.marinemammalhabitat.org/imma-eatlas/), and is waiting for further evidence to qualify as an IMMA. In this regard, results from this study may be used in the future to reassess the status of the entire region.
This study has shown that the area has a year-round presence of cetacean species and more than half of these are reliant on the inshore habitat, which makes them more exposed to human impacts. These extensive urbanized, highly populated coasts are also recognized to provide an important and thriving economic and recreational resource, supporting activities (e.g. tourism and fisheries) that may adversely influence its ecosystems and threaten cetacean species. Thus, the baseline information generated here, at least within the extent of the area surveyed, may enable early recognition of adverse effects from human impacts on cetaceans, highlighting main sources of risks and selecting areas for protection and human activities management (spatial management measures), or more detailed research. For example, bottlenose dolphin, classified as Vulnerable on the IUCN Red List because of its decline in the Mediterranean Sea, may receive targeted attention since the species is exposed to a wide variety of threats (chemical and plastic pollution, bycatch, reduced prey availability caused by overfishing, and habitat degradation, including acoustic disturbances from noise and vessel traffic) in the coastal areas investigated.
Commission Decision (EU) 2017/848 specified that, for marine mammal species, both state and pressure indicators (bycatch, contaminants, and marine litter) should be developed to help interpret changes in the populations. However, the abundance and the conservation status of cetacean populations in the study area, as well as actual pressures and potential threats for each species, fundamental requisites for conservation, still have to be assessed. In the absence of more robust data and management frameworks, the following operational recommendations are offered to cope with cetacean species in the region: (1) Implement further, targeted studies and monitoring activities that branch from the present work. (2) Enforce specific conservation measures (sensu Habitat European Directive 92/43/CEE) to maintain or restore ‘favourable conservation status’ for the species that are included in Annexes II and IV of the Habitats Directive. (3) Actively regulate, restrict, or prevent disturbance sources that may cause temporary displacement from key habitats, disruption of the animals' natural behaviours, and stress, such as shipping and boat traffic (including nautical tourism) and regulated or unregulated whale/dolphin watching (e.g. adjustment of navigation speed and observance of safety distance to the animals). (4) Develop and implement a code of conduct/guidelines to be promoted among tour boats and nautical tourism companies as well as among the large community of recreational boaters. (5) Develop dedicated initiatives to increase local public awareness and knowledge regarding the effects of human activities on cetacean species. (6) Develop a dedicated mobile phone app (with strict criteria for the input and incorporation of photograph/video, effort time, species, and simple behaviour categories) to obtain more structured sighting data from an engaged public. Regarding point (1), coordinated research activities in the study region due to a networking effort between the universities of Rome, Tuscia, and Pavia, combined with ISPRA, are currently ongoing. In addition, the use of passive acoustic monitoring in some specific areas is taking place, adding opportunities to collect data on cetacean presence and possible noise disturbance.
This study produced valuable scientific data for the cetacean case study offshore of the Lazio region in the central Tyrrhenian Sea. Opportunistic sightings collected by citizens were important to further understand the complexities and level of importance of different areas. Using cetacean research and social media combined datasets, new and interesting occurrence information and distribution results were obtained, especially for the species with more selective habitats (bottlenose dolphins, and fin, sperm, and beaked whales). The approach used here may be opportunistically applied in other regions worldwide, given the necessary caution that must be taken when using and analysing data and interpreting results of such data.
ACKNOWLEDGEMENTS
We are extremely grateful to FB and YT users for providing us with detailed information on their cetacean encounters, to Oceancare (Sigrid Luber and Silvia Frey) for supporting CETYS and TASM projects by the University of Rome, and to Roma Natura - Tor Paterno Marine Protected Area (Cinzia Forniz and Maurizio Gubbiotti) for collaboration and assistance. We are also thankful to all the ferry companies participating in the research program and especially to Corsica-Sardinia Ferries, Grimaldi Lines, and Tirrenia, who kindly hosted the researchers on board. Statistical advice by Giovanna J. Lasinio (Dept. of Statistical Science, Sapienza University of Rome) is also gratefully acknowledged. Thanks to Andrea Belluscio, Edoardo Casoli, Claudio Cordaro, Alessandro Criscoli, Roberto Crosti, Graziano Di Giovanni, Gino and Orietta Domenici, Carlo Fortunato, Michela Podestà, Giampietro Silvestri, Massimo Del Monte and Simone Piperno (Kairos) Guido Sirolli and Giorgia Talucci (Marine Village), Pier Paolo Tomassi and Luca Pelo (Miceli Vela), Daniela Taliana, and Daniele Ventura for their help during this work. Special thanks to Maria Cristina Gambi, Caterina Lanfredi, Arianna Azzellino, Raffaella Tizzi, Francesca Triossi, Elena Papale, Tiziana Chieruzzi, and Sabina Airoldi for their constant support and encouragements. We are also extremely grateful to Pasquale Raicaldo, Eleonora De Sabata, Maria Pia Pezzali, Maddalena Jahoda, Lucio Biancatelli, Aldo Marinelli and all the Italian scientific journalist community for their daily dedication to the diffusion of news and information on cetaceans and Mediterranean Sea conservation. Indications and support for conservation strategies by Luca Marini and Elena Santini (Lazio Region) are gratefully acknowledged. We finally thank Kelsey Horvath who revised the English language and grammar.




