Upcycling of Postconsumer PET Using Serinol: Green Preparation of a New Multifunctional Initiator for PLA
Funding: The authors received no specific funding for this work.
ABSTRACT
Chemical recycling is attracting more and more interest as it can transform a plastic scrap into a high-value material. This work shows that the aminolysis of postconsumer poly(ethyleneterephthalate) (PET) with serinol, a nontoxic and biosourced hydroxyamine, leads to a novel multifunctional initiator for poly(lactic acid) (PLA). The reaction was performed at 180°C, without the need of catalysts, and resulted in a substantially complete aminolysis of PET, selectively yielding the corresponding diamide of terephthalic acid (terephthalic acid serinol bis-amide, TASBA), together with ethylene glycol as the co-product. TASBA holds four hydroxy groups and was used as multifunctional initiator in the polymerization of lactide to PLA, with 0.1%, 0.5%, and 1% w/w of TASBA with respect to L-lactide. TASBA led to a multiarm PLA, whose molecular weight and thermal and rheological properties could be tuned with the amount of initiator. The lowest amount of initiator left substantially unaffected the molecular weight and led to higher melt viscosity and to lower crystallinity. Lower molecular weight and melt viscosity were obtained by increasing the amount of TASBA. This work therefore proposes a synergy between the most important polyesters, the most diffused PET and the compostable PLA.
1 Introduction
The total amount of resins and fibers produced every year has been constantly increased since 1950 with a compound annual growth rate (CAGR) of about 2.5 times the CAGR of the global gross domestic product, and exceeded the total of 9 billion ton already in 2017 [1-3], with a global production of plastics that, in 2022 alone, reached 400.3 Mton [4]. At the end of their life, the management of these materials is problematic leading, especially in developing countries, in prevailingly landfilling or leakage with impact on the environment [1-3], in terms of pollution and effects on the climate change [5]. The impressive growth of plastics production, moreover, is expected to prevail on the efforts to mitigate plastic pollution that have been recently set up by many Countries in the World [6]. Plastic waste represents a problem also by an economical point of view: it was reported that 95% of plastic packaging material value is lost to the economy after a short first use [7]. Hence, recovery of plastic is mandatory, but plastics recovered via mixed waste collection mainly go to landfill or energy recovery and only a separate plastic collection makes recycling the best option, albeit with some intrinsic limits [8]. Plastics recycling occurs via mechanical, dissolution/precipitation and chemical routes [9-12]; up to now mechanical recycling has been prevailingly applied [13]: even if economic and relatively fast, it has revealed remarkable drawbacks such as the need of preprocessing and cleaning steps, and the thermomechanical degradation of many polymers due to multiple processing steps. It is acknowledged that, differently from mechanical recycling, the chemical recycling to monomer or to low molecular mass chemicals can allow the so-called upcycling (i.e., the conversion of low value chemicals to higher value ones) and an ideal circular economy in the polymer and plastic field, as it represents an efficient way to transform plastic waste into added-value intermediates and products with a significant reduction in the environmental impact of plastic use [14-19]. In this context, several protocols, including pyrolysis, photoreforming, gasification, bioconversion, mechanical and chemical reprocessing, have been developed to transform plastic waste in valuable products [20-24].
Thus, the upcycling of plastic can be considered as a multidisciplinary approach in which a wide variety of depolymerization methods and techniques are applied [25].
Poly(ethyleneterephthalate) (PET) is one of the most diffused polymers on the market, with a worldwide market volume in 2022 of about 22.5 Mton: it finds large application on fibers and thermoformed packaging. The global warming potential of end-life PET in landfill was estimated of about 45 kgCO2 eq. kg−1 [26]. Recycling of PET is thus increasingly performed, and it is fundamental to lower its environmental impact. It was estimated that PET bottles and fibers, produced from waste, could reduce by 60% the greenhouse gas emissions and by 85% the fossil resource scarcity reduction [27].
PET can be recycled by mechanical or chemical procedures [26-29], with mechanical processes currently being the most widely used. Unfortunately, as with all polyesters, PET partially hydrolyzes during processing due to moisture present in the environment and therefore a straight recycle is hardly achieved. This leads to the necessity of finding ways to make the molecular weight come back to the original values to limit PET downcycling during processing. Chemical recycling, on the other hand, includes hydrolysis, glycolysis, ammonolysis, and aminolysis and can allow for the production of fine chemicals to be used in many different fields [30-40].
In the last years, mathematical models have been developed to better understand the kinetics of these recycling methods, depending on the reaction conditions, such as the use of catalysts, the kind of solvents, and heating modes, aiming to better compare the depolymerization and/or degradation performances of the various methods and their suitability by the circular economy point of view [41, 42].
It was reported that, among the chemical recycling techniques, aminolysis, hydrogenolysis, and glycolysis using propylene glycol have the lowest global warming potential [26]. Aminolysis of recycled PET (R-PET) consists of its reaction with amines, leading to the formation of the corresponding diamides of terephthalic acid [43-47]. The reaction is highly versatile and can be performed in the presence or in the absence of catalyst. As the diamides can be further used as building blocks to produce new added value materials, this process represents an upcycling [40]. In particular, ethanolamine was used to prepare BHETA, a dihydroxy diamide that has been used in the synthesis of polyurethanes [48, 49], of polyesters [50], as additive in asphalt [51], as corrosion inhibitor [52], as additive in hot melt adhesives [53] or in the synthesis of plasticizers [54] (Figure 1a).
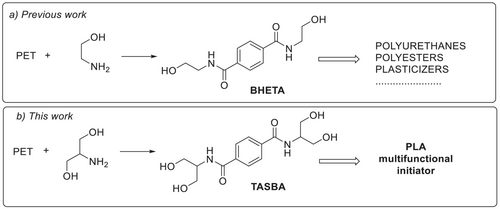
Tetrahydroxy terephthalamides were also obtained by aminolysis of PET with diethanolamine and used in coatings [55]. Very recently imidazole or substituted β-hydroxyamine have been used in the aminolysis of PET [56, 57].
Serinol (2-amino-1,3-propanediol) is a nontoxic and biodegradable amine that occurs in sugar cane. It can be prepared both from fossil-based derivatives [58] and from natural ones such as glycerol [58-60]. It is used in medicinal chemistry [61], and was used by some of the authors as a building block for the regioselective synthesis of imines and oxazolidines [62], and for the Paal–Knorr synthesis of pyrrole compounds [63, 64].
The use of serinol for the aminolysis of postconsumer PET could be of great interest for the sustainability of the entire upcycling process. Moreover, serinol has two primary hydroxyl groups, and this allows us to obtain, from the PET aminolysis, a novel molecule, a diamide containing four hydroxy groups, that can be used as a polyfunctional molecule.
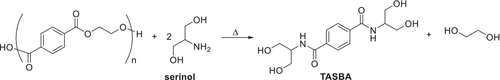
In this work, serinol was for the first time used for the aminolysis of postconsumer PET, through a green synthesis, in the absence of solvents and catalysts, to obtain a tetrahydroxy terephthalamide: terephthalic acid serinol bis-amide (TASBA). This molecule was tested as a multifunctional initiator in the synthesis of PLA. The two main steps of the research are summarized in Figure 1a,b respectively.
The use of multifunctional initiators in the ring-opening polymerization (ROP) of lactide yields star-shaped polymers that are characterized by unique thermal and rheological features [65, 66]. The properties of the resulting materials depend not only on the nature of the multifunctional initiator but also on its amount. For this reason, different loadings of TASBA were initially tested as initiators in lactide's ROP and the effects of the different quantities on the properties of the final material were investigated.
2 Results and Discussion
2.1 Depolymerization of PET With Serinol
2.1.1 Depolymerization of Bottle-PET
Depolymerization with serinol was performed by investigating the serinol/PET molar ratio, reaction temperature, and time.
2.1.1.1 Effect of the Reaction Temperature
The initial screening was performed by heating postconsumer bottle-PET flakes in serinol (Scheme 1) at different temperatures and with the same serinol/PET ratio (Table 1, entries 1–3), obtaining TASBA and ethylene glycol as co-product. Values of temperature, time, and analytical yield of TASBA in the crude, calculated versus an internal standard, are in Table 1.
| Entry | Serinol/PET molar ratio b | t (min) | T (°C) | Analyt. yield c (%) |
|---|---|---|---|---|
| 1 | 6 | 90 | 160 | 61 |
| 2 | 6 | 50 | 180 | 96 |
| 3 | 6 | 20 | 200 | 89 |
| 4 | 4 | 90 | 180 | 98 |
| 5 | 3 | 120 | 180 | 57 |
| 6 | 10 | 30 | 180 | 89 |
| 7 | 10 | 50 | 180 | 99 |
- a Reactions were performed on 250 mg of PET.
- b Amine/PET molar ratio based on the repeating unit of PET.
- c Yields were calculated using 1H NMR spectroscopy, details in Section 4 and Supporting Information.
In the experiment performed at 160°C (Table 1, entry 1), a sticky solid was obtained after 90 min. The yield was 61% and did not significantly increase for longer reaction times. Substantially quantitative yield of diamide TASBA was achieved at 180°C within 50 min (entry 2), and the final mixture was homogeneous. In the reaction performed at 200°C (entry 3), the reaction mixture was substantially homogeneous after 20 min, but it turned dark and it was stopped, achieving a lower yield compared to entry 2 in Table 1.
Reactions were then performed exploring different serinol/PET molar ratios and reaction time at 180°C. Reaction with a lower amount of serinol (Table 1, entry 4) gave substantially complete conversion after 90 min. The experiment, performed with a serinol/PET 3:1 molar ratio (entry 5) gave low yields after 2 h. It is to be noted that, in this latter case, after 50 min the reaction mixture turned solid and unreacted PET was still present after the workup. Thus, the serinol/PET ratio of 4:1 seems to be the minimum one to have an almost quantitative yield, making possible an effective mixing of PET flakes with serinol.
Reactions were also performed with a higher serinol/PET molar ratio, that is, 10:1 (Table 1, entries 6 and 7) at 180°C. A yield of about 90% and quantitative yield were obtained at 30 and 50 min as the reaction time, respectively. As the yields were substantially the same (entries 2 and 7), the use of a higher amount of serinol is not convenient.
2.1.1.2 Effect of the Reaction Time
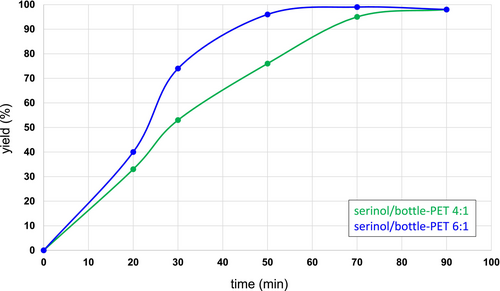
The effect of the reaction time on the depolymerization of bottle-PET flakes was thus studied at 180°C, by using serinol/PET in a molar ratio of 4:1 and 6:1. Values of molar ratio, reaction time, and analytical yield are in Table 2: the experiments of entries 1–5 are with a 4:1 molar ratio and those of entries 6–10 are with a 6:1 molar ratio. The yields of TASBA versus time are plotted in Figure 2. As expected, the formation of TASBA was faster with a higher amount of serinol. In all the experiments, in which the yield was lower than 90%, unreacted PET flakes were still present. This suggests that the formation of the diamide is very efficient with respect to the dissolution of PET.
| Entry | Serinol/PET molar ratio b | t (min) | Analyt. yield c (%) |
|---|---|---|---|
| 1 | 4 | 20 | 33 |
| 2 | 4 | 30 | 53 |
| 3 | 4 | 50 | 76 |
| 4 | 4 | 70 | 95 |
| 5 | 4 | 90 | 98 |
| 6 | 6 | 20 | 40 |
| 7 | 6 | 30 | 74 |
| 8 | 6 | 50 | 96 |
| 9 | 6 | 70 | 99 |
| 10 | 6 | 90 | 98 |
- a Reactions were performed on 250 mg of PET.
- b Amine/PET molar ratio based on the repeating unit of PET.
- c Yields were calculated using 1H NMR spectroscopy, details in Section 4 and Supporting Information.
2.1.1.3 Recyclability of Serinol
The recyclability of the excess of serinol was tested. Details of the experiments are in the Section 4. The aminolysis was carried out at 180°C for 90 min, with 4:1 as the serinol/PET molar ratio, thus reproducing the experimental conditions of entry 5 in Table 2, which allowed us to achieve an analytical quantitative yield in the crude. Three consecutive runs were performed, TASBA was recovered at the end of each run by work-up, and the isolated yields were collected for each run in Table 3.
| Entry | Run b | Moles of serinol added per mole of PET | TASBA recovered (%) |
|---|---|---|---|
| 1 | 1 | 4 | 77 |
| 2 | 2 | 2 | 70 |
| 3 | 3 | 1 | 62 |
- a Reactions were performed using 2 g of bottle-PET, at 180°C, for 90 min each run.
- b Consecutive runs.
After the first run (Table 3, entry 1), 77% of TASBA was recovered after work-up, performed as follows. The crude was refluxed in water, obtaining a clear solution, and TASBA was recovered as precipitate after cooling the solution. The aqueous solution recovered after filtration of TASBA was evaporated and fresh serinol and PET were added to the solid residue, which contained the excess of serinol, ethylene glycol and the residual TASBA. The mixture was heated to 180°C and the second run (Table 3, entry 2) was performed by using half of the amount of serinol with respect to run 1 (2:1 serinol/PET molar ratio). Differently from the first run, the residue after evaporation was liquid also at room temperature. This gave the chance to further reduce the amount of serinol (1:1 serinol/PET molar ratio) (entry 3). As shown in Table 3, the quantity on the recovered TASBA decreased, however not dramatically. After the three runs the overall ratio serinol/PET used was 2.3:1 and the overall yield was 70%. In this way, at regime, it was possible to use serinol in almost stoichiometric amount. Recycling was not further optimized.
2.1.2 Depolymerization of R-PET Powder
Depolymerization was then performed using R-PET powder in place of bottle-PET, on a scale of 10 g and 60 min as reaction time, obtaining TASBA in 70% yields with high purity (~97%); the remaining ~3% was only serinol. A second recrystallization allowed us to obtain the diamide with higher purity (> 99%). Water, recovered by distillation contains only traces of ethylene glycol and can be reused in the process, confirming that can be recycled and reused.
As serinol can be easily recycled, this experiment was performed at 180°C using a molar ratio serinol/PET of 6:1, in order to have a more efficient mixing of the mixture.
2.2 Depolymerization of PET With Serinol: Green Metrics Calculations
In order to evaluate the sustainability of the protocol, Atom Economy (AE), Process Mass Intensity (PMI), and Environmental Factor (E-factor) were calculated considering the reaction of Table 3, entry 1 [67]. AE resulted to be 83%, PMI = 12.7, and E-factor 1.3 (water used for purification was not considered as it can be recovered); considering the three consecutive runs (Table 3), the E-factor decreases to the better value of 0.6 (details about calculation in Tables S1–S4).
2.3 TASBA As Multifunctional Initiator for the ROP of Lactide
TASBA was tested as a multifunctional initiator for the ROP of L-lactide.
2.3.1 ROP
In Scheme 2, the polymerization with this novel initiator is represented.
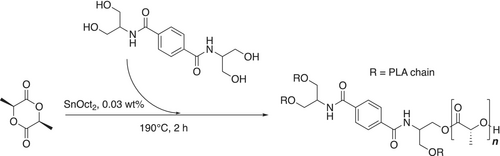
The synthesis was carried out with different amounts of diamide, namely, 0.1%, 0.5%, and 1% w/w (PLA-TASBA0.1, PLA-TASBA0.5, and PLA-TASBA1.0, respectively) with respect to L-lactide. The polymerizations were carried out in bulk, with Sn(Oct)2 as the catalyst (details in the Section 4). All samples were compared to a PLA synthesized in the same conditions without using the multifunctional initiator. The samples were characterized through differential scanning calorimetry (DSC), rheological analyses to determine the effects of the increasing amount of TASBA on the final properties of the materials, and size exclusion chromatography to determine the molecular weight.
An image of the TASBA-initiated PLA materials compared to commercial PLA is reported in Figure S4.
2.3.2 Rheological Characterization
Melt viscosity curves of the synthesized samples are shown in Figure 3.
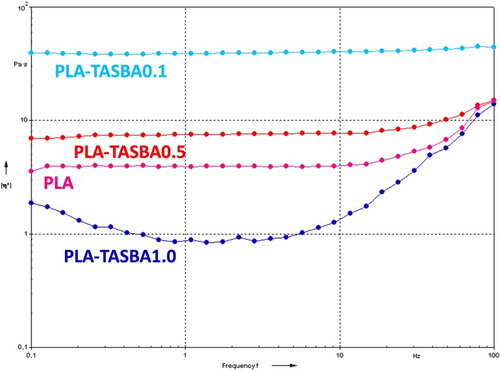
Considering PLA as a reference, both PLA-TASBA0.1 and PLA-TASBA0.5 showed the increase of the melt viscosity, with PLA-TASBA0.1 showing the greatest increase. The reduction of the melt viscosity was observed in the case of PLA-TASBA1.0. It is known that the polymerization reaction in the presence of a multifunctional initiator yields a complex mixture of products, comprising both free linear chains and chains bonded to the multifunctional initiator, which are the stars' arms. The formation of star-shaped macrostructures is known to have profound effects on the melt viscosity properties of the materials [66]. A higher quantity of initiator leads to shorter arms and, in turn, to lower viscosity due to the low quantity of entanglements forming between all the macromolecules. At low initiator concentration, the arms of the stars are long enough to provide entanglements, thus causing a high viscosity of the melt. Due to the presence of the central “core,” that is, the multifunctional comonomer (TASBA in this case), the star-shaped polymers can be characterized by more interchain interactions with respect to a linear macromolecule, resulting in higher melt viscosity. Hence, to account for the findings shown in Figure 3, one could consider that alongside promoting the formation of star-shaped macromolecules, TASBA may exert a toughening effect, provided by its rigid aromatic core. The melt viscosities obtained with different amount of TASBA could be considered as a result of the combination of the two separate and opposite effects, arising from the PLA macrostructure and the TASBA toughening effect.
2.3.3 Calorimetric Analysis
DSC analysis was carried out as described in detail in the Section 4. In brief: a first heating (not shown in Figure 4) was performed to cancel the thermal history of the samples, then cooling and heating cycles were made. The superimposed DSC thermograms of the PLA and PLA/TASBA samples, to be seen in Figure 4, show the three cycles.
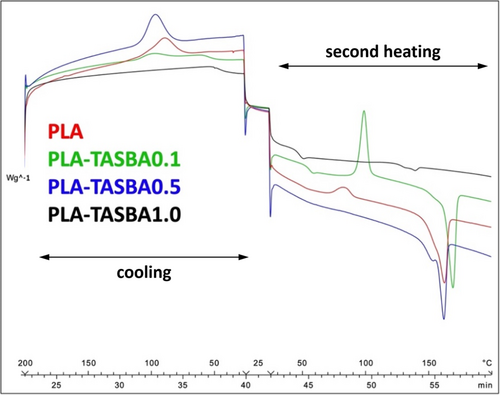
The temperatures relative to crystallization (T C), glass transition (T g), cold crystallization (T CC), and melting (T M) are reported in Table 4.
| Sample | T C (°C) b | T g (°C) c | T CC (°C) c | T M (°C) c |
|---|---|---|---|---|
| PLA | 115 | 49 | 83 | 163 |
| PLA-TASBA0.1 | 105 | 55 | 98 | 171 |
| PLA-TASBA0.5 | 102 | — | — | 161 |
| PLA-TASBA1.0 | — | 47 | — | 140 |
- a Temperature of crystallization (T C), glass transition (T g), cold crystallization (T CC), and melting (T M).
- b Recorded during the cooling scan.
- c Recorded during the second heating scan.
Standard PLA showed a well-defined crystallization transition in the cooling step and residual crystallization and melting in the second heating scan. The use of 0.1% of TASBA in polymerization resulted in a reduced crystallization during cooling and in a more evident cold crystallization and melting, shifted at higher temperatures compared to PLA. The increase of the diamide concentration in polymerization to 0.5% led to the complete crystallization of the material during the cooling step, as indicated by the absence of cold crystallization peaks during the second heating scan, and to the decrease of the melting temperature. The 1.0% concentration of TASBA in polymerization caused the increase of the amorphous character of the material. Indeed, the DSC trace did not reveal any crystallization transition and the melting transition was barely visible.
To account for these findings, the macrostructure of the PLA and PLA-TASBA samples and the PLA-TASBA interaction, commented above, should be taken into account. The viscosity of the PLA-TASBA0.1 sample, which is the highest of all the samples, as shown above in Figure 3, reasonably hinders the crystallization in the first cooling. The 0.5% TASBA concentration reasonably leads to lower molar mass arms, hence to lower viscosity (as shown in Figure 3), thus enhancing the crystallization ability of PLA. The interaction of TASBA, in higher amounts, with PLA could account for the lower melting point. The highest amount of TASBA is expected to cause a further reduction of the arm length. This macrostructure and the interaction of PLA with the TASBA molecules could explain the poor crystallization ability and the lower melting point. These findings confirm that complex polymeric architectures do affect the formation of crystalline structures [65, 66].
2.3.4 Size Exclusion Chromatography
The molecular weight of the samples was determined to understand how the addition of TASBA could affect the growth of the chains. As anticipated, TASBA is a multifunctional monomer that could, in principle, act as a multifunctional initiator in the ROP of L-lactide. For this reason, the introduction of different amounts of TASBA in the feed could affect significantly the outcome of the reaction, not only in terms of macromolecular architecture but also molecular weight. Table 5 reports the molecular weight data of the synthesized samples.
| Sample | D | ||
|---|---|---|---|
| PLA | 20,000 | 33,000 | 1.6 |
| PLA-TASBA0.1 | 19,400 | 31,000 | 1.6 |
| PLA-TASBA0.5 | 6500 | 8600 | 1.3 |
| PLA-TASBA1.0 | 5000 | 9000 | 1.8 |
As data show, the introduction of TASBA strongly affects the molecular weight of the samples. The introduction of 0.1% of multifunctional initiator has only limited effects, resulting in just a modest decrease of molecular weight. On the other hand, as expected, the molecular weight drops, with increasing loading of TASBA. Remarkably, alongside the decrease of molecular weight, the dispersity decreases as well for PLA-TASBA0.5 in comparison to standard PLA used as reference. A further increase in concentration up to 1.0% resulted in an increase of dispersity. These observations may hint the possibility that 0.5% could be close to the maximum amount of TASBA to be loaded as higher concentrations could have detrimental effects on the properties of the materials.
3 Conclusions
Nowadays, one of the major drawbacks of PET is its global warming potential when its end-of-life is in landfills. The recycling of PET is mandatory and chemical recycling gives the chance for an ideal circular PET economy, leading to monomers and low molecular mass functional molecules. In this context, serinol has been used in the aminolysis of post-consumer PET obtaining a polyhydroxylated diamide that has been successfully used as a multifunctional initiator for PLA, a well-known biopolymer. The high efficiency of the aminolysis, which is performed under atmospheric pressure without the need for catalysts, followed by a simple work-up procedure in which only water is required, together with the fact that serinol is a biosourced and nontoxic amine, allows the whole process to meet the Green Chemistry principles development. Moreover, green metrics such as AE, PMI, and E-factor have been also calculated.
The potential applicability of the diamide as a multifunctional initiator in the ROP of lactide has been demonstrated. Different amounts of diamide result in effects on thermal and rheological properties. These results pave the way for the possibility of applying this strategy for tuning the properties of PLA materials depending on the intended final application, increasing the versatility of this class of polymers. In this regard, as results show, the different amounts of TASBA have significant effects on the thermal and rheological properties of the final materials. Being able to control such properties could eventually lead to new applications for PLA-based materials, widening its array of properties.
4 Experimental Section
4.1 Materials
Flakes of bottle PET were obtained from postconsumer water bottles. Bottles were washed with water and detergents, rinsed with distilled water, and dried at 80°C for 2 h in the oven. The washed bottles were cut into flakes of about 5 mm × 5 mm. Powder of R-PET was provided by Montello SPA (Montello—BG, Italy) and was used without further treatment. Serinol was kindly provided by Bracco (Milano, Italy).
NMR was acquired on a Bruker 400 MHz instrument equipped with a 5 mm multinuclear probe with reverse detection; 1H and 13C chemical shifts (δ) are given in ppm relative to the signals of the solvent.
Yields are based on the repeating unit of PET.
4.2 Depolymerization of Bottle-PET With Serinol for Analytical Yields
Bottle-PET flakes (0.25 g) and serinol were mixed in a 10 mL round-bottom flask and heated under slow stirring (see Table 1). After the reaction time, the mixture was cooled, 3 mL of water was added, and then 80–90 mg of hydroquinone as internal standard was added after the complete dissolution of the white solid. A sample was withdrawn, diluted in DMSO-d6, and analyzed by 1H NMR. Details about the yield calculation in Supporting Information. The structure was confirmed by comparison with the data reported in the literature [68].
4.3 Depolymerization of Bottle-PET With Serinol and Recycle
Bottle-PET flakes (2.0 g, 10.4 mmol based on repeating unit) and serinol (3.8 g, 41.7 mmol) were mixed in a 50 mL round bottom flask and heated under slow stirring at 180°C for 90 min. After this time, the mixture was cooled at about 90°C (a precipitate was formed) and 22 mL of distilled water was added. The resulting mixture was refluxed under vigorous stirring until complete dissolution of the solid. Then the mixture was left at room temperature overnight, the white precipitate was filtered, washed with 4 mL of distilled water, and dried obtaining 2.51 g of TASBA as a white powder (77%). The water contained in the mother liquor of the filtration was removed under reduced pressure recovering the unreacted serinol together with ethylene glycol and residual diamide.
To this recovered mixture, bottle-PET flakes (2.0 g, 10.4 mmol based on repeating unit) and fresh serinol (1.90 g, 20.9 mmol) were added and the reaction protocol was performed as reported above. The recovered solid was recrystallized from water (40 mL) obtaining 1.93 g of TASBA as the first crop. A second crop of diamide (0.16 g) was obtained by concentration under the reduced pressure of the mother liquor. The overall amount obtained in this step was 2.12 g of diamide. The water contained in the mother liquor was removed under reduced pressure.
To the second recovered mixture, bottle-PET flakes (2.0 g, 10.4 mmol based on repeating unit) and fresh serinol (0.96 g, 10.4 mmol) were added and the same protocol was followed obtaining, after recrystallization, as first crop 1.63 and 0.24 g of diamide as second crop after concentration of the mother liquor. Other 0.16 g of diamide were recovered after concentration of the recrystallization mother liquor. The overall amount of diamide obtained in this third step was 2.03 g.
After the three runs 6.66 g of TASBA were recovered with an overall yield, based on the repeating unit of PET, of 70%. TASBA (N 1,N 4-bis(1,3-dihydroxypropan-2-yl)terephthalamide, TASBA) 1H NMR (DMSO-d6) δ = 8.06 (d, J = 7.9 Hz, 2H, NH), 7.93 (s, 4H), 4.65 (t, J = 5.7 Hz, 4H, OH), 4.02–3.93 (m, 2H), 3.60–3.48 (m, 8H) ppm; 13C NMR (DMSO-d6) δ = 165.5, 136.7, 127.1, 60.3, 53.9 ppm [68].
4.4 Gram Scale Depolymerization of R-PET Powder With Serinol
In a two-necked flask equipped with a mechanical stirrer and a condenser, 10 g of R-PET powder (52 mmol, based on the repetitive unit) was added to 28.5 g (313 mol) of serinol. The mixture was heated and kept at 180°C for 60 min. After this time, the mixture was cooled down to 90°C–100°C, and 125 mL of distilled water was added. The resulting mixture was refluxed until complete dissolution of the solid. The solution was then poured into a flask and left standing overnight. The solid was filtered, and washed with distilled water obtaining 11.4 g (36.5 mmol, 70% yield) of TASBA, a purity of 97%. The solid was then recrystallized from water (150 mL) and dried, obtaining 9.5 g of TASBA as a white powder with > 99% purity.
4.5 Polymerization of Lactide in the Presence of TASBA
The selected amount of TASBA depending on the chosen % w/w loading was placed in a 250 mL three-necked round bottom flask. Then, L-lactide (0.1 mol) and Sn(Oct)2 (0.03 wt%, 0.01 mol%, with respect to the monomers) were added and mechanical stirring was applied (40 rpm). The reaction was carried out in a closed oven at 190°C for 2 h under N2 flux, and then the polymer was left to cool overnight. Also, a polymerization of L-lactide without TASBA was performed in order to have a standard PLA synthesized in the same conditions acting as a comparison. In this case, the moisture present in lactide was enough to act as an initiator and start the ROP.
4.6 Characterization by DSC
DSC analyses were conducted using a Mettler Toledo DSC1 on samples weighing from 5 to 10 mg each. Melting and crystallization temperatures were measured using the following temperature cycles: (1) heating from 25°C to 200°C at 10°C/min; (2) cooling from 200°C to 25°C at 10°C/ min; (3) heating from 25°C to 70°C at 10°C/min; and (4) heating from 70°C to 200°C at 5°C/min. The first cycle was made to erase the thermal history of the samples.
4.7 Characterization by Rheological Analyses
Rheological analyses, conducted using frequency sweep experiments, were performed using a Physica MCR 300 rotational rheometer with a parallel plate geometry (diameter = 25 mm, plate gap = 1 mm). The strain was set equal to 5%, which was previously checked as being in the linear viscoelastic region for the samples, and curves of complex viscosity as a function of frequency were recorded, taking 30 points ranging from 100 to 0.1 Hz with a logarithmic progression, at 180°C.
4.8 Characterization by Size Exclusion Chromatography
The molecular weight of synthesized polymers was evaluated using an size exclusion chromatography (SEC) system having a Waters 1515 Isocratic HPLC pump and a four Waters Styragel columns' set (HR3-HR4-HR5-HR2) with a UV detector Waters 2487 Dual λ Absorbance Detector set at 230 nm, using a flow rate of 1 mL/min and 60 μL as injection volume. Samples were prepared dissolving 50 mg of polymer in 1 mL of THF and filtering the solution on 0.45 μm filters. Given the relatively high loading, a check was performed using a lower concentration of polymer (5 mg/mL), in order to verify that no column overloading was observed. Higher loadings were preferred as the UV signal of PLA is relatively weak. Molecular weight data are expressed in polystyrene (PS) equivalents. The calibration was built using 16 monodispersed PS standards, having a peak molecular weight ranging from 1,600,000 Da to 106 g/mol (i.e., ethyl benzene). For all analyses, 1,2-dichlorobenzene was used as an internal reference.
Author Contributions
Ada Truscello: conceptualization (equal), data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), software (equal), supervision (equal), validation (equal), writing – original draft (equal), writing – review and editing (equal). Chiara Sartiano: conceptualization (equal), data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), supervision (equal). Maurizio Stefano Galimberti: conceptualization (equal), data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), supervision (equal), validation (equal), writing – original draft (equal), writing – review and editing (equal). Cristian Gambarotti: conceptualization (equal), data curation (equal), formal analysis (equal), funding acquisition (equal), investigation (equal), methodology (equal), resources (equal), supervision (equal), validation (equal), writing – original draft (equal), writing – review and editing (equal). Stefano Gazzotti: conceptualization (equal), data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), software (equal), supervision (equal), validation (equal), visualization (equal), writing – original draft (equal), writing – review and editing (equal). Marco Aldo Ortenzi: conceptualization (equal), data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), software (equal), supervision (equal), validation (equal), visualization (equal), writing – original draft (equal), writing – review and editing (equal).
Acknowledgments
The authors are grateful to Montello SPA (Montello—BG, Italy) for providing Recycled PET and to Bracco (Milano, Italy) for providing serinol. Open access publishing facilitated by Politecnico di Milano, as part of the Wiley – CRUI-CARE agreement.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.




