A review: Multidimensional internal plasticization of molecular structure in energetic polymer
Yanan Li and Wenfang Zheng contributed equally to this work.
Abstract
In the field of propellant and polymer-bonded explosives (PBX), energetic polymers may bring poor mechanical properties for bulky groups and polarity. To get rid of the defects, plasticizers with low molecular weight can enhance the flexibility and processability of propellants through monotonous mixing. However, the migration of plasticizers over time may severely disrupt the mechanical property of energetic polymers. In contrast to general plasticization, internal plasticization can regulate flexibility from the perspective of molecular structure. In this context, this review focuses on the internal plasticization of energetic polymers from different dimensions. Hopefully, this review can provide some inspiration for designing energetic polymers to optimize the mechanical properties from various dimensions and integrate them for further research.
1 INTRODUCTION
Proverbially, the flexibility of energetic polymer is obstructed by bulky and polar energetic groups, bringing about the decline of mechanical properties.1-11 To eliminate the defect, plasticizers are traditionally blended to interact physically with polymers, thus improving their flexibility, rheology, and processability. However, highly plasticized propellants may suffer from the migration of plasticizers, which may bring about poor aging behavior or severe safety concern.12, 13 Being distinguished from external plasticization, internal plasticization can alter the molecular structure and avoid migration to optimize mechanical behavior.14
The most frequently used internal plasticization method in linear polymers is to break the regularity through copolymerization with flexible chains to improve the internal molecular force.15-17 3,3-Bis(azidomethyl)oxetane-tetrahydrofuran (BAMO-THF) attaches great attention among numerous energetic linear copolymers due to its terrific combustion performance and low-temperature mechanical properties.18-20 However, as the prepolymer of thermosetting elastomers, random copolymers were accompanied by problems like curing effect, difficult recovery, and insecurity. Considering these drawbacks, energetic thermoplastic elastomer (ETPE) with block polymer enables component recovery owing to its viscosity sensitivity to temperature.21-25 On the other hand, flexible side chains were considered to improve the intermolecular force from the two-dimensional perspective instead of linear structure limited.26-28 Generally, two common strategies exist to introduce side chains. One method is to introduce side chains into the monomers to polymerize. Lim et al.27 introduced carboxylic acid into poly (3,3 bis (3-chloromethyl) oxetane) (PBCMO) to obtain PBAMO with partial branched carboxyl groups for better thermal properties, lower Tg, and higher combustion heat than PBAMO. Another method is to connect plasticizers with polymers. In 2019, Asghar Bodaghi traced a series of newly reactive plasticizers in his article, including energetic plasticizers.29 Moreover, to realize the synchronous improvement of internal plasticization and energy level, energetic polymers with hyperbranched or cross-linking network structure have attracted more attention for their excellent mechanical properties from the 3D perspective.30, 31 It is worth noting that interpenetrating polymer networks may mix individual characteristics through permanent entanglement, which was expected to solve the problem of polymer incompatibility, thus enhancing the mechanical strength and thermal degradation resistance for interpenetrating and entanglement of polymer chains.
Herein, we will focus on introducing the sequence and structure through different dimensions and furtherly comparing the effects of structural changes on mechanical and thermal properties. In particular, to inspire researchers for the simultaneous optimization of energy levels and mechanical properties, we will discuss the ability of different dimensional sequence structures to optimize mechanical properties.
2 UNI-DIMENSION
It has been a frequently-used method to plasticize skeleton internally through linear copolymerization, which aims to break the regularity of molecular chain and improve intramolecular force.32 Around the 20th century, massive research has been carried out in the field of linear copolymerization due to its great potential in improving compliance. In early synthesis, epoxy butane and epoxy propane connected with energetic groups were generally used as the monomers of energetic copolymers (Figure 1a), and their polymerization mechanism was commonly cationic polymerization (Figure 1b).24 Notably, the selection of soft and hard segments is a relative concept, which means the monomers without highly symmetrical structures like GAP (glycidyl azide ether) and AMMO (3-azidomethyl-3-methyloxetane) can be either of the two segments in the copolymer.33-36
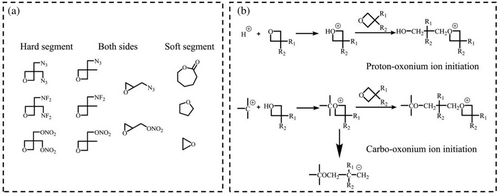
2.1 Random copolymerization
Preliminary work in copolymerization with energetic binder focused primarily on Manser et al.37-39 They synthesized a variety of energetic copolymers, which were obtained with significant improvement in compliance and processing performance. Compared with the homopolymer tending to be in the solid or viscous state, copolymers were mostly in the liquid state. Subsequently, their polymerization mechanism, combustion or pyrolysis progress, physical and chemical performance, and process optimization have been widely discussed.40, 41
Difluoroamino group is of great interest in promoting the development of propellant for its high energy specific impulse.42 As typical difluoroamino monomers, 3-difluoroaminomethyl-3-methyloxetane (DFAMO) and 3,3-bis (difluoroaminomethyl)oxetane (BDFAO) have been successfully synthesized by Manser et al.39-42 Unfortunately, owing to the great polarity of NF2, the flexibility of these polymers was unsatisfactory for practical use. Li et al.8, 43-45 synthesized a series of copolymers and studied their pyrolysis progress, physical and chemical properties (BDFAMO-THF, DFAMO-THF, DFAMO-BAMO, DFAMO-AMMO, and DFAMO-NIMMO). Compared with BDFAMO-THF and DFAMO-THF, BAMO, AMMO, NIMMO (3-nitratomethyl-3-methyloxetane) were of no great benefit to the flexibility of NF2 polymers.
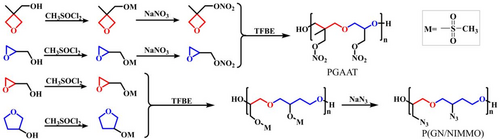
Meanwhile, endowed with the small size and low cost of the methanesulfonate group (Figure 2), Dong et al.34, 47 synthesized poly (glycidyl azide-r-3-azide tetrahydrofuran) (PGAAT) (Tg = −60°C) and poly (glycidylnitrate-r-3-nitratomethyl-3-methyloxetane) (P(GN/NIMMO)) (Tg = −37.9°C) through nucleophilic substitution of mesylate precursors, which exhibit superior flexibility than GAP (glycidyl azide ether), PGN (polynitrate glycidyl ether) and NIMMO. Significantly, the synthesis of PGAAT solves the difficulty of polymerizing substituted THF through any initiator due to the slight change in the free energy of polymerization and the steric factors. This polymerization method provides the feasibility for more difficult ring opening cyclic ethers.
However, it is rather complicated to figure out sequence arrangement in skeleton in light of the uncertainty of random copolymerization. BCMO/ε-caprolactone (CL) copolymerization was studied by Jutiter et al.,46 who proved AB block copolymer through the random feeding method. Zhai et al.20 analyzed the chain structure of BAMO-THF random copolymer and found that the ideal chain structure can be obtained by controlling the molar feed ratio of monomers to improve the mechanical properties of the polymer.
2.2 Block copolymerization
Compared with random copolymers, block copolymers are mostly applied in ETPE, which possesses high-temperature viscosity sensitivity, microphase separation, and low vulnerability.48, 49 In addition, block copolymerization is an effective method to reduce the glass transition temperature of polymers for breaking the regularity of polymers.24 Generally, energetic block copolymers are divided into two block modes in terms of arrangement: ABA triblock polymer and (AB)n multi-block polymer.24, 50
Different from the energetic block copolymers, hydroxyl-terminated polyether (HTPE) is multiblock copolymers copolymerized through oxonium coupling of polytetrahydrofuran PTHF (Mn = 650–1000 g mol−1) and polyethylene glycol (PEG) (Mn = 200 g mol−1). Granted with excellent low-temperature mechanical properties and insensitive characteristics, HTPE has been applied and demonstrated on a variety of tactical missiles in the United States.51, 52 Owing to the eminent mechanical properties of inert polymers, HTPB (polybutadiene) and HTPE are given rise to candidates for copolymerization with energetic binders. Wang and Qian prepared new energetic adhesives PNIMMO-HTPB-PNIMMO, PNIMMO-HTPE-PNIMMO, and PGN-HTPB-PGN with HTPB and HTPE as initiators.7, 53, 54 The research demonstrated that they exhibit superior performance than PNIMMO. Cappello et al.55 first reported four different ether butadiene ether triblock copolymers, which initiator was HTPB (Figure 3a). The prepared polymers are endowed with the advantages of HTPB and azide polymer. Surprisingly, the performance of the adhesive is adjustable by regulating the type and length of the energetic block, even though the HTPB block complicates the azidation and reduces the storage stability of azide copolymers.
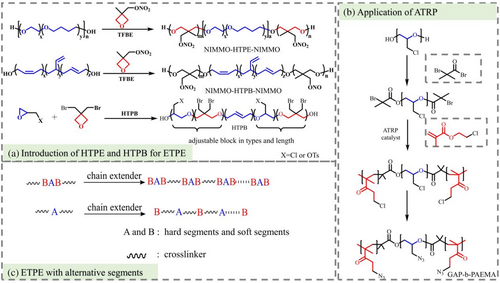
It has been acknowledged that the polarity of lactone is greater than ether, which can endow the structure with better mechanical properties.59 PGN-PCL-PGN and PCL-GAP-PCL were synthesized through ring-opening copolymerization of poly(ε-caprolactone) (PCL) with PGN and GAP. The DSC result shows that their glass transition temperature decreased remarkably.36, 58 Practically, as early as 2012, the energy block copolymer of GAP and poly(azidoethyl methacrylate) (GAP-b-PAEMA) was synthesized through atom transfer radical polymerization (ATRP) and characterized (Figure 3b).56 Moreover, lactone-based polymers were traced in the review of Sikder et al.24 in 2013.
Multi-block copolymers adjust their properties by measuring sequence structure, thus achieving better physical and chemical properties.49 Xu et al.50 synthesized PBAMO-PNMMO-PBAMO tri-block copolymers and (PBAMO-PNMMO)n multi-block copolymers with their rheological and mechanical properties tested, respectively. (PBAMO-PNMMO)n were entailed with higher solid modulus and lower melt viscosity, while PBAMO-PNMMO-PBAMO displayed better mechanical properties. Additionally, compared to random block copolymer which irregular alignment may damage mechanical properties of the material for cluttered arrangement, alternating copolymerization exhibit preferable tensile strength and mechanical behavior to random ETPE. Luo et al.14, 57 prepared alternative BAMO-AMMO and random BAMO-AMMO multi-block. Which former exhibits higher tensile strength than the latter. Currently, two general methods to alternate blocks by chain extension reaction are available (Figure 3c). One approach is to prepare the ordered structure through bonding agents based on tri-block polymer, and the other is to connect the chain extender with one homo-polymer for further alternating reaction. More detailed glass transition temperature and mechanical properties of liner copolymers were gathered and listed in Table 1 for intuitive reference.
| Sample | Molecular weight (g/mol) | Tg (°C) | Tensile strength (MPa) | Elongation (%) | |
|---|---|---|---|---|---|
| Mn | Đ | ||||
| PGAAT47 | 2300 | 1.65 | −60 | - | - |
| P (GN/NIMMO)34 | 1200 | 2.06 | −37.9 | - | - |
| PNIMMO-HTPB-PNIMMO53 | 6700–12,500 | −76 | - | - | |
| PNIMMO-HTPE-PNIMMO7 | 5900–9200 | 1.4–1.5 | −68.9 to −68.2 | - | - |
| PGN-PCL-PGN36 | 1300 | 2.20 | −56.2 | - | - |
| PCL-GAP-PCL58 | 1800 | 1.59 | −64.3 | - | - |
| GAP-b-PAEMA56 | 11,400 | 1.64 | −18.4 | - | - |
| PBAMO-PNMMO-PBAMO50 | - | - | −3.4 | 2.96–5.25 | 100–683 |
| Alternative (BAMO-AMMO)n13 | 26,100 | 2.53 | - | 6 | 500 |
| Random (BAMO-AMMO)n13 | 28,800 | 2.61 | - | 4 | 500 |
3 TWO DIMENSION
The mechanical properties of energetic groups were mainly optimized by linear copolymerization. Nevertheless, there is no doubt that the increase of flexible chains seriously reduces the content of energetic groups, thus limiting the majorization of mechanical properties in 1D internal plasticization. Therefore, researchers have started to further improve the mechanical properties via the introduction of plasticizers in the side chain.60, 61 Considering the migration of plasticizers, functional groups were gradually drawn into the polymers to modify their intermolecular force.27, 30 The literature statistics are listed in Table 2 to offer more intuitive data to analyze.
| Sample | Molecular weight (g/mol) | Tg (°C) | Tensile strength (MPa) | Elongation (%) | |
|---|---|---|---|---|---|
| Mn | Đ | ||||
| PPG-GAP-PPG62 | 1600 | 1.50 | −63 | - | - |
| PPG-PGN-PPG63 | 1900 | 2.02 | −58 | - | - |
| P(GN-ran-PO)64 | 1200 | 1.27 | −47 | - | - |
| PEPH-GAP-PEPH26 | 1000–1900 | 1.33–2.25 | −60 to −47 | - | - |
| Poly (TFEE-r-GA)28 | 2900 | 1.47 | −49.5 | 5.52 | 162.8 |
| (PBFMO-PNMMO)57 | 6900 | 1.22 | −20.4 | 10.54 | 723 |
| P (FPO/NIMMO)65 | 3000 | - | −42.7 | - | - |
| GAP66 | 1900 | 1.16 | −49 | - | - |
| Carboxylated GAP66 | 2300–2600 | 1.15–1.17 | −55 to −48 | - | - |
| PBAMO27 | 2000 | 1.21 | −37 | - | - |
| Carboxylated-PBAMO27 | 2000–2400 | 1.22–1.3 | −51 to −43 | - | - |
3.1 Monomer grafting
Researchers have found that introducing plasticizers into the monomer is one solution to side chain grafting. The copolymerization of propylene oxide with alkane side chains has been extensively studied. Compared with the symmetrical structure of PEG, PPG (poly (epoxy propane)) behaves better flexibility (Mn = 2000 g/mol, Tg = −70°C). Because the existence of branched-chain methyl group brings about the optimization of inter and intramolecular force.62
In 2018, Hafner synthesized the linear copolymer of epichlorohydrin and 1,2-epoxidated hexane (EpH) by cationic ring-opening polymerization.26 The introduction of non-polar alkyl side chains in GAP effectively resulted in lower viscosity and glass transition temperature. Bayat et al.62-64, 67 prepared linear energetic triblock copolymer PPG-GAP-PPG (Tg = −63°C), PPG-PGN-PPG (Tg = −58°C), and random copolymer P(GN-ran-PO) (Tg = −47°C). They are all accompanied by extraordinary thermal stability and lower glass transition temperature than GAP (Tg = −45°C) and PGN (Tg = −32°C) (Table 2). In addition, it is worth studying the influence of non-polar alkane chains with different lengths on mechanical properties. Unfortunately, it is difficult to visually compare PPG-GAP-PPG with PEPH-GAP-PEPH considering the differences in molecular weight, sequence alignment, and nitrogen content. The relevant synthesis of PPG and EpH as grafted monomers is shown in Figure 4. Molar masses have been rounded in case of the presence of measurement errors.
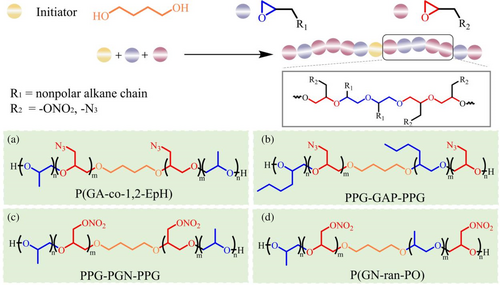
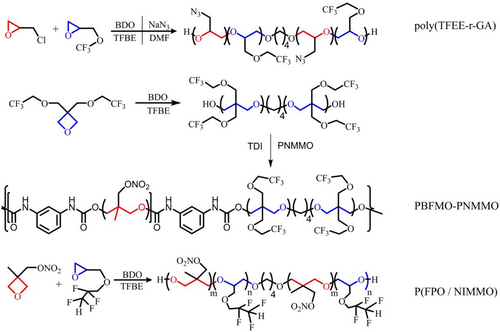
It has been highly valued that fluoropolymer is the candidate for high density and wide operating temperature range.68, 69 To improve the mechanical properties of GAP-based polyurethane network binders, Xu et al.28 synthesized (2,2,2-trifluoro-ethoxymethyl epoxy-r-glycidyl azide) copolymer (poly (TFEE-r-GA)) through cationic copolymerization of epichlorohydrin and 2,2,2-trifluoro-ethoxymethyl epoxy, followed by azidation. Compared with GAP, the polyurethane network based on poly (TFEE-r-GA) is endowed with superior mechanical properties (Table 2).
Subsequently, they successively reported poly[3,3-bis (2,2,2-trifluoroethoxymethyl)] oxetane block poly (3-nitromethyl-3-methyloxetane) (PBFMO-PNMMO) and a novel fluorine-containing copolymer of 2-(2, 2, 3, 3-tetrafluoro-propoxymethyl)-oxirane and 3-nitratomethyl-3-methyloxetane (P(FPO/NIMMO)) (Figure 5).57, 65 It has been confirmed that they are both provided with superior mechanical properties compared to PNMMO (Table 2).
3.2 Click reaction in grafting
Unlike monomer grafting, reactive plasticizers are grafted to polymers and have received extensive interest, supplying the grafted more selectivity. In 2019, Asghar Bodaghi reviewed alkyne compounds connected with binder through click reaction between azide and acetylene groups in Figure 6a.29
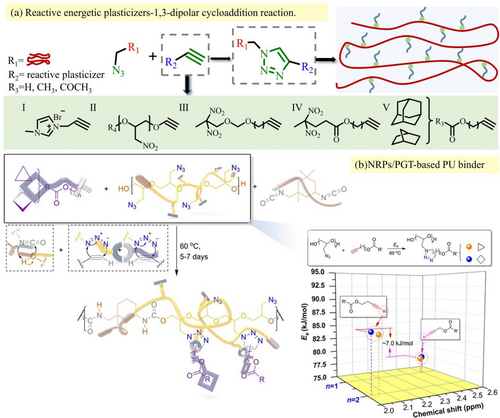
In 2020, Kwon attached two norbornane-based reactive plasticizers (NRPs) to poly (Glycidyl Azide tetrahydrofuran) based polyurethane, preventing the harm from migration.71 In the study, reactive monocyclic plasticizers (RMCPs) were chemically incorporated into GAP to reduce the instability of cured polymer adhesive.70 After adding RMCPs, the tensile properties and impact sensitivity of GAP-based polyurethane adhesive were enhanced due to the transformation of azide into triazole groups. The increase in tensile strength may result from the triazole group and bulky norbornane moiety, which belong to sterically hindered species (Figure 6b). Simultaneously, instead of the click reaction between azide and acetylene groups, Lim et al.27, 66 prepared carboxylated GAP and carboxylated PBAMO, which are granted with lower Tg values than GAP and PBAMO, providing superior processing performance (Figure 7).
4 THREE DIMENSION
Furthermore, considering the 3-D structure, hyper-branched polymer and interpenetrating network structure were prepared according to different molecular interaction mechanisms. Hyper-branched polymer reduces the entanglement of molecular chains through anchors, thereby improving flexibility. Besides, the interpenetrating network structure aims to connect the mixed soft and hard segments through chemical bonds, improving compatibility.30, 31
4.1 Hyper-branched polymer
Hyper-branched polymer is provided with less inter-molecular interaction and greater compliance, attributed to its smaller arrangement size of atomic space, multiple anchoring points, and less entanglement between molecules. Significantly, they are prone to deformation during extrusion or rolling and improve the rheological properties of materials.72-75 There are generally two formations for hyper-branched polymers: dendritic polymer (Figure 8a) and star-shaped polymer (Figure 8b).74-77
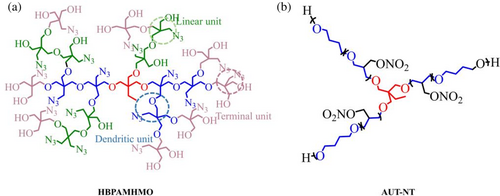
In dendrimer, Zhang et al.74 synthesized poly-3-azidomethyl-3-hydroxymethyloxane (HBPAMHMOS) through cationic ring-opening polymerization (Table 3). The Tg value of HBPAMHMO (Tg = −54°C) was lower than PAMMO (Tg = −44°C). Furthermore, the research indicates that tensile strength and elongation of HBPAMHMO-based film (EBPAMMO) are better than PAMMO-based film in a wide temperature range (−40 to 50°C), especially at low temperature.
| Sample | Molecular weight (g/mol) | Tg (°C) | Viscosity (mPa s) | |
|---|---|---|---|---|
| Mn | Đ | |||
| HBPAMHMOS | 2800 | 1.12 | −54 | 3472 at 25°C, 1013 at 50°C |
| PAMMO | 3300 | 1.21 | −44 | 3219 at 25°C, 907 at 50°C |
| Tensile strength (MPa) | Elongation (%) | Impact sensitivity (J) | Friction sensitivity (J) | Tg (°C) | |
|---|---|---|---|---|---|
| BPAMHMO-2 | 8.98 | 6.44 | 35 | 324 | −45 |
| BPAMHMO | 8.22 | 4.26 | 25 | 288 | −37 |
Zhang et al.75 prepared a series of hyper-branched copolymers based on PBAMO (R-POB-m) by adjusting the molar ratio of monomer BCMO and EHO, followed by cationic polymerization and azide reaction. The results prove that R-POB-m polymers are amorphous and exhibit lower viscosity and Tg value than PBAMO. Moreover, R-POB-4 possesses the lowest Tg value, which is connected with four linear chains (Table 4).
Star-shaped polymers are special hyper-branched polymers, extending more than three linear chains radially from one point.77 In 2018, Wang et al.76 prepared and studied an allyl urethane tetrahydrofuran copolyether (AUT-NT) structure containing three linear polymers. As a result, the tensile strength of AUT-NT increases from 1.0 to 3.0 MPa, and the elongation increases from 135% to 300%.
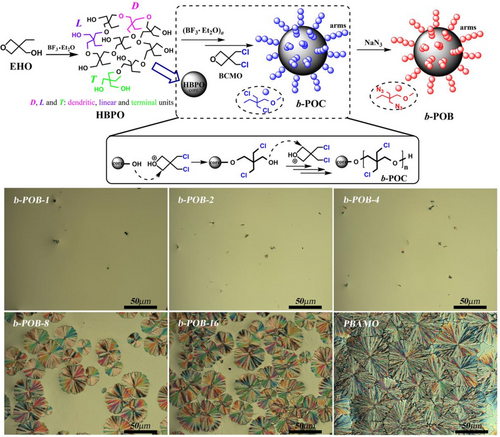
Surprisingly, hyper-branched polymers with the spatial arrangement of three-dimensional spherical molecules are difficult to migrate and tangle with considerable molecular weight. Luo et al.78-81 further introduced BAMO and GAP cyclic ether to the terminal hydroxyl group of hyper-branched poly (3-ethyl-3- (hydroxymethyl) oxane) (HBPO) and prepared hyper-branched star copolymers POG-n and POB-n (Figure 9, n = number of linked linear chains). Consequently, POB-n and POG-n are endowed with lower sensitivity and better mechanical properties than PBAMO and GAP. POB-4 and POG-8 exhibit the lowest Tg value. The film composed of HTPE and POG-8 (at 30 wt%) exhibits the best mechanical performance (2.48 MPa, 212.6%).
4.2 Energetic interpenetrating polymer networks (EIPNs)
Besides, in terms of cross-linking network, EIPNs can blend the properties of individuals through networks and interpenetrating polymerization. Inspired by IPNs, Tanver et al.31, 82 designed a series of newly prepared EIPNs to enhance the mechanical properties of GAP. In 2015, they developed two strategies to realize the EIPNs between GAP and HTPB in succession (Figure 10). The former one (Sample 1) is to facilitate cross-linking interspersed through mixed curative agents, wherein 1,3 dipole ring is cured through azide of GAP and hydroxyl is connected with HTPB and DDPM (dimethyl 2,2-di (prop-2-ynyl) malonate). The latter (Sample 2) hybrid cross-linking is promoted occasionally by hexamethylene diisocyanate biuret trimer (Desmodur N100). As a result, Sample 1 and 2 exhibit excellent mechanical properties (Table 5) and thermal stability. In particular, the mechanical strength of Sample 2 is the largest in the binder system of GAP and HTPB studied.
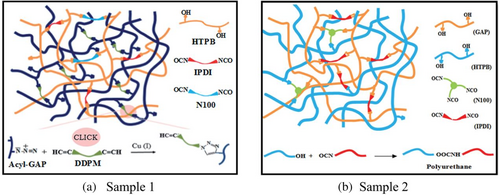
In 2016, Tanver et al.83 designed a new EIPNs-based nanocomposite (Sample 3) composed of functionalized MWCNTs (multi-walled carbon nanotubes), HTPB and GAP. The evidence suggests that Sample 3 possesses excellent mechanical properties (Table 5), accounting for the good dispersion of fMWCNTs in the polymer matrix and the entanglement of strong interfacial bonds with the cross-linking network during in-situ polymerization.
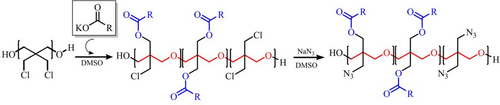
In replacement of HTPB, Ou et al.84 successfully prepared HTPE semi-interpenetrating network GAP/dimethyl dipropylmalonate (Sample 4) by catalytic carbamate reaction (Figure 11). The structure of Sample 4 is connected with four azide groups by DDPM through toluene diisocyanate. The decomposition behavior studied by thermal analysis proves that gas originating from azide groups decreased significantly, showing appreciable thermal stability with the increase in temperature.

5 CONCLUSION
Great efforts have been made to optimize the overall energy level and mechanical properties simultaneously due to the importance of the general energy level and mechanical properties in practical propellant formulation systems. Based on the fundamental aim of achieving internal plasticization of high-energy polymers, massive polymers have been reviewed and proved effective, including the regulation in 1D linear copolymerization, 2D grafted copolymers, entanglement reduction, and improved compatibility from the 3D perspective.
However, there are still many challenges to overcome. For example, it is still hard to flexibly adjust the ratio of alternating units to block units free from chain extenders in linear copolymers. The effects of side chain length, content, and linkage positions on various energy-based polymers are still partially unclear. Besides, there are still many possible mechanisms to achieve internal plasticization from the 3D perspective. Energetic polymers are the hinge that enhances propellant energy level and mechanical properties. Here, we summarize the internal plasticization in polymers from different dimensional perspectives, providing more research interest for researchers in designing the molecular structure of energetic polymers.
AUTHOR CONTRIBUTIONS
Yanan Li: Conceptualization (lead); investigation (lead); writing – original draft (lead). Wenfang Zheng: Conceptualization (supporting); investigation (lead); writing – original draft (lead). Wenxi Li: Investigation (equal); writing – review and editing (equal). Renming Pan: Supervision (lead); writing – review and editing (equal). Xiangyang Lin: Supervision (equal); writing – review and editing (supporting).
CONFLICT OF INTEREST
We declare that we have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Biographies

Miss Yanan Li is a PhD student in chemical engineering at Nanjing University of Science and Technology (NJUST). The main research is the preparation of energetic binder in propellant.

Mr. Wenfang Zheng is the associate professor of Nanjing University of Science and Technology (NJUST). Mainly engaged in the research of energetic materials.

Miss Wenxi Li is presently pursuing a master's degree in chemical engineering at Nanjing University of Science and Technology (NJUST). The main research is the process optimization of adhesives.

Mr. Renming Pan is a leader of energetic materials group at NJUST. His researches mainly focus on preparation and application of energetic materials and fire protection.

Mr. Xiangyang Lin is the associate professor of Nanjing University of Science and Technology (NJUST). Mainly engaged in the preparation and testing of energetic materials.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




