Recent developments in P(O/S)–N containing flame retardants
ABSTRACT
Synthesis of organophosphorus compounds containing nitrogen as heteroatom and its application as a flame retardant (FR) have attracted much attention in the academic and industrial communities over the past decade. Such compounds are relatively easy to synthesize and offer advantages such as high thermal stability, which is useful for high temperature processing, improved char stability, density, and yield during the thermal decomposition of polymer, and release phosphorus species active in the flame inhibition process. Though a variety of phosphorus–nitrogen (P–N) compounds can be found in the literature, this review mostly summarizes the recent (since 2013) development in phosphorus (O)-nitrogen containing flame retardants which have been published in peer-reviewed journals. General strategies of synthesizing P(O)–N compounds as FRs from various phosphorus-based starting materials are highlighted in this review. Some of the most common classes of researched P(O)–N containing compounds as FRs include the phosphinamides, phosphonamides, phosphoramides, phosphoramidates, phosphorodiamidates, phosphonamidates and their thio counterparts which are usually obtained via a one- or two-step synthetic strategy. Incorporation of these compounds as FRs in various polymer systems such as polyurethane, epoxy resins, polyamides, cellulose, polylactide, poly(butylene terephthalate), polycarbonates, and acrylonitrile–butadiene–styrene are discussed in detail in this review. Special emphasis on the various fire and thermal performances of the new materials are also summarized. The mechanical performance of new materials and the influence of these additives on polymer processing are also briefly discussed. © 2019 Wiley Periodicals, Inc. J. Appl. Polym. Sci. 2020, 137, 47910.
INTRODUCTION
In a modern society, polymeric materials are increasingly used in diverse sectors such as construction, transportation, electronics, packaging, industrial machinery, aerospace, and so forth. Increased use of organic polymers in such materials heighten the risk of fire, as most of them ignite easily. Thus, the development of effective flame retardant (FR) strategies, which guarantee the public health and safety, motivates academia and industry. In addition, the development of such technologies helps lower the danger of fire accidents and loss of property.
Some FRs based on halogens which were extensively used in the past but have been proven to be toxic and thus banned from use today.1 New phosphorus-based FRs (P-FRs) have been considered as an alternative to halogenated FRs, and their usage in various applications have been explored extensively.2-4 In addition, the development of sustainable halogenated FRs has also risen dramatically.1 The versatile nature of phosphorus, that is, high efficacy (low loadings in polymers and coatings), chemical diversity, and ability to exhibit multiple mode of action, makes it a key element for a new FR development.
The mode of action of an FR additive is crucial when developing the right formulations of polymer materials to meet specific fire tests. Mode of action of the FR strongly depends on its relationship with the polymer matrix. Generally, the mode of action of FRs is classified into two phases, that is, the condensed phase and the gas phase, with P-FRs exhibiting both mechanisms.5, 6 In the condensed phase, P-FRs facilitate the formation of char via crosslinking, facilitating cyclization, aromatization, or graphitization by dehydration of the polymeric material. Additionally, some P-FRs act via intumescence, where the residue acts as a protecting sheet, reduce the heat transfer speed to the underlying polymeric material.7-9 It is also claimed that for some P-FRs, gas-phase flame inhibition mechanism and condensed-phase mode of action occur in parallel during the thermal decomposition of the material.10
All compounds discussed in this review are either incorporated into the polymer matrix as complete polymer chains or as a part of the copolymer chain as a comonomer. Their incorporation in the material can also be done via surface modification or blending or by mixing of unreactive additives. Thermosets contain functional groups such as alcohols, amines, epoxy, halogens, and so forth, which allow for the incorporation of reactive additives during curing.3 Usually, reactive FRs have an advantage since they are covalently bonded to the polymer matrix and therefore have a minor effect on the physical properties of the material. In contrast, addition of nonreactive FR additives results in a decrease in the glass-transition temperature and the possibility of leaching is obvious. However, nonreactive additives dominate the market because of their lower price and easy processability. In addition, reactive FRs require careful formulation of various ingredients, whereas nonreactive FR additives could be used in various polymer matrices without much modification.2, 11
P-FRs containing heteroatoms, for instance, silicon, nitrogen, boron, and sulfur offer a vast choice of specific beneficial interactions compared to the phosphorus compounds made only of pure hydrocarbons.12 Such interactions lead to the reduction of the load of FRs in the material and improved FR efficacy.13-18 More recently, DOPO-based phosphonamidate (6,6'-(ethane-1,2-diylbis(azanediyl))bis(dibenzo[c,e][1,2]oxaphosphinine 6-oxide) [EDA-DOPO]) has been shown to be nontoxic and successfully upscaled in the industry.19, 20 The use of phosphorus–nitrogen (P–N) compounds as FRs are extensively studied, and their FR effect in many cases is attributed to P–N synergism. It is believed that P–N synergism encourages the crosslinking of polymeric chains during the thermal decomposition process. It boosts the retention of phosphorus in the condensed phase and produces thermally stable char. Additionally, the density of the residue formed at elevated temperatures increases and the matrix is more likely to be retained during the combustion process, consequently contributing to condensed-phase action and giving higher char yields.21 Cone calorimetry and thermal experiments of polyurethane (PU) foams containing P(O)–N compounds have indicated their possible gas phase flame inhibition effect.22, 23 Recently, gas phase species such as phosphorus nitride (PN) and other PN species have been detected during the thermal decomposition of dimethyl phosphoramidate. The flame inhibition effect of such species is unknown.24
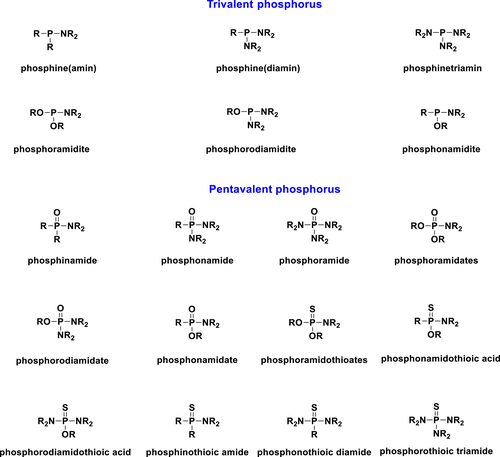
NOMENCLATURE OF P–N CONTAINING PHOSPHORUS COMPOUNDS
A wide variety of P–N containing organophosphorus compounds are known in the literature. The general structure of various P–N bonds containing trivalent and pentavalent organophosphorus compounds is summarized in Figure 1.
SYNTHESIS OF P(O)–N COMPOUNDS
General Synthesis Methods
A straightforward and common way to synthesize P(O)N and P–N compounds is by nucleophilic substitution reactions of P(O)─Cl and P─Cl compounds respectively.25, 26 Thiophosphorylated aminoheterocycles have been synthesized using this methodology.27 The P(O)–N containing compounds are also commonly synthesized from the P─H (phosphonates and phosphonates) using carbon tetrachloride (CCl4) and an amine via an Atherton–Todd (AT) reaction where it is believed that a P─Cl bond is formed in situ as an intermediate.28 However, regardless of the easy handling of this reaction, it has found no commercial successes as CCl4 is a carcinogen and depletes the ozone.29, 30 More recently, one pot two-step approach has been employed to convert P─H bonds to P(O)–N bonds. In these cases, P(O)─H bond has been transformed to P─Cl via the use of several chlorinating agents such as tert-butyl hypochlorite (t-BuOCl),31 chlorine gas (Cl2),32-34 copper(II) chloride (CuCl2),35 sulfuryl chloride (SO2Cl2),36 n-chlorosuccinimide (NCS),22, 37 and trichloroisocyanuric acid (TCCA).23, 36, 38-40 and P(O)─Br via N-bromosuccinimide.41 Furthermore, P(O)─Cl bond formation has also been investigated via Michaelis–Arbuzov rearrangement of trialkyl phosphite with Cl2.42 Moreover, synthesis of P(O)–N bonds can also be achieved via an oxidative coupling method using iodine (I2)43, 44 or copper (Cu)45, 46 as catalysts. However, upscaling of these synthetic procedures is challenging because of their exothermic nature, expensive raw material as well as side products and moderate reactions yields. As a possible alternative, the Staudinger-phosphite reaction has also been proposed to achieve P(O)–N bond.47-50 This synthesis procedure involves the reaction of alkyl azides with trialkyl phosphite to produce phosphorimidates, followed by rearrangement to their corresponding phosphoramidates. Another approach to the synthesize P(O)–N bond is metal-catalyzed C─H bond amidation by using phosphoryl azides51-54; however, the use of hazardous organic azides is a major limitation of this method. Recently, the synthesis of phosphoramidates was carried out via a green synthetic methodology using an organic dye as a photocatalyst. However, this synthetic methodology is only useful for diethyl phosphite derivatives.55
Synthetic Approaches of P(O)–N Compounds Used as a Flame Retardants
The general strategy of synthesizing P(O)–N FR compounds from various phosphorus-based starting materials is summarized in Scheme 1. P(O)–N bond creation using chlorinating agents from various phosphorus-based starting materials are the most commonly used methods in academics for development of FRs. Some methods discussed in this review are also commercially suitable.
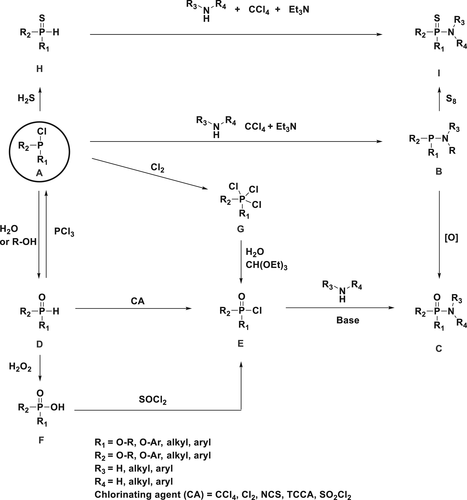
There are two major synthetic pathways to obtain P(O)–N compounds; the first pathway is the nucleophilic substitution of trivalent phosphorus Compound A, and the next step is the oxidation of Compound B to achieve the desired product C as shown in scheme 1.25 The second possibility synthesizing P(O)–N compounds is the oxidation of Compound A followed by chlorination of Compound D using various chlorinating agents followed by nucleophilic substitution to obtain the desired product C. The method to synthesize compound C involves the chlorination of F in boiling thionyl chloride for 18 h to produce Compound E, which is then reacted with an amine in the presence of a base.26 Another approach to obtained Compound C involves the reaction of A with chlorine (Cl2) gas,56 to form G intermediate, which upon hydrolysis yields intermediate E. The synthesis of thiophosphorus P(S)–N derivative involves a two-step strategy, starting with the reaction of A with hydrogen sulfide (H2S) to generate Compound H followed by an AT reaction which yields the desired Compound I. Other possibility of synthesizing thiophosphorus compounds involves the nucleophilic substitution of A in the first step to obtain B followed by an oxygen sulfur exchange using S8.25
The following sections describe the application of P(O)–N-based compounds as FRs for specific polymers.
Polyurethanes
PUs are a broad class of materials used widely in many applications in various forms such as foams (rigid or flexible), coatings, adhesives, sealants, solid components, and etc.57 Rigid PU foam (RPUF) is often used as an insulation material, especially in buildings, due to its superior physical and mechanical properties and low thermal conductivity, while flexible foams are used for application in a variety of consumer and commercial products, including bedding, furniture, automotive interiors, carpet underlay, and packaging.58-63 Due to their extensive use in everyday life, it is necessary to treat these materials with FR additives especially for fire safe applications. It is required that these FRs should not have negative influence on the physical properties of the materials and the environment. Phosphoramidates64-66 and phosphonates67 are well studied as FRs for PU foams. These compounds produce active PO species in the gas phase during the thermal decomposition process, which interferes with the combustion process of the flammable volatiles.
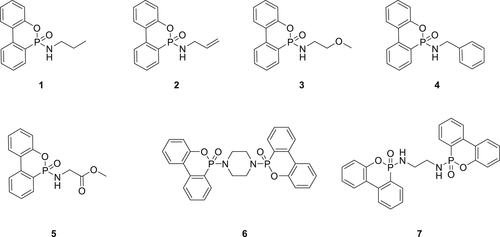
The toxic nature of the CCl4 limits the industrial upscaling of P(O)–N-based DOPO derivatives via AT reaction. To replace CCl4, especially for industrial upscaling, different chlorinating agents were explored. Along with CCl4 two compounds, SO2Cl2 and TCCA were investigated as alternative chlorinating agents. The TCCA reaction is exothermic and it was suggested that it might not be suitable for use in industrial-scale synthesis.36 In contrast, SO2Cl2 releases toxic gasses (HCl and SO2) as byproducts.36 The fire performance of P(O)–N containing DOPO derivatives 1, 2, 4, 6, and 7 was studied by incorporating them in polyether-based flexible PU foams and compared to the commercially available FRs (TCPP, DOPO, and Exolit OP 560). FR evaluation based on UL-94 HB test on the PU foams showed that PU foam with bis-DOPO-based phosphonamidates display superior fire performance in contrast to the commercial FRs. Compound 7 was shown to exhibit the best FR behavior compared to the other DOPO-phosphonamidate compounds (1, 2, 4, and 6). A concentration of only 5 wt % (based on the weight of polyol) of Compound 7 was needed to achieve HF1 rating in the UL-94 fire test.68
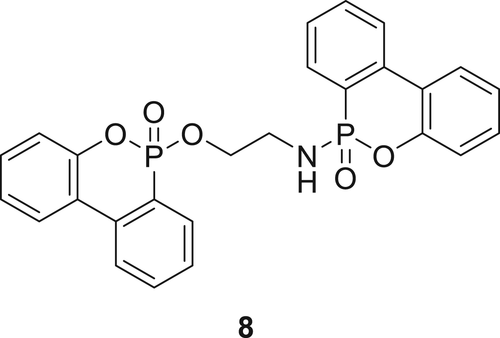
A similar DOPO-phosphonamidate derivative ETA-DOPO (8)69, 70 as shown in figure 3 was incorporated in the RPUFs and evaluated for flame retardancy. Small-scale fire tests show that by addition of 2 wt % phosphorus in RPUFs a HF1 rating in the UL-94 test and a limited oxygen index (LOI) of 22.2% can be achieved. Based on the cone calorimetry and thermogravimetric analysis (TGA) tests, RPUF containing Compound 8 exhibited a higher char yield and lower smoke production. Compound 8 is active in both phases, that is, in the gas phase by formation of low active phosphorus species and in the condensed phase by catalyzing tough char, which act as a barrier for fire.
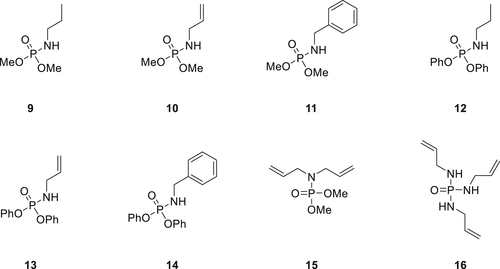
A library of phosphoramidate derivatives (9–16) as shown in Figure 4 have been synthesized and investigated for their flame retardancy in FPUFs. Being liquids, these compounds exhibit good compatibility with the PU matrix. Fire test results of the PU foams showed Compounds 10 and 13 have superior FR performance compared to their structural analogs. The TGA and pyrolysis combustion flow calorimeter analysis showed that except for Compound 16, all other compounds are mostly active in the gas phase.23
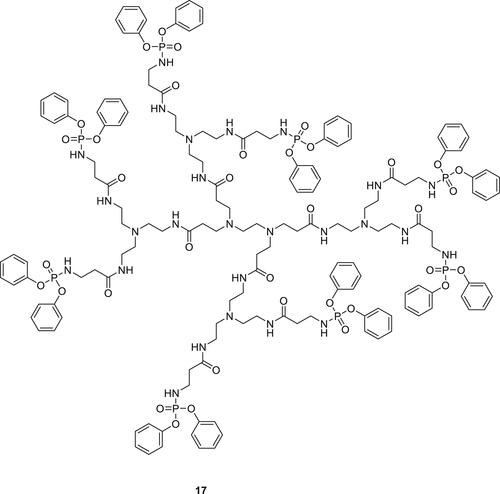
Dendrimer diphenyl phosphoryl chloride (DPC)–poly(amido amine) (PAMAM; 17) containing a P–N unit (Figure 5) was synthesized and integrated into the PU matrix via in situ polymerization to form a gel-phase network. Incorporation of the Compound 17 in the PU matrix significantly improves the tensile strength of the PU material. TGA and cone calorimetry results showed that the insertion of Compound 17 enhances the thermal stability and improves the flame retardancy of the PU matrix. The scanning electron microscope (SEM) analysis of residual char obtained from cone calorimeter experiments indicated a compact and uniform char layer for 17/PU matrix compared to the char residue of the blank PU. This suggests that DPC–PAMAM works primarily in the condensed phase during combustion.71
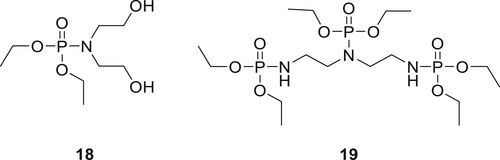
A reactive additive diethyl-N,N-bis(2-hydroxyethyl) phosphoramide (DEPA) (18) as shown in figure 6 was synthesized from diethyl phosphate and diethanolamine (DEA). Compound 18 together with 8 wt % expandable graphite (EG) was incorporated in RPUFs. LOI value of such RPUF improved by 10% as compared to the blank foam.
It was proposed that EG and Compound 18 acted via the two-phase mechanism (gas phase and condensed phase).72 In the gas phase, the material produces water (H2O) and carbon dioxide (CO2) along with PO* species and suppress the production of flammable gasses such as ether and benzene. In the condensed phase, continuous and dense char layers are formed with phosphorus-rich residues. Due to this two-phase mechanism, EG/DEPA system exhibits excellent flame retardancy in RPUF. In a similar work, Compound 19 (figure 6) was synthesized from diethyl phosphonate and diethylenetriamine via AT reaction. Addition of 19 to RPUF alters its thermal decomposition process and helps increase the char formation in TGA experiments. The LOI values of RPUF containing 8 wt % EG and 16 wt % of 19 were around 30%. This study suggested that EG/19 interaction enhances the formation of a stable char layer on RPUFs which act as a fire obstacle during the combustion process. Microscale combustion calorimetry (MCC) experiments on the RPUFs containing EG/19 showed a lower heat release rate for it compared to the neat RPUF. The SEM and energy dispersive X-ray analysis results on the chars obtained for RPUFs containing EG and 19 show a compact and phosphorus-rich layer which improve the barrier property of the char.73
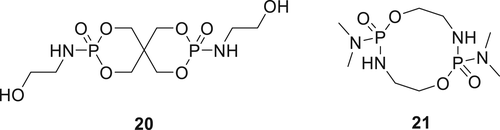
A bisphosphoramidate derivative 20 (figure 7) was synthesized via a two-step reaction, which involves the synthesis of spirocyclic pentaerythritol bisphosphonate disphosphoryl chloride (SPDPC) in the first step, followed by its reaction with ethanolamine in the presence of a base. RPUF was prepared with different concentrations of Compound 20 with the best fire results achieved at a loading of 25 wt % of 20 (i.e., V-0 rating in the UL-94 HB test and an LOI value of 27.5%).74 Compound 20 was also incorporated in a waterborne PU (WPU) dispersion and its fire performance was evaluated. For the WPU matrix containing 9 wt % of 20, 1.62 wt % char content in TGA measurements were observed, and a higher LOI value of 26% was achieved. The LOI and cone calorimetry experiments indicated that by increasing the concentration of Compound 20 in the matrix, the flame retardancy of WPU films was improved. It was proposed that Compound 20 is active in two phases, that is, in the gas phase, it produces nonflammable gases, and in the condensed phase, it produces phosphoric acids. Further, phosphoric acid condenses into polyphosphoric acid to enable char formation via the carbonization process.75
A novel P–N containing FR, octahydro-2,7-di(N,N-dimethylamino)-1,6,3,8,2,7-dioxadiazadiphosphecine (21) as shown in figure 7 was synthesized in three steps. In the first step, phosphorus oxychloride (POCl3) reacts with dimethylamine hydrochloride in dichloromethane solution in the presence trimethylamine base. In the second step, ethanolamine was reacted with intermediate dimethylphosphoramidic dichloride in dichloromethane solution using trimethylamine as a base followed by decompressing and distillation of the intermediate to acquire the final product 21.
In the next step, Compound 21 was used as a chain extender to formulate waterborne P–N synergistic FR PU (phosphorus–nitrogen synergistic halogen-free flame-retardant waterborne polyurethane (DPWPU)). PUs were prepared with the different concentrations of Compound 21 (5–20 wt %) and V-0 rating in the UL-94 test and an LOI value of 30.2% was achieved at a loading of 10 wt %. For the DPWPU matrix containing 15 wt % of 21, an LOI value of 30.6% was achieved; on the other hand, it was observed that when FR 21 content increased up to 20 wt %, LOI value drops down to 29.8%. Moreover, it was found that the char residue increased with the increase of FR contents at 480 °C.76 A firm and highly crosslinked reside formed during the burning tests was attributed to P–N synergism and keeps the material parts in the condensed phase for a long time.
Epoxy Resins
Epoxy resins (EPs) exhibit strong mechanical and adhesive properties, satisfactory electrical insulation, resistance toward chemicals and heat, making them ideal matrix for applications such as adhesives, composites, and coatings.77-80 However, EPs are also highly flammable polymers and therefore requires protection against fire by suitable chemical modifications or by incorporation of FR additives.81-87 A new multielement Compound 21 containing phosphorus/nitrogen/sulfur elements were designed and manufactured (Figure 8) via AT reaction and reacted with the EPs. The modified EPs showed lower thermal stability as well as lower glass-transition temperature (Tg) as compared to the blank EPs.
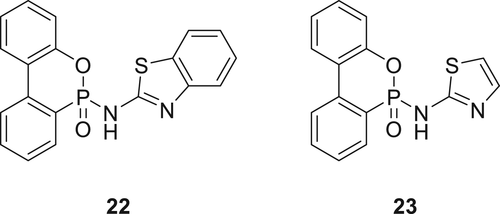
The tensile strength of the modified EPs was lower compared to the virgin polymer; however, the flame retardancy of these EPs is significantly improved and successfully achieves a V-0 rating in the UL-94 test and an LOI value of 33.5%. Moreover, Compound 22 showed activity in reducing heat release and reducing smoke discharge in cone calorimetry experiments. Evolved gas analysis using coupled techniques such as TGA–Fourier transform infrared (TGA–FTIR) spectroscopy and pyrolysis–gas chromatography/mass spectrometry (PY–GC/MS) showed that Compound 22 is active in the gaseous phase through N2/S volatiles. Volatiles such as ammonia, hydrogen cyanide, nitric oxide, H2S, sulfur dioxide, and sulfur trioxide along with oxygen and phosphorus-based free radicals were also observed in these experiments. A similar Compound 23 was also synthesized (Figure 8) via AT reaction from DOPO and 2-aminothiazole and combined with EPs. When 5 wt % of 23 used, an LOI value of 34.7% was achieved, and the material successfully passed V-0 rating in the UL-94 test. For EPs containing 10 wt % of 23, a 20% increase in tensile strength was observed compared to the blank EP. It was hypothesized that its activity in the gas phase plays a vital role in stopping the heat discharge of EP during combustion.88
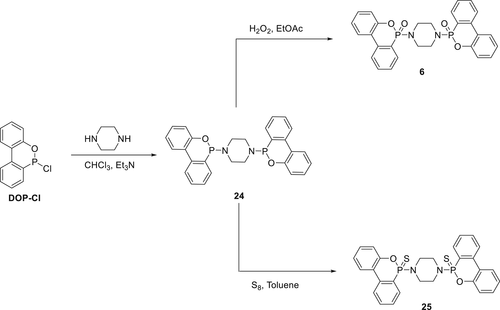
6-Chloro-6H-dibenz[c,e][1,2]oxaphosphorin (DOP─Cl) is an important precursor to synthesize DOPO and its derivatives. DOP─Cl is an intermediate formed in the DOPO manufacturing process and therefore commercially available. DOP─Cl is highly reactive, and thus it is widely used as a starting material to react with other nucleophiles to synthesize P-heteroatom compounds. For instance, the reaction of DOP─Cl with piperazine in dry chloroform yields Compound 24 (Scheme 2).89 Subsequently, by using hydrogen peroxide (H2O2), 24 was oxidized in ethyl acetate solvent to yield Compound 6. By reacting 24 with sulfur (S8) in toluene as a solvent, 6,6′-(piperazine-1,4-diyl)-bis(6H-dibenzo[c,e][1,2]oxophosphinine-6-sulphide) (25) was obtained (Scheme 2).90, 91
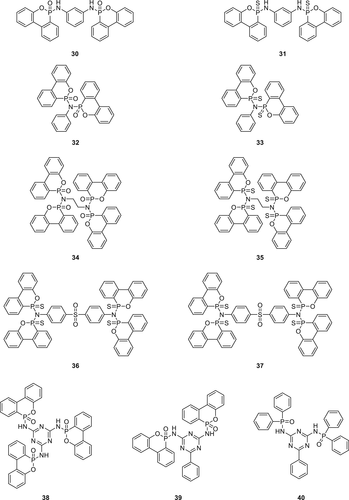
A series of P(O)–N containing bridged compounds were synthesized from corresponding phosphorus chloride and nitrogen-based nucleophiles, followed by oxidation with tert-butyl hydroperoxide (Scheme 3) to the pentavalent P(O)–N 25 or heated with elemental sulfur (S8) in toluene to obtain the di(thiophosphon) amidate (26), di(thiophosphin) amide (27), and di(thiophosphor) amidate (28), respectively. DOPO─Cl shows a different reactivity compared to DOP─Cl. When DOPO─Cl is reacted with a primary amine, it forms phosphonamidate (29), compared to DOP─Cl with the same amine leads to the formation of corresponding diphosphonamidates 25 (i.e., primary amine reacts with two DOP─Cl molecules). The thiophosphonamidate 30 was prepared via two-step methodology; in the first step, nitrogen-based nucleophiles (m-phenylenediamine) and DOPAM-3-propyl were transformed into phosphonamidite intermediate via a condensation reaction under reduced pressure. In the final step, phosphonamidite was reacted with S8 in toluene to form thiophosphonamidate (30).
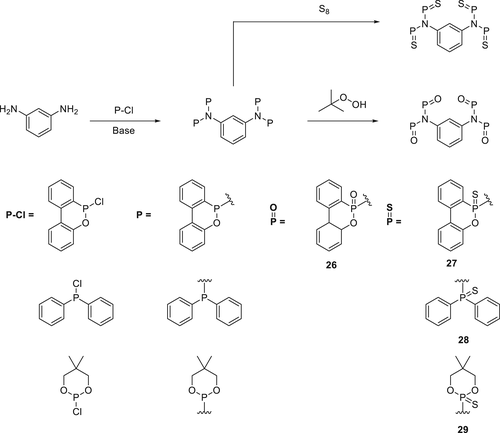
The advantages of diphosphonamidates (25 and 26) over phosphonamidates (29 and 30) are their higher thermal stability, higher phosphorus contents, and higher molecular weight. Their fire performance was investigated in DEN438/DICY/Fenuron-based EPs, and the UL-94 test results were found to be dependent on the FR loading levels. For EPs containing Compounds 25, 26, and 30 (1.5 wt % P) a V0 classification was achieved, as compared to EPs containing 27 and 29 for which only a V1 rating could be achieved at similar phosphorus loading. Compound 29 does not showed adequate FR effect in EPs and is thus not classified in the UL-94 test. The char yields (TGA experiments in N2) of FR containing EPs improved from 20 to 40 wt % at 700 °C as compared to the virgin EP (30 wt %). A highest char yield of 40 wt % was observed for EPs containing 29 which supports the hypothesis that Compound 29 is active mainly in the condensed phase.93 For EPs containing Compounds 26, 27, 28, 30, and 31, a lower amount of char formation was observed compared to the blank EPs, indicating their gas phase flame inhibition activity.77, 94
Recently, phosphorylated melamine-based derivatives were synthesized from DOP─Cl or diphenyl phosphine (DPP)─CI as starting materials followed by oxidation in H2O2 and ethyl acetate as a solvent to afford pentavalent P(O)–N derivatives (38, 39, and 40) as shown in figure 9. The fire performance of Compound 38 was investigated in EPs (DEN438/DICY/Fenuron) and for comparison, commercial available DOPO was chosen. For EPs containing DOPO (1.6 wt % P), a V0 classification was achieved, whereas EPs with 1.4 wt % P (Compound 38) V0 rating could be achieved.95
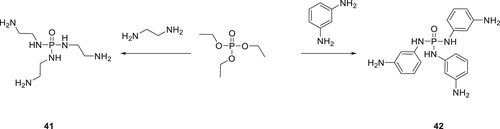
Phosphortriamidates 41 and 42 were synthesized by reaction of triethyl phosphate with an excess of ethylene diamine and benzene-1,3-diamine, respectively (Scheme 4). Both compounds were successfully used as crosslinkers for the EPs. Their FR behaviors were investigated, and UL-94 tests showed that for the EPs with 2 wt % P a V-0 rating and LOI values of 30% could be achieved.96
A series of phosphorus nitrogen containing Compounds (43–48) as shown in figure 10 were prepared from phenylphosphonic dichloride and different amines in the presence of triethylamine (TEA) as a base. Among these compounds, two compounds (43 and 46) were selected and tested for flame retardancy in EP diglycidyl ether of bisphenol A (type EP) along with 4,4-diaminodiphenylsulfone (curing agent). At a loading of 30 wt % of 46 in cured EP, a V-0 rating in UL-94 test and an LOI value of 31% could be achieved. For the cured EP with a loading of 30 wt % of Compound 43, a V-1 rating in a UL-94 test at and an LOI value 28% was achieved. In addition, in cone calorimetry experiments, lower peak heat release rate (pHRR) was observed for EP/46 compared to the EP/43 system. It was proposed that Compound 46 is active in two-phase; however, 43 is only active in the condensed phase. In the condensed phase, thermal decomposition of both compounds produces acid compounds (H3PO3 and H3PO4) which acted as a source of char formation with epoxy matrix. In the gas phase, thermal decomposition of 46 produced PO* radicals responsible for flame inhibition. Furthermore, the small loading of Compounds 43 and 46 had no significant impact on the Tg, tensile strength, and storage modulus of EPs.97
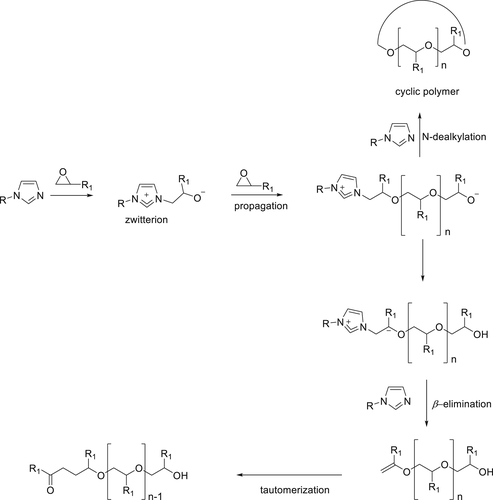
Imidazole-based phosphorus derivatives (49 and 50) (Figure 11) were synthesized and used to cure EPs.98 These compounds ensure not only long pot life during storage of EP and fast curing during application but also excellent FR effects. For EPs containing 15 wt % of 49, an LOI value of 31% was achieved compared to 21% for a virgin resin. Moreover, in the UL-94 test, a V-0 rating for 1.6 mm thick samples could be achieved. A similar result was found in the UL-94 test and a higher LOI value of 38% was observed for EPs containing 15 wt % of 50. Cone calorimetry tests showed that 49/EP and 50/EP formulation had excellent flame retardancy and 49/EP clearly demonstrated improved smoke suppression due to its dominant activity in the condensed phase. By loading 15 wt % of 49, the total smoke production (TSP) of 49/EP dropped to 13.3 m2 m−2 compared with blank EP (25 m2 m−2), and the char residue yield was higher (37.3%). However, TSP value of 15 wt % of 50/EP increased (28 m2 m−2) as compared to that for the blank sample, and it was thus suggested that Compound 49 is more active in the vapor phase.98
The proposed curing mechanism for P(O)–N/EP components is shown in Scheme 5. First, the epoxy group of the prepolymer reacts with tertiary nitrogen of the imidazole derivative to form an adduct (zwitterion); in the next step, further reactions lead to an epoxy chain propagation. Additionally, there are two options for the following reactions, the first possibility is the N-dealkylation reaction which generates a cyclic epoxy macromolecule, and the second possibility is the β-elimination which yields an unsaturated macromolecular structure which is finally converted into a carbonyl group. Consequently, it was proposed for Compounds 49 and 50, the electron-deficient imidazole ring detaches from the macromolecule and further takes part in other reactions as a catalyst during the curing process.
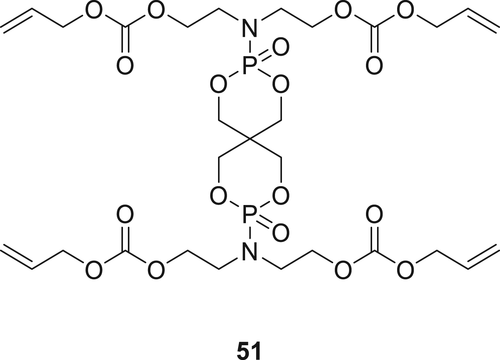
A multifunctional P(O)–N containing diluent 51 (Figure 12) was synthesized using multistep reactions starting from spirocyclic pentaerythritol bisphosphonate phosphoryl chloride followed by its reaction with DEA, and the final product 51 was achieved by reaction with allyl chloroformate. The synthesized Compound 51 was successfully incorporated in epoxy acrylate oligomer in various concentrations (5–25 wt %) together with a photo-initiator, applied on wood via coating and finally cured with ultraviolet (UV). In the vertical burning test (UL-94), coated wood (10 × 7 cm2) achieves a V-0 classification along with a significant increase in LOI values. When the treated wood was exposed to 5–10 s of flame, a self-extinguishing behavior of the wood was observed.99 By increasing the concentration of Compound 51 (5–25 wt %) in the coating formulation, the LOI of the matrix was also increased from 23 to 35%. The char data obtained from TGA show that by increasing the phosphorus (P), nitrogen (N), and aromatic content of the polymer matrix, char yield increase from 2.53 to 17.9 wt % which acts as a barrier against fire.
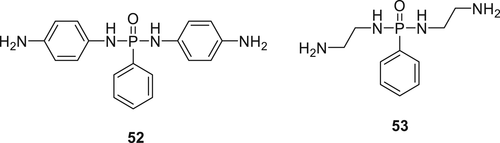
Two phosphorodiamidates (52 and 53) as shown in Figure 13 were synthesized via a condensation reaction of corresponding diamines with phenylphosphonic dichloride. Due to the bifunctional nature of these compounds, it makes them useful candidates as a crosslinkers of EP. Flammability and thermal studies on the modified EPs show that the incorporation of Compound 53 at 2.3 wt % phosphorus content produces high char yields along with an LOI of 27% (around 6% higher than untreated EP). Furthermore, Compound 52, which contains higher aromatic content display excellent FR activity, an LOI value of 31% was obtained at 2.1 wt % P in the EP.100
The P–N synergism in 52 and 53 plays a vital part in forming P–N rich char during the thermal decomposition of the epoxy polymer. EPs containing 52 gives higher and more stable char compared to that cured with 53.
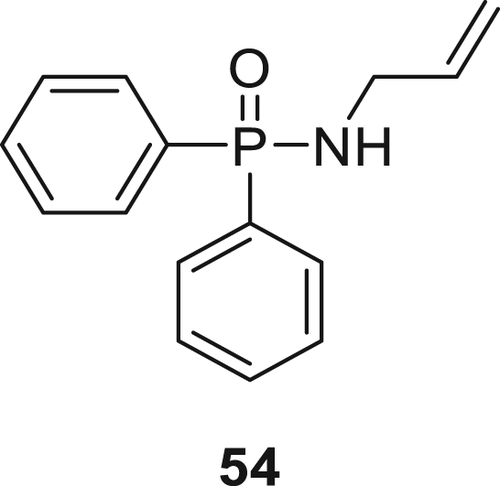
Compound 54 (Figure 14) was synthesized by the reaction of diphenylphosphinic dichloride with allylamine in the presence of trimethylamine as a base. It was then incorporated in the epoxy acrylate along with octamercaptopropyl polyhedraloligomeric silsesquioxane (POSS-8SH). Various formulations using different weight ratios of Compound 54 and POSS in an epoxy acrylate resin was prepared. The incorporation of Compound 54 into an epoxy acrylate reduced the double-bond conversions; in contrast, the addition of POSS-8SH improved the conversion of unreacted double bonds. Moreover, with the addition of Compound 54 flame retardancy of the EPs increased but with the addition of POSS, flammability decreased and failed to pass the UL-94 test. Also as the amount of Compound 54 in the polymer matrix increased, the tensile strength of the coatings reduced.101 EPs with 20 wt % 54 loading achieved a V-1 rating in the UL-94 test.
Phenylphosphonic derivative 55 and its tautomer 56 (Figure 15) was synthesized by reaction of 2-aminobenzothiazole with phenylphosphonic dichloride using TEA as a base and crosslinked in EPs.102 For EP containing 0.69 wt % of P, a V-0 rating in UL-94 test and 31% LOI value was observed.
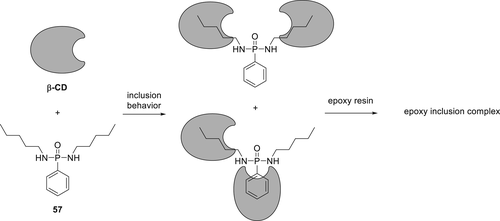
A phenylphosphonic derivative 57 (Figure 16) was synthesized and combined with a bio-based molecule beta-cyclodextrin (β-CD) to form an inclusion complex (IC). The IC was obtained by dissolving β-CD in water at 65 °C, followed by dropwise addition of Compound 57 in water at the same temperature (65 °C) for 2 h and possible inclusion structures were proposed according to the results of 1H NMR spectra.
As a comparison to IC, the physical mixture (PM) of Compound 57 and β-CD prepared by mixing them in dimethyl sulfoxide at room temperature and evaporation of the solvent. The results showed that the addition of 2 wt % loading of 57 and β-CD obtained via PM in the EPs increased LOI values to 24.5%, whereas the blank resin had an LOI value 22%. However, PM/epoxy system was not able to pass the UL-94 test. By using the same concentration (2 wt %) of IC in EP, higher LOI values (26.5%) were achieved; however, the IC/EP system also showed no rating in UL-94. The TGA results under nitrogen (N2) showed that the char formation of IC was higher (25.9%) than those of PM (16.8%) at 600 °C. Moreover, the thermal decomposition temperature of IC was much higher than PM. The flammability of EP with IC was lower as compared with EP and PM/epoxy system. These results indicate that IC acts in both condensed and gas phase. In the condensed phase, Compound 57 probably acted as an acid source along with β-CD, which functions as a char precursor. On the other side, a decrease in the heat of combustion values of EP/IC and increased values of CO/CO2 in cone calorimetry experiments compared to the blank EPs showed that IC is active in the gas phase.103
Subsequently, in a separate work, 57 was added into the epoxy matrix and in a glass fiber reinforced epoxy composite (GRE). On addition of 5 wt % of Compound 57, the epoxy matrix showed a significant increase in the LOI from 22% (blank) to 32% and a V-1 rating in the UL-94 test was obtained. Upon addition of 12 wt % of 57, the highest LOI values (36%) was achieved and the material passed with a V-0 rating. LOI values of blank-GRE (25%) were higher compared to the blank epoxy matrix (22%). With the addition (5 and 12 wt %) of Compound 57 in GRE, the LOI increased (29 and 33%, respectively); however, the impact of Compound 57 on LOI of GRE was lower as compared to the epoxy matrix at the same loading. In the UL-94 test, FR glass fiber reinforced epoxy composites (FGRE) achieved no ratings, but with the addition of 12 wt % of 57 in GRE, a V-0 rating could be achieved. In addition, pHRR of FR 12 wt % 57/epoxy matrix dropped to 66% in comparison to the blank epoxy matrix. In TGA experiments, the char residue of flame-retarded EPs was lower than that of FGRE. Compound 57 was proposed to be active in both the condensed and gas phases. In the gas phase, the thermal decomposition of 57 released PO* radicals and PO* radicals are able to recombine H* and OH* radicals. In the condensed phase, thermal decomposition of 57 produced the acidic phosphorus compounds which catalyze char formation. Mechanical characterization involving nanoindentation test results showed that the addition of 57 did not disturb the interfacial strength and hardness of epoxy matrix and GRE.104
Polyphosphonamide (PPDA) derivatives were synthesized via nucleophilic substitution reaction of phenlyphosphinic dichloride and various amines, as shown in Scheme 6, combined with the EPs and subsequently investigated for their fire performance. All synthesized PPDAs display low flammability and excellent thermal stability. On addition of PPDAs, the flammability of the epoxy composites was significantly improved. It was found that the thermal properties of the final material are highly dependent on the chemical environment of the PPDAs. The synthesized PPDAs were combined with EPs at 80 °C for 10 min, poured in a PTFE mold and cured for 2 h at 80 °C followed by postcuring for 2 h at 120 °C. All samples were then gradually cooled down to room temperature. All formulations prepared with same concentration (15 wt %) of PPDA. Pure EP produced poor char residue and no rating in the UL-94 test. However, the addition of PPDAs in EP, LOI, and char residue increased. When 15 wt % of 59 was incorporated into EP, the LOI value of cured EP increased to 28.9% and a V-1 rating was achieved in the UL-94 test. Upon incorporation of 15 wt % of Compounds 58 and 60 in EP, the LOI values increased to 29.5 and 29.6%, respectively, V-0 rating achieved in the UL-94 test in both cases. These investigations show that the combination of the heteroatom in 58 and 60 in the phosphorus structure results in enhanced flame retardancy compared to 59. The PPDAs are active in both the condensed phase and gas phase. In the condensed phase, the thermal decomposition of PPDAs into acid compounds (H3PO3) which promote the char forming process in EP, provide an additional protective layer. This is evident from the increased char yield (21 wt %) for the EP/PPDA composites compared to the blank EP (4.8 wt %) as observed from cone calorimetry test. In the gas phase, PPDAs showed the predicted flame inhibition effect during ignition. It is known that phosphorus-containing polysulfone acts as toughness modifier for EPs.105 However, for EP/59 and EP/60 systems, no serious drop in toughness was observed.106
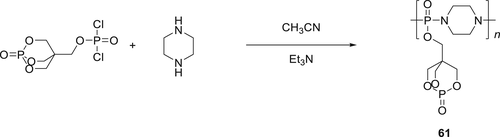
A polymeric crosslinker poly(pentaerythritol phosphate phosphinic acyl piperazine) (PPAP) (61) was synthesized as shown in Scheme 7 and its fire performance tested on EPs. At 20 wt % PPAP loading in the EP, an LOI value of 35% was observed compared to the blank EP which has an LOI value of 19%. Furthermore, TGA showed an improvement in the thermal stability of the composite. In cone calorimetry experiments for 20 wt % loading of 61, EPs improved the char formation (92.7 wt %) and a 70% decline in pHRR and 60% decrease in the THR was observed. Dynamic mechanical analysis of the EPs showed that crosslinker 61 also improves the mechanical property of EP.107
Polyamide 6 and Polyamide 66
Polyamide 6 (PA6) and polyamide 66 (PA66) engineering plastics exhibit high abrasion resistance, excellent mechanical strength, good processability, and high chemical resistance. These properties make them suitable substrates for electronics, carpets and textile clothing, automotive, and electrical parts. Even though polyamides show slow-burning behavior, their flame resistance is not enough for most fire safe applications. Due to these risks, polyamides require to be protected against fire.108, 109 Most common method used to decrease the flammability of polyamides materials is to add an FR additive in the material via melt processing.
Commercially available diethyl aluminum phosphinate (Exolit OP 1230) and Compound 7 (Figure 2) were incorporated in PA6 via an extrusion process. A V-0 rating in the UL94 test was observed for the PA6 formulation containing both additives. In addition, an LOI of 31.7% was observed for PA6/7 formulation.110
A P(O)–N containing monomer DOPO–DAAM (62) as shown in figure 17 was synthesized, grafted on PA66 fabric via an UV treatment which was shown to improve its flame resistance. Prior to the FR treatment, the PA66 fabric surface was first modified via an HCl treatment to enhance the grafting of the FR molecules. A pass was achieved in a vertical burning test for PA66 fabric treated with 20 wt % of 62. TGA test of the grafted PA66 fabric samples showed a decrease in the thermal decomposition temperature compared with the blank fabric. Cone calorimetry experiments and TGA of the treated fabric, residual char analysis showed that the 62 decomposed earlier than the virgin PA66 and in such system, a gas phase flame inhibition activity is dominant compared to the condensed phase activity.112


Phosphorus-containing PA66 was synthesized via polymerization of the PA66 prepolymer and monomer 63 (Figure 18). A two-step reaction procedure was used to synthesize monomer 63. Phenyl phosphinic dichloride first added dropwise to acrylic acid, followed by an addition of aminobenzoic acid to obtain the desired monomer 63. On addition of 5 wt % 63 as a comonomer, the modified PA66 passed the UL-94 test with a V-0 rating and achieved an LOI value of 28%.113

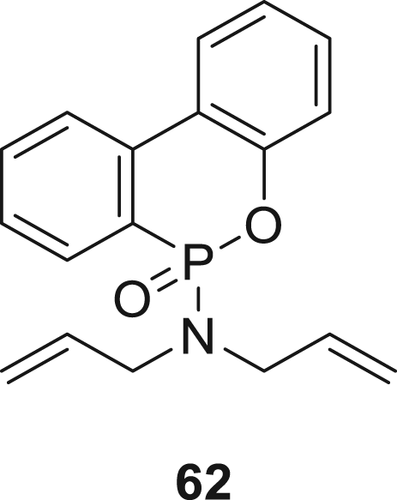
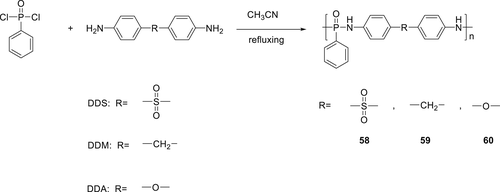
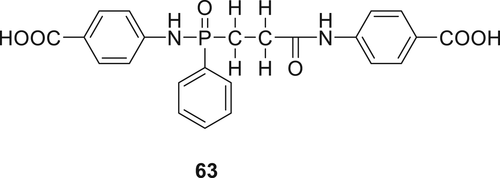
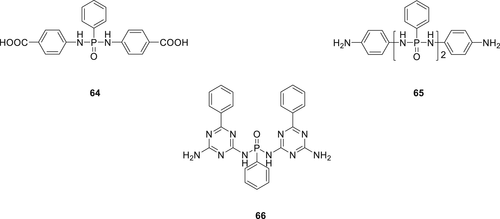
In a subsequent work, several new derivatives of phosphorus and nitrogen-containing compounds (64–66) (Figure 19) having carboxyl or amino end groups were synthesized and incorporated via copolymerization in PA66.114 Synthesis of monomer 64 was carried out via acylation reaction of phenylphosphonic dichloride with aminobenzoic acid. Compound 65 was prepared by the reaction of 1, 4-benzenediamine with phenylphosphonic dichloride by a dropwise addition and TEA was used to trap chlorine fumes. The reaction of benzoguanamine with phenylphosphonic dichloride resulted in 66. By using 5 wt % of these monomers, all modified PA66 could successfully achieve V-0 rating and PA66 containing monomer 65 afforded the highest LOI value of 29%.
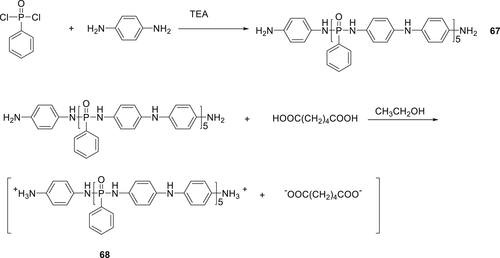
P(O)–N containing poly-N-aniline-phenyl phosphamide (67) reactive macromolecule as shown in Scheme 8 was synthesized. By using adipic acid, prepolymer 68 was prepared followed by the polymerization of PA66 with prepolymer 68. For PA66 containing 4.5 wt % prepolymer 68, a V-0 rating in UL-94 test along with LOI value of 28% was achieved. However, compared to the virgin PA66, the TGA results of the modified PA66 showed a decrease in the thermal stability of the modified PA66 by 43 °C.115

Cellulose Fibers
Cellulose is one of the most commonly used biopolymer and widely used in the manufacturing of textiles, paper, and architectural products. Like many organic polymers, cellulose is inherently flammable and mostly flame retarded by incorporation of FR additives in bulk or as coatings. P-FRs especially the P(O)–N containing compounds are very popular for flame retardation of cellulose.116 In many cases, it is claimed that P(O)–N compounds offer synergism and improved flame retardancy of cellulose.116-118
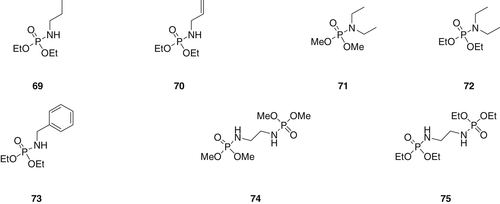
A series of alkyl ester phosphoramidates derivative (69–75) as shown in figure 20 were synthesized and incorporated in cellulose fibers obtained from a different source (peat and cotton fibers). Phosphoramidates with smaller phosphoester group display significantly higher condensed phase activity compared to the phosphoramidates with larger phosphoester groups. Furthermore, the bis-substituted phosphoramidates derivatives showed higher fire performance as compared to the monosubstituted phosphoramidates (69, 70, 71, 72, and 73).116
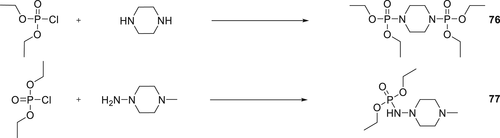
Two piperazine derivatives 76 and 77 as shown in Scheme 9 were synthesized, and their flame retardancy was investigated on a cellulose substrate (cotton fabric). Various thermal and chemical analytical techniques such as TGA–FTIR, PY–GC/MS, and TGA–FTIR were used to understand the mechanism of the thermal decomposition for the cellulose treated with 76 and 77. The analysis showed that the phosphorus additives were stable to the treatment conditions. The untreated cellulose generates more flammable gasses compared to the cellulose containing Compounds 76 and 77.119 Cotton fabrics treated with 7–13 wt % of 76 and 77 passed the vertical flammability. For the corresponding treatments, the LOI values of the treated cotton fabrics with Compound 77 were 26.7–33.1% and for 76 were 23.7–31.0.120
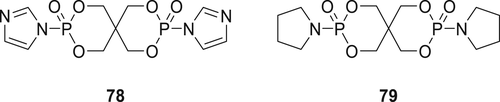
Spirocyclic phosphoramidate derivate 78 (Figure 21) was synthesized via one-step reaction of imidazole with spiral phosphodichloride (SPDPC)121 and coated on cotton fabrics with various aqueous formulations of 78, cyanuric acid and phosphoric acid. Before treatment, the cotton fabric was boiled in water for 30 min and dried at room temperature. Then, blank cotton fabric was immersed in an aqueous solution of different formulations of 78. In these formulations, Compound 78 was used as an intumescent FR, cyanuric acid as a crosslinking agent and phosphoric acid (H3PO4) as a catalyst. Five different formulations were used from 10 to 30 wt % loading of 78. In fire tests, the treated cotton fabrics with 30 wt % of Compound 78 exhibited self-extinguishing behavior and achieved an LOI value of 36.6%. The cured cotton fabric exhibited improved thermal stability and 13.3% decrease in the tensile strength compared to the untreated cotton fabric. Cone calorimetry experiments on the treated cotton showed a decrease in heat release rate by 57% and total heat formation decreased by 60% compared to the blank cotton fabric. Furthermore, a similar precursor of phosphoramidate derivate 79 (Figure 21) was synthesized by reacting pyrrole with di(phosphate monochloride) (SPDPC) in the presence of TEA as a base in acetonitrile. The TGA data of 79 showed the initial decomposition temperature was around 270 °C, along with 5% char yield at 600 °C and may be useful as FRs for some polymer during melt processing.
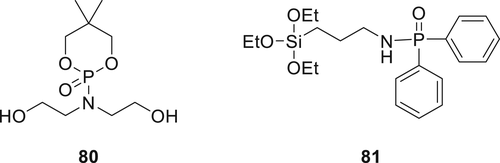
Hydroxyl containing spirocyclic phosphoramidate 80 (Figure 22) was prepared by reacting neopentyl glycol with POCl3 in acetone, followed by dropwise addition of DEA and TEA in acetone at 0–5 °C.122 Cotton fabrics were treated with various concentrations (10, 15, 20, 25, and 30%) of Compound 80 in acetone/water (1:4 v/v) solutions. The treated fabrics passed the vertical burning tests and achieved higher LOI values (27.0–29.3%), respectively, compared with neat cotton (19%). The cone calorimetry results showed that improved flame retardancy of treated cotton fabrics with Compound 80 was due to the phosphorylation reaction, which promotes phosphorus-rich char formation layer in the condensed phase and restricts the release of combustible gases in the gaseous phase.
A silicone containing phosphoramidate 81 (Figure 22) was synthesized by reacting diphenylphosphinic chloride with (3-aminopropyl)trimethoxysilane in the presence of a base. The cotton fabric was treated with various concentrations of Compound 81 in ethanol/water solutions. Subsequently, the treated fabrics were dried at 140 °C and evaluated for their thermal stability and flame retardancy. TGA measurements showed increased char formation for treated cotton fabrics above 500 °C. Treated cotton fabrics exhibited self- extinction as soon the flame source was removed. The treatment was shown to be durable to several washing cycles.123
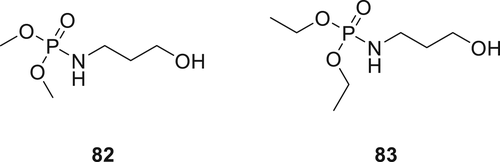
Two hydroxyl functional phosphoramidate derivatives, dimethyl 3-hydroxypropylphosphoramidate 82 and hydroxypropylphosphoramidate 83 as shown in figure 23 were synthesized and applied on cotton twill fabric at 5–20 wt % loadings.124 Cotton twill fabric with 5 wt % of both compounds showed self-extinction behavior. Furthermore, all fabrics treated with 82 exhibited higher LOI values (27.0–37.2%) compared to the 83 treated fabrics (25.8–33.4%). In the MCC test, lower values for THR and pHRR were observed for all fabrics treated with Compound 82 as compared to the fabrics treated with Compound 83. Furthermore, the THR (3.9–3.8 kJ g−1) of 82 treated samples decreased with increasing add-on, while the THR (4.6–6.3 kJ g−1) of 83 treated cotton increased with increasing add-on. TGA results showed that the char yield increased for 82 (33–36%) and 83 (28–31%) treated fabrics with the increasing add-on. Evolved gas analysis using TGA–FTIR showed that Compound 82 decomposes to yield a terminal amine. Further, this terminal amine could react with decomposing cellulose to enhance its char formation or volatilize into an active species in the gas phase. Additionally, the FR efficacy of 82 was related to the ability of O-alkyl group to form a covalent bond with cellulose.
Various phosphoramides and phosphoramidates (Figure 24) were synthesized, applied together with a thermal initiator on cotton fabrics and cured at high temperature.125 Compounds 85 and 87 showed a lower degree of grafting (DG) and char formation in TGA experiments compared to Compounds 84, 86, and 88. The DG for each monomer was estimated at three different concentrations of monomers. It was observed that the DG increased by increasing the monomer concentration. Monomers (84, 86, and 88) containing unsaturated (CC) bonds have the ability to graft to the cotton fabrics. TGA/differential scanning calorimetry results of the treated cotton showed an enhancement in the char formation and confirmed the action of monomers in the condensed phase.
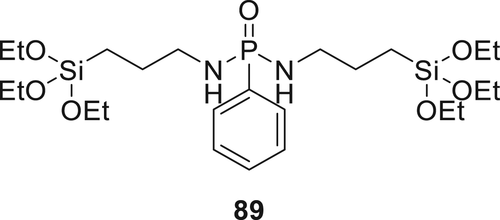
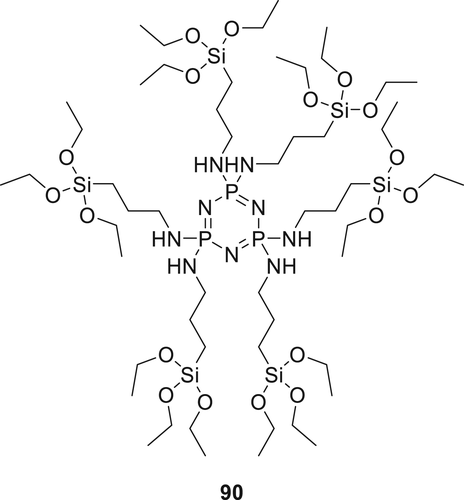
Triethoxysilane derivative 89 (Figure 25) was synthesized and applied on cotton fabric. The cotton fabrics were treated with a 10 wt % solution (water and ethanol, v/v, 1:1 of 89 for different treatment times. The treated cotton fabrics instantly self-extinguished once the flame source was detached.126 Hybrid materials prepared by combining Compound 89 and thiol–ene photocurable curable resin. The sol–gel precursor was synthesized by reaction of methacryloxypropyltrimethoxysilane with an alkoxide in ethanol. Water was used for hydrolysis in the presence of a catalyst (p-toluene sulfonic acid). The combination of sol–gel precursor and Compound 89 not only increases the thermal stability of the materials but also improved the flame retardancy of the hybrid material (LOI of the hybrid material was 26.5%).127 Similar synthetic methodology was used to synthesize 90 (Figure 26) and subsequently used to coat the cotton fabrics. However, lower thermal stability for treated fibers was observed compared to the fibers treated with the previously synthesized FR hybrid system. An increase in char residue was observed for treated fibers with increasing concentration of Compound 90. Fire performance of the treated and untreated fabrics was investigated via LOI and vertical test. When add-on of 80 in fabrics was increased from 5.4 to 18.7%, the LOI value improved from 20.5 to 24.5% respectively.
Thus, hybrid Compound 90 improved the flame retardancy of the cotton fibers by improving char formation, which stops the decomposition of the underlying substrate and restricts the formation of flammable gases throughout the thermal degradation process. The fabric's treatment durability was also confirmed as there was no change observed in phosphorus content of the fabrics after the laundry experiments.128
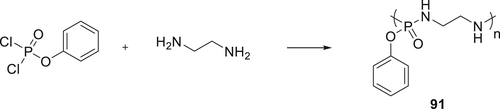
Poly(phosphorodiamidate) 91 was synthesized by the reaction of ethylenediamine with phenyl dichlorophosphate (Scheme 10) and was used to coat cotton fabrics. The cotton fabrics were immersed treated with various concentrations of 91 along with tetraethyl orthosilicate (TEOS) which acts as a crosslinker to increase the networking of 91 on the fabric. The treated cotton fabrics were dried at 160 °C and were laundered before further fire tests. Vertical flame tests were used to investigate the flammability of treated cotton fabrics. Based on phosphorus–nitrogen synergism, oligomer 91 exhibited excellent flame retardancy on treated cotton fabrics compared to the untreated cotton fabrics. The fire test showed, for a loading of 3.5 wt % of 91 and 1 wt % TEOS, an LOI value of 27.2% achieved. TGA results showed that treated cotton fabrics with 91 were more thermally stable as compared with blank cotton fabrics.129
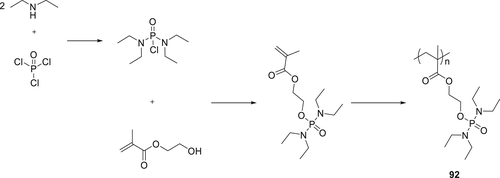
Polymer 92 was synthesized from the polymerization of methacryloyloxyethylorthophosphortetraethyl diamidate via a free-radical polymerization with benzoyl peroxide as an initiator. The monomer was synthesized via a three-step reaction shown in Scheme 11. Different concentrations of aqueous solutions of polymer (92) were used to treat the cotton fibers in the presence of a crosslinker and a binder (vinyl acetate).
The cotton fabrics treated with polymer 91 (3–4 wt %) and vinyl acetate as a binder, their LOI values range 18.6–19%, respectively, as compared with blank cotton fabric (17%). Some improvement in LOI values (2–3%) was observed but it was considered not enough to act as flame barriers.130
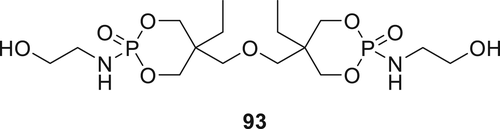
Recently, Compound 93 was synthesized using ditrimethylolpropane with phosphoryl chloride as starting materials (Figure 27) and was applied to polyester, cotton, and nylon fabrics via a dipping method. The fabrics were dried first and then cured at 160 °C. The fire performance of the cured fabrics was tested using vertical flame tests and LOI measurements, and the results show that treated nylon fabrics possess better flame resistance compared to other treated fabrics.131
Poly(lactic acid)
Poly(lactic acid) (PLA) is known for its good biodegradability and biocompatibility as well as having a relatively high melting point compared to other biopolymers. Because of these properties, PLA and its copolymers are used in nonwoven and woven textiles, packaging, electronics, and agriculture.132-135 Due to these useful properties, it is expected that in the future, PLA will be increasingly used in sectors such as electronic equipment and transportation were a high level of flame retardancy is required. The flammability of PLA is reduced by mixing it with phosphorus-containing compounds, antimony trioxide, halogenated, and nitrogen compounds.136-139
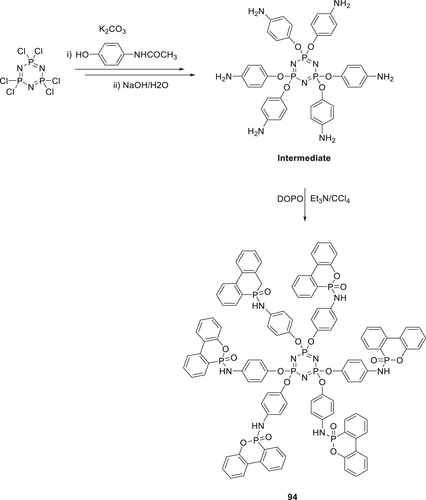
A series of phosphazene and phosphaphenanthrene derivatives (94–97) as shown in Schemes 11 and 12 have been synthesized as FR additives for PLA. The synthesis of 94 was performed via a three-step strategy. In the final step, DOPO reacted with amino containing triazine intermediate using CCl4 in the presence of TEA via AT reaction to form 94 as shown in Scheme 12. Furthermore, condensations of this intermediate by three different types of aldehydes along with DOPO via Kabachnik–Fields reaction with an imine linkage yields Compounds 95, 96, and 97 ,respectively, as shown in scheme 13.140
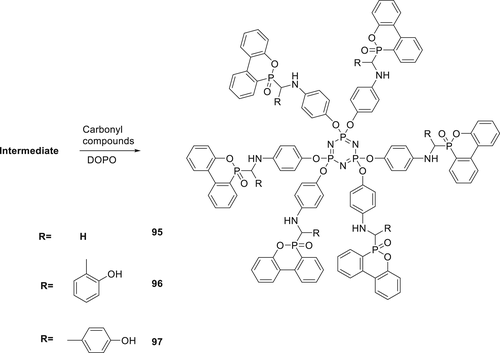
The fire performances of Compound 96 were investigated by mixing with PLA via melt blending. An LOI value of 30% was achieved at a 5 wt % loading of Compound 96. However, for PLA composite with 5 wt % 94, an LOI value of 34.7% and a V-0 rating in UL-94 test was achieved.141
Poly(butylene terephthalate)
Poly(butylene terephthalate) (PBT) is widely used as an insulator in the electronics and electrical industries. PBT is the semicrystalline polymer that is resistant to shrinkage and solvents as well as being mechanically and thermally stable up to 150–200 °C. Flame retardancy of this material can be achieved by incorporating FR additives.
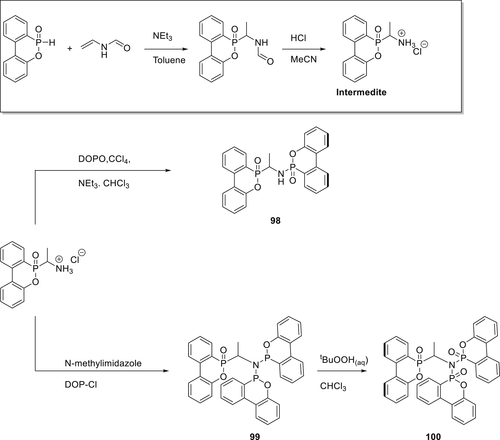
Phosphorylated ethylamine derivatives (98 and 100) were synthesized as shown in scheme 14 and investigated for their FR properties in PBT. Initially, an intermediate was synthesized via an addition reaction between DOPO and N-vinylformamide as a catalyst. Further, an acidic workup gives a stable intermediate, which was used further to synthesize storable, stable amines derivatives (98 and 100). The synthesis of 98 was performed via AT reaction using CCl4 as chlorinating agent. Compound 100 was synthesized via multistep synthesis procedure, which involves the reaction of trivalent DOP─Cl with the intermediate in the presence of a base to form 99. Afterward, oxidation of 99 produces the desired product in high yield. PBT composite produced via injection molding containing 20 wt % of 100 achieves V-2 classification in a UL-94 fire test. The TGA results show that the decomposition temperature of 99 is relatively low to be suitable for engineering plastics and thus it was not further tested.142
Polycarbonates
Polycarbonate (PC) is extensively applied in the electronics, construction, and automotive industries because of its outstanding properties such as stiffness, good hardness, transparency, impact strength, thermal stability, and dimensional stability. In the UL-94 test, even if the PC displays a V-2 rating, lower FR ratings are required for electronic and electric applications. Therefore, many FR additives have been developed to improve the fire performance of PC.143-145
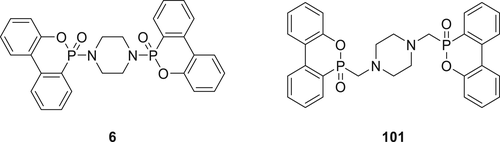
The fire performance for the PCs containing Compound 101 (Figure 28) has been investigated and compared to PC containing 6. Only 3% of Compound 101 in PC is required to achieve V-0 classification in the UL-94 test. In comparison, a 10% loading of 6 is required to achieve the same (V-0) classification in the UL-94 test. Regardless of the similarities in the structures, Compound 6 is far less active.146 Even though both FRs show some FR activity in the gas phase, 101 is believed to offer excellent flame retardancy because of the catalyzed degradation of PCs through the active tertiary amine group.
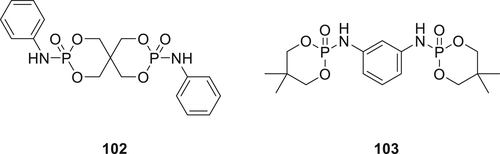
Two intumescent FRs (IFRs) 102 and 103 were synthesized as shown in Figure 29 and incorporated in PC.147 The synthesis of IFR 102 was achieved via reaction of pentaerythritol with POCl3 in acetonitrile followed by a second reaction with aniline. The IFR 103 was obtained via a two-step reaction involving the synthesis of 2-chloro-2-oxo-5, 5-dimethyl-1, 3, 2-dioxaphosphorinane (DOPC) via reaction of neopentyl glycol with POCl3 in chloroform followed by DOPC with m-phenylenediamine in the presence of a base as a catalyst. PC containing 5 wt % IFRs passed the UL-94 burning test with V-0 classification with an LOI value of approximately 35%. TGA experiments proved that both IFRS showed increased char forming capability when tested alone or together with PC.
Acrylonitrile–Butadiene–Styrene
Acrylonitrile–butadiene–styrene (ABS) composites are well known for their chemical resistance, excellent mechanical properties, and easy processing. ABS resins are widely used in electrical appliances, office supplies, and in the automobile industry. However, flammability of ABS resins is a critical issue. Therefore, it is vital to improve the flammability of ABS resins, which is mostly obtained by incorporating suitable FR additives.
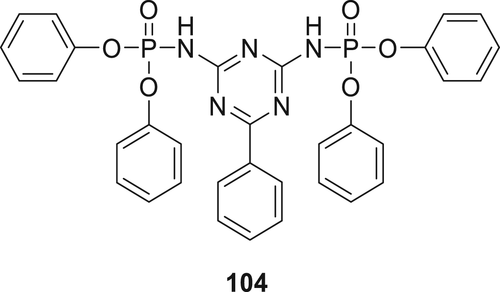
4-(Diphenoxyphosphorylamino)-6-phenyl-[l,3,5] triazin-2-y1]-phosphoramidic acid diphenyl ester (104) as shown in figure 30 was synthesized via a reaction of benzoguanamine with diphenyl phosphorochloridate in THF in the presence of a base. To study the flame retardancy of this additive, various formulations of ABS resins were studied. These formulations consisted of several concentrations of LDHs (Mg─Al─Co—layered double hydroxides) and additive 104. However, in the UL-94 test, only a V-2 classification could be achieved for these formulations. LOI values of ABS/104 and ABS/104/LDHs composites was 23.9 and 24.7%, respectively, which is higher compared to the blank control ABS (18.1%).148
Polyolefins and Related Polymers
Polypropylenes (PPs) and polyethylene (PE) are relatively inexpensive materials that offer many benefits such as physical flexibility, useful electrical, mechanical, and thermal properties as well as corrosion resistance, natural processability, and chemical stability. These properties make them attractive candidates for application in the electronics, automotive, and electrical industries. PP and PE are highly flammable polymers because they contain high amount of carbon content, which burns straightforwardly with a fast fire spread. During combustion, they produce toxic gases, which restrict them to be used in high-risk applications. Therefore, it is essential to improve the flammability of PP and PE.149-153
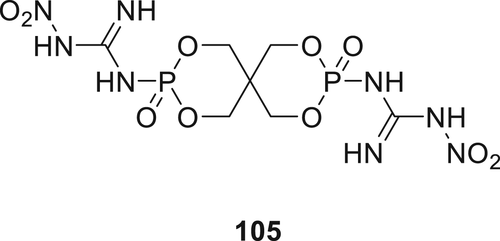
P(O)–N containing IFR 105 (Figure 31) was synthesized and incorporated into linear low-density PE (LLDPE). At a 30 wt % of IFR 105 in LLDPE composite, the LOI values increased to 31% compared to 17% for the blank. The TGA results of the additive showed improved thermal stability and the char formation for LLDPE.154
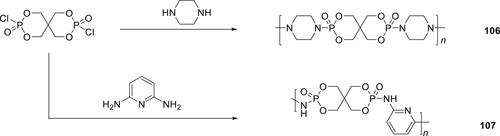
P–N containing polymeric IFR poly(piperazine spirocyclic pentaerythritol bisphosphonate) (PPSPB; 106) was synthesized as shown in scheme 15 and applied to PE)/ethylene(vinyl acetate) copolymer (LDPE/EVA) mixture. Nanocomposite containing Compound 106 and organically modified montmorillonite (OMMT) LDPE/EVA was also studied. The combined effects of PPSPB and OMMT on thermal stability and flame retardancy were investigated by TGA and cone calorimetry.155 The mixture of 106 and OMMT enhanced the thermal stability and significantly decreased the flammability of the composites. The SEM results showed that upon burning, a dense and compact char was obtained for the LDPE/EVA/IFR/OMMT nanocomposites. Compound 106156 was also combined with a triazine-based polymer as a char forming agent (CFA) and ammonium polyphosphate (APP) and melt blended in PP to reduce its flammability. For formulation containing a total of 30 wt % of FR [106:APP:CFA (3:6:2 wt %)] loading, a V-0 rating in UL-94 test along with an LOI value 39.8% was achieved. Moreover, cone calorimeter analyses showed that pHRR and the THR of the 106/PP formulations decreased significantly compared to the pure PP.
P(O)–N containing oligomeric Compound 107 was synthesized and grafted on multi-walled carbon nanotube (MWNT), and EVA used as a copolymer.157 The EVA/MWNT-g-107 nanocomposites containing 0.2, 0.5, 1.0, and 2.0 wt % of MWNT–107 were prepared through melt compounding at 100 °C. UL94 tests showed that grafted EVA/MWNT-g-107 nanocomposites failed to pass the flame retardancy tests.
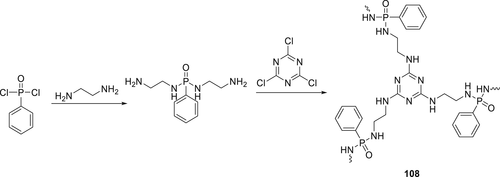
A novel polymeric FR 108 containing phosphorus oxynitride, phenyl, and triazine groups was synthesized from phenyl phosphonic dichloride, cyanuric chloride, and ethylenediamine starting materials (Scheme 16) and incorporated in PP along with APP.158 By using 25 wt % of APP: PTPA (2:1) in PP, an LOI value of 34.0% and V-0 rating in UL-94 was obtained. In the cone calorimetry tests, the pHRR values decreased by about half on the addition of either APP or 108 separately, compared to the pure PP. However, combining 108 with APP, the PHRR was reduced to almost 3 times (121 kW m−2) compared to the blank PP. The TGA results show that 108 undergoes two-stage thermal decomposition process, the first step of the decomposition process occurred at 269–359 °C and the second step at 359–700 °C. Cone calorimeter test and SEM of char residue indicate that decomposed products of 108 and APP promote the formation of the firm and compact char layer. In a different work, Compound 108 was combined with APP and was used to prepare an FR composite of 10 wt % 108/60 wt % wood fiber/APP (20 wt %).159 The LOI values of 31.5% and V-0 rating were achieved for these composites.
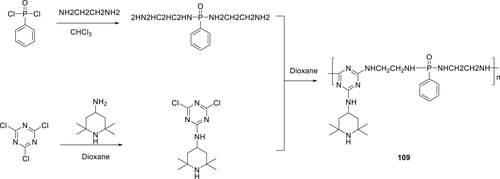
A macromolecular additive containing P–N group (109) (Scheme 17) with the capability of free-radical quenching was synthesized and used as an FR additive together with APP for PP. The thermal stability and flammability of PP/APP /109 composites were studied by LOI, TGA, UL-94, and cone calorimetry experiments. For 25 wt % content of 109/APP (mass ratio of 109 to APP was 1:1) in PP a UL-94, a V-0 rating along LOI of 29.5% was achieved. In cone calorimeter test, for blank PP, a pHRR of 916.5 kW m−2 was observed at 210 s. However, when 12.5 wt % of 109 and 12.5 wt % APP was incorporated in PP, the matrix showed a decrease (72.1%) in the pHRR.160
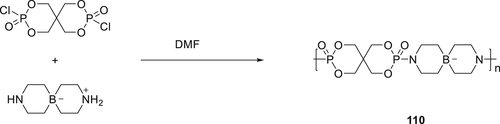
Polymeric FR 110 containing boron was synthesized by the reaction of SPDPC with diethanolamine borate in DMF solvent as shown in scheme 18. Compound 110 was combined with APP and incorporated in LLDPE. Improved thermal stability was observed for the modified LLDPE. For a 30 wt % APP/poly(pentaerythritol spirocyclic phosphorusoxy spirocyclic diethanolamine borate) (PPSPSDB) loading in LLDPE, an LOI of 29.6% and V-0 rating in UL-94 test was observed. The addition of 30 wt % polymeric FR 110 into LLDPE increased the thermal stability and char residue up to 15.06% at 800 °C. The reason for higher flame resistance of 110 is the synergic effect of the P, N, and B elements. In the condensed phase, LLDPE/110/APP systems thermally decompose, the phosphorus oxide (PO) bonds provide the acid resource, the nitrogen (N) atoms turn into incombustible gas (N2), and ammonia (NH3) and finally, the boron (B) atoms get converted into boric anhydride (B2O3) or boric acid (H3BO3). In the condensed phase char layer consist of PO, B─O, P(O)–N, and P─O─P, and so forth substructures. Meanwhile, APP decomposes to produce phosphoric acid and promote the formation of a compact and stable char layer.161
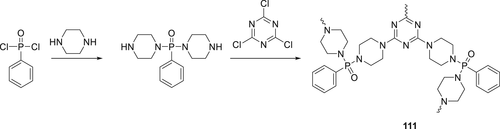
A P(O)–N containing hyperbranched macromolecule (HCFA) 111 was synthesized (Scheme 19) and used with the combination with APP as fire retardant PP. PP composite along with IFR and APP passed UL-94 V-0 rating and achieved an LOI value 31% at 20 wt % IFR/APP (1/2, w/w). Besides, 111 was compared with another hyperbranched CFA (hyperbranched and phosphorus-containing charring-foaming agent [HPCFA]).162 HPCFA (111) and HCFA have similar chemical structure, differing only in the main chain phosphorus containing unit of HPCFA. Cone calorimetry showed that the incorporation of 20 wt % 111/APP (ratio 1:2) reduces pHRR of PP by 86.24%. Analysis of char residue showed that 111/APP system forms a dense, compact char layer compared to the HCFA/APP system.163
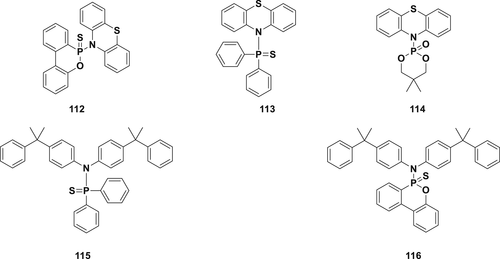
A series of P–N containing compounds (112-116) as shown in figure 32 were designed and synthesized from corresponding phosphorus chloride and nitrogen-based nucleophiles in the presence of lithium source (diisopropylamide or n-butyllithium) followed by oxidation with elemental sulfur (S8) to obtain the FRs and were used as a stabilizer with PP. The results indicate that it is possible to process PP with these additives in the melt (extrusion, injection molding, etc.) without any degradation of PP.164
CONCLUSIONS
Phosphorus is a central focus for the development of new FRs due to its chemical versatility, activity in both phases (condensed and gas phase) and ability to function as a reactive component or additive. The different oxidization states of phosphorous and synergistic propensity with various elements make phosphorous an ideal candidate for the advancement of FR materials. P(O)–N bond formation using chlorinating agents such as CCl4, t-BuOCl, Cl2 gas, CuCl2, SO2Cl2, NCS, and TCCA from various phosphorus-based starting materials are the most commonly used methods in academia for the development of FRs. Some methods that involve the use of TCCA, NCS discussed in this review is commercially feasible if the FR properties outweigh its cost of production. Furthermore, the use of other synthetic strategies such as Michaelis–Arbuzov rearrangement, oxidative coupling methods using iodine or copper, the Staudinger-phosphite reaction, metal-catalyzed amidation by using phosphoryl azides, and green synthetic methodology, are seldom used by researchers to synthesize P(O)–N containing organophosphorus FR derivatives. One can find numerous literature that focuses on the development of P(O)–N compounds such as phosphinamide, phosphonamide, phosphoramide, phosphoramidates, phosphorodiamidate, phosphonamidate, pentaerythritol bisphosphonate, and P(S)–N containing phosphorus compounds such as phosphoramidathioates and thiophosphinamide as FR additives on various polymers, however, their commercialization is still limited. They have been shown to be very effective in flame retarding diverse class of polymers such as on PU, EPs, polyamides, polyolefins, cellulose, PLA, PBT, PCs, and ABS. With increased demands toward recyclablility, nontoxicity, and sustainable materials, phosphorus-containing compounds have the potential to fulfill all the requirements of future FR additives. Future FR research will focus on sustainable synthetic methodologies, development of polymer-based FR additives and the use of bio-based resources. Furthermore, investigations into synergism of phosphorus (P) with various moieties (P─Si, P─B, P─P, etc.) and multicomponent arrangements will keep evolving with the central goal to decrease the FR loading and increase FR performance.
Biographies

Dr. Rashid Nazir obtained his Ph.D. from the Warsaw University of Technology in 2015, under the Supervision of Prof. Dr. Daniel T. Gryko. In July 2017 he joined the group of Dr. Sabyasachi Gaan as a Postdoctoral researcher at the Empa, Swiss Federal Laboratories for Materials Science and Technology. His research interests are mainly focussed on the synthesis of initiators, flame retardant additives and crosslinker, polymer, organic-functional dyes and pH sensitive gels.

Dr. Sabyasachi Gaan obtained his Ph.D. in Chemistry from UC Davis, California in 2007. He is currently working as a Group Leader in Empa, Swiss Federal Laboratories for Materials Science and Technology. His research interest include development of additives for polymers, synthesis of functional gels and high thermally stable polymers, metal-polymer composites, functional coatings for metals and textiles.




