Zirconium catalyzed amide formation without water scavenging
Abstract
A scalable homogeneous metal-catalyzed protocol for direct amidation of carboxylic acids is presented. The use of 2–10 mol% of the commercially available Zr(Cp)2(OTf)2·THF results in high yields of amides at moderate temperature, using an operationally convenient reaction protocol that circumvents the use of water scavenging techniques.
1 INTRODUCTION
The amide functionality is one of the most fundamental chemical units that constitutes the backbone of proteins and is found in synthetic products such as pharmaceuticals, polymers and agrochemicals.1 Commonly, amides are formed with the use of stoichiometric coupling reagents to activate and protect the carboxylic acid. As a result, these processes display low atom economy and green methodologies for the transformation have been determined as a key green research area by the ACS Pharmaceutical Roundtable in both 2007 and 2018.2 Catalytic condensation of carboxylic acids and amines to form amides is a highly sustainable reaction, since one equivalent of water is formed as the sole by-product. Despite the atom efficiency, the number of catalytic protocols for this transformation is limited3 and the formation of ammonium carboxylate salt is considered one of the main challenges (Scheme 1).
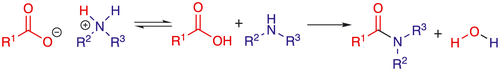
Typically, catalysts for direct amidation are Lewis acidic compounds based on boron or transition metals.4 In the latter class, Group (IV) metal complexes constitute the most well-documented catalyst type.5 A frequent complication of homogeneous catalytic direct amidation is that the generated water deactivates the catalyst and/or reactive intermediates. For this reason, water scavenging techniques such as azeotropic distillation or addition of molecular sieves are commonly employed, where the latter find their use in mild protocols at low reaction temperatures. Although convenient enough on a laboratory scale, the need to continuously remove water using molecular sieves is a major drawback from a scale-up perspective. For this reason, robust and water tolerant amidation catalysts are of high importance to enable catalytic formation of amides under mild homogeneous conditions. Trifluoromethanesulfonate (triflate, OTf) complexes of rare earth metals are a class of catalysts that meet these requirements and have been employed for a variety of reaction types.6 Recently, the work of Li et al. demonstrated that polyfluorinated alkylsulfonate complexes of zirconium were able to activate carboxylic acids, nitriles and primary amides to afford secondary amide products under non-dry conditions.7 However, a significant limitation of Li′s protocol is the use of perfluorooctanesulfonate (PFOS) counterions. PFOS is listed as a persistent organic pollutant (POP) under the Stockholm Convention and is regulated under the European Chemicals Regulation (REACH EC No. 1907/2006) and other international regulations due to its persistent nature, and bioaccumulative and toxic properties.8 In contrast, the considerably smaller triflate unit displays no such restrictions.
2 EXPERIMENTAL
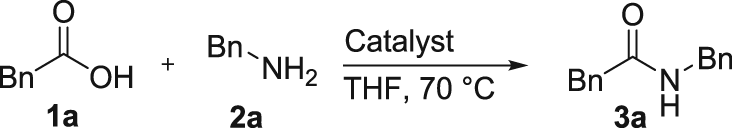
Phenylacetic acid 1a (0.5 mmol) and Zr(Cp2(OTf)2·THF (5.9 mg, 0.01 mmol, 2 mol %) were added to an oven dried 10 ml microwave tube equipped with a magnetic stir bar and sealed with a crimp-on cap with septum. The atmosphere was exchanged for N2 by three consecutive vacuum cycles after which THF (1.25 ml) was added. The reaction mixture was heated to 70 °C in an oil bath, benzylamine 2a (1 mmol) was added and the reaction was stirred for 48 hrs. The crude reaction mixture was filtered through a pad of silica (~15 g) in a glass filter funnel, rinsed with 100 ml ethyl acetate:triethylamine (20:1 mixture) and concentrated under vacuum to afford amide 3a in 94% yield. 1H-NMR (400 MHz, CDCl3) δ 7.22–7.38 (m, 8 H), δ 7.15–7.20 (m, 2 H), δ 5.66 (bs, 1 H), δ 4.42 (d, J = 5.9 Hz, 2 H), δ 3.64 (s, 2 H). 13C-NMR (100 MHz, CDCl3) δ171.0, 138.3, 134.9, 129.6, 129.2, 128.8, 127.6, 127.6, 127.5, 44.0, 43.7.
3 RESULTS AND DISCUSSION
We have previously reported that chloride complexes based on hafnium,5 zirconium5 and titanium5 are active as direct amidation catalysts in the presence of molecular sieves at 27–70 °C, and were interested in studying the activity of the corresponding triflate complexes under non-dry conditions. Hence, a series of commercially available metal triflate compounds were evaluated as catalysts for the condensation of phenylacetic acid 1a and benzylamine 2a, using the same solvent and reaction temperature that was previously employed using ZrCl4 in catalytic amounts.5 Gratifyingly, it was found that both Hf(OTf)4 and Zr(Cp)2(OTf)2·THF were catalytically active (Table 1, entries 6 and 7), resulting in approximately 50% yield after 72 hours. For comparison, ZrCl4 was evaluated under the same conditions and reaction time, resulting in 17% yield (Table 1, entry 9). This result is similar to that obtained in the absence of any catalyst (Table 1, entry 10) and indicates that the catalytic ability of the zirconium chloride complex is hampered by the water formed in the reaction, in contrast to the metallocene triflate compounds. The higher solubility in organic medium and the lower molecular weight of the zirconocene complex compared to the hafnium compound prompted us to continue our study using the former as catalyst.
 |
||||
|---|---|---|---|---|
| Entry | Catalyst | Mol% | Time (h) | 3ac (%) |
| 1a | La(OTf)3 | 10 | 108 | <5 |
| 2a | Yb(OTf)3 | 10 | 108 | <5 |
| 3a | Y(OTf)3 | 10 | 108 | <5 |
| 4a | LiOTf | 10 | 72 | <5 |
| 5a | Ba(OTf)3 | 10 | 72 | <5 |
| 6a | Hf(OTf)4 | 10 | 72 | 49 |
| 7a | ZrCp2(OTf)2·THF | 10 | 72 | 49 |
| 8b | ZrCp2(OTf)2·THF | 2 | 48 | 94 |
| 9a | ZrCl4 | 10 | 72 | 17 |
| 10a | - | - | 72 | 12 |
| 11b | - | - | 48 | 16 |
- a [1a] and [2a] = 0.1 M, 70 °C, THF, N2 atmosphere.
- b [1a] = 0.4 M, [2a] = 0.8 M, 70 °C, THF, N2 atmosphere.
- c isolated yield.
In analogy with previously reported Group IV metal-catalyzed amidation protocols,5, 9 excess amine was beneficial for the reaction outcome and enabled a decrease in catalyst loading (see Supporting Information). As a result, 94% yield was observed in 48 hrs using 2 mol% catalyst and starting concentrations of 0.4 M and 0.8 M for 1a and 2a, respectively (Table 1, entry 8). In the absence of catalyst, thermal amidation resulted in 16% yield of 3a under otherwise identical conditions (Table 1, entry 11). A decrease in reaction temperature from 70 °C to 50 °C resulted in lower yield of 3a after 72 hrs (95 and 51%, respectively, see Supporting Information). High yields of 3a were observed under normal atmosphere using non-dried THF (85% yield after 72 hr), however accompanied by the formation of imine 4a, resulting in a less clean reaction. For this reason, dry and inert conditions were henceforth used. Oxidative dimerization of 2a to form dibenzylimine 4a is a known reaction (Scheme 2) and has previously been reported to be mediated by catalysts based on e.g. cobalt,10 copper11 and ruthenium.12 Full conversion of 1a into 3a was observed as monitored by HPLC for standard conditions with and without addition of molecular sieves or one equivalent of water after 72 hours. Addition of molecular sieves resulted in a faster reaction rate, whereas addition of 5 and 10 equivalents of water slowed down the rate considerably (see Supporting Information).
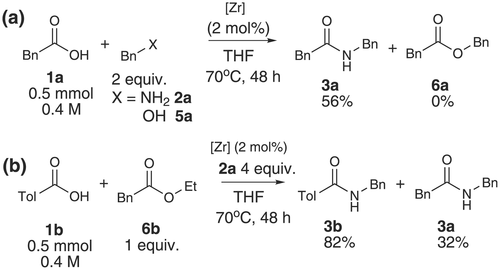
We have previously reported that alcohols inhibit Hf- and Zr-catalyzed amidation.5 Interestingly, this phenomenon was not observed using Zr(Cp)2(OTf)2·(THF) as demonstrated by a competition experiment using two equivalents of benzylamine 2a and benzyl alcohol 5a. The reaction proceeded with full selectivity towards amide product with a 56% yield after 48 hours (Scheme 3A). Furthermore, the catalyst was found to favor amidation of carboxylic acids over esters (Scheme 3B). A competition experiment using p-tolylacetic acid 1b and ethylphenylacetate 6b in the presence of 2a resulted in 82% yield of 3b compared to 32% yield of 3a. Similar selectivity was observed in hafnium-catalyzed amide formation at room temperature,5 while efficient zirconium-catalyzed aminolysis of esters at 100 °C has previously been reported.13
An evaluation of the substrate scope was carried out, demonstrating that the protocol works well with both benzylic and aliphatic amines (Figure 1A). The reaction also proceeded smoothly using heteroaromatic amines (3j and 3 k), whereas aromatic amines failed to form product. Secondary aliphatic amines reacted sluggishly under standard conditions; however, by increasing the reaction temperature to 100 °C the nitrogen-rich amide 3l was isolated in 66% yield. The reaction proved to be scalable from 0.5 mmol to 10 mmol without problems, affording amide 3a in 92% isolated yield using a round-bottomed flask and reflux condenser. An iodo-substituted carboxylic acid was also smoothly converted into its corresponding benzyl amide 3b in good yield, with the halide serving as a useful handle for further functional group manipulations. Both Cbz and Boc protecting groups are compatible with the reaction conditions (3d-e) whereas Fmoc-protected glycine resulted in trace amounts of amide product. Aromatic acids reacted sluggishly even in the presence of a large excess of 2a and extended reaction times with benzoic acid giving rise to higher yields compared to analogues with either electron withdrawing or donating substituents (3f-h). This finding stands in contrast to the previously reported protocol catalyzed by ZrCl4, where electron-poor benzoic acids reacted faster than more electron-rich analogues.9 Although unexpected, this difference does not necessarily indicate different operating mechanisms between the dry and the non-dry Zr-catalyzed protocols and may result from differences in equilibria of ammonium carboxylate salts and/or other off-cycle species.
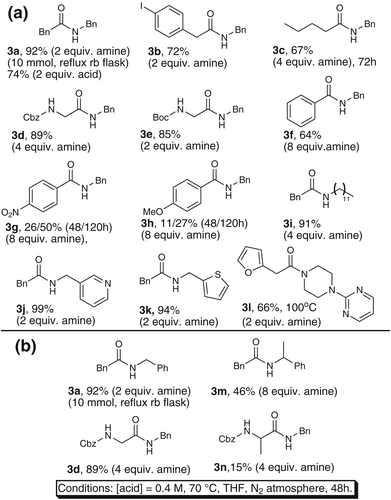
As demonstrated in Figure 1B the reaction is sensitive towards steric hindrance in both the amine and the carboxylic acid. Even in the presence of 8 equivalents of amine, amide 3m was isolated in only 46% yield as compared to 92% of amide 3a after the same reaction time. Similarly, amide 3d was isolated in 91% yield whereas amide 3n was isolated in merely 15% yield. The observed drop in yield for the substrates with a methyl group in the neighbouring position of either the carboxylic acid or amine compared to their H-analogues is analogous to the previously reported hafnium- and zirconium-catalyzed protocols under dry conditions.5
4 CONCLUSIONS
To conclude, a practical and scalable homogeneous protocol for direct amidation using a commercially available zirconium catalyst has been developed that circumvents the use of water scavenging techniques. The system shares several characteristics with previously developed Group (IV) metal-catalyzed protocols for direct amidation, such as higher yields using higher amine concentrations and a preference for amidation of carboxylic acids over esters. The tolerance towards alcohols and stoichiometric quantities of water are however distinct differences of this system compared to previous protocols. Group (IV) metal chloride complexes are known to be hydrolytically unstable and form the corresponding oxides in the presence of water. The observation that fluorosulfonate analogues of water-sensitive chloride complexes can be used as catalysts under non-dry conditions is intriguing and makes these Lewis acidic metal compounds a highly interesting class of robust catalysts for further exploration.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Stockholm University, the K. & A. Wallenberg Foundations and the Swedish Research Council for financial support.




