Non-Natural MUC1 Glycopeptide Homogeneous Cancer Vaccine with Enhanced Immunogenicity and Therapeutic Activity
Graphical Abstract
A chemically defined vaccine is prepared by combining the structural design of a non-natural glycopeptide antigen with a site-specific protein modification. The resulting vaccine, which contains a single copy of the antigen, is both prophylactically and therapeutically effective. Importantly, this approach refutes a prevailing dogma that glycoconjugate vaccines require multivalent presentation of antigens.
Abstract
Glycopeptides derived from the glycoprotein mucin-1 (MUC1) have shown potential as tumor-associated antigens for cancer vaccine development. However, their low immunogenicity and non-selective conjugation to carriers present significant challenges for the clinical efficacy of MUC1-based vaccines. Here, we introduce a novel vaccine candidate based on a structure-guided design of an artificial antigen derived from MUC1 glycopeptide. This engineered antigen contains two non-natural amino acids and has an α-S-glycosidic bond, where sulfur replaces the conventional oxygen atom linking the peptide backbone to the sugar N-acetylgalactosamine. The glycopeptide is then specifically conjugated to the immunogenic protein carrier CRM197 (Cross-Reactive Material 197), a protein approved for human use. Conjugation involves selective reduction and re-bridging of a disulfide in CRM197, allowing the attachment of a single copy of MUC1. This strategy results in a chemically defined vaccine while maintaining both the structural integrity and immunogenicity of the protein carrier. The vaccine elicits a robust Th1-like immune response in mice and generates antibodies capable of recognizing human cancer cells expressing tumor-associated MUC1. When tested in mouse models of colon adenocarcinoma and pancreatic cancer, the vaccine is effective both as a prophylactic and therapeutic use, significantly delaying tumor growth. In therapeutic applications, improved outcomes were observed when the vaccine was combined with an anti-programmed cell death protein 1 (anti-PD-1) checkpoint inhibitor. Our strategy reduces batch-to-batch variability and enhances both immunogenicity and therapeutic potential. This site-specific approach disputes a prevailing dogma where glycoconjugate vaccines require multivalent display of antigens.
Introduction
Mucin-1 (MUC1) is a highly O-glycosylated glycoprotein expressed on the surface of epithelial cells. Its extracellular domain consists of tandem repeats of 20 amino acids (AHGVTSAPDTRPAPGSTAPP) containing five potential O-glycosylation sites (shown as bold letters in the peptide sequence).1 While this protein displays complex oligosaccharides in healthy tissues, MUC1 is decorated with simple and truncated carbohydrates in tumor cells, where its expression is dramatically increased2 due to malfunction or translocation of GalNAc-transferases,1b or mutations in COSMC, a chaperone required for glycosyltransferase C1GalT activity.3 As a result, the immunogenic epitope APDTRP4 and several tumor-associated carbohydrate antigens (TACAs) such as Tn (αGalNAc-Ser/Thr, hereinafter Tn-Ser and Tn-Thr, respectively), Thomsen–Friedenreich (TF, βGal-(1,3)-αGalNAc-Ser/Thr) or sTn (αNeu5Ac-(2,6)-αGalNAc-Thr antigens become exposed and may elicit a weak immune response.5 In this regard, several studies have reported that cancer patients can develop anti-MUC1 antibodies at early stages of the disease that recognize this tumor-associated MUC1 (TA-MUC1).6 These findings have contributed towards the development of MUC1-based vaccines, most of which carry the complete sequence of MUC1 glycosylated at one or more sites with Tn or other TACAs conjugated to protein carriers, liposomes, or nanoparticles, among others.7 This strategy favors the multipresentation of the antigen but, on the other hand, leads to a heterogeneous conjugate and may result in poorly reproducible formulations and variable efficacy.8 Despite these efforts, there have been no successful clinical applications to date,7e likely because aberrantly glycosylated proteins can be present at low concentrations on healthy cells, leading to immune system tolerance and, consequently, poor immune response in pre-clinical mouse models. Another potential reason for this immune tolerance could be interactions between Siglecs, which are known modulators of the immune response, and sialic acid.9
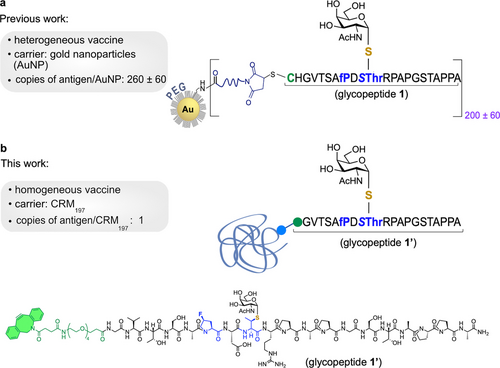
We and others are developing one approach to potentially overcome immune tolerance which is based on the use of non-natural MUC1 antigens.10 These derivatives should be more immunogenic by design, as they are synthetic and stable towards enzymatic degradation.11 We recently reported12 an unnatural MUC1 glycopeptide containing the unnatural (4S)-4-fluoro-l-proline residue and a Tn surrogate with an S-glycosidic linkage (S-(α-d-GalNAc)-thiothreonine) that replace the first proline and the Tn-Thr antigen at the alanine-proline-aspartic acid-threonine-arginine-proline (APDTRP) epitope, respectively (Figure 1a). This derivative was displayed randomly in the surface gold nanoparticles (AuNP) and, when administered prophylactically, elicited a potent humoral immune response in mice.
A site-specific approach for the construction of non-natural MUC1 protein conjugate vaccines for cancer vaccination and treatment.
With the goal of developing a formulation with definite advantages regarding standards and manufacturing costs relative to heterogeneous displays, we decided to site-specifically conjugate a potent non-natural antigen to the clinically approved the CRM197 (Cross Reactive Material 197) protein, a non-toxic mutant of diphtheria toxin (Figure 1b). This genetically detoxified protein carrier is commercially available, its structure and molecular properties are well defined,13 and it is widely used as a carrier for polysaccharides and haptens. CRM197 has been effectively used to generate immunity in infants and young children. For example, it is a key component in Menveo®, a tetravalent conjugate vaccine that protects against Neisseria meningitidis serogroups A, C, W135, and Y.14 It is also used in Prevnar®, a pneumococcal vaccine that conjugates the capsular polysaccharide of the most common types of Streptococcus pneumoniae to CRM197.15 This carrier protein remains the most used carrier protein for human vaccine development.16 To date, most conjugate vaccines built using CRM197 have employed random conjugation of lysine residues to achieve heterogeneous multivalent display of antigens on the protein surface. In one example, conjugation of multiple copies of a mimetic of the Tn-Thr antigen enhanced antibody titers and slowed the progression of triple-negative breast cancer in mice,17 but produced detrimental structural and pharmacological effects to CRM197. More recently, disulfide-re-bridging approaches have emerged to site-specifically modify solvent exposed disulfide bond between cysteine186 and cysteine201 (C186–C201) relative to C461–C471.13, 18 These strategies generate homogeneous constructs that retain CRM197 structural integrity while preserving its immunogenicity.18, 19 In one study,19 conjugation of Salmonella O-antigen to C186–C201 resulted in significantly higher antibody titers in mice relative to heterogeneous conjugation to lysine residues 37 and 39, which highlights the importance of the conjugation site and correct antigen presentation to the immune system.20 In addition, this study showed that a single copy of the antigen at a precise site may be sufficient to elicit high antibody levels.
Considering this compelling experimental evidence, we set out to design homogeneous vaccines that feature CMR197 site-specific disulfide re-bridging with the use of a non-natural MUC1 glycopeptide (glycopeptide 1’, Figure 1b). The homogeneous non-natural MUC1-derived vaccine produced strong immune responses in mice, and the elicited antibodies selectively recognized the naturally occurring antigen in human cells. In addition, vaccine-mediated activation of Th1 immune response and generation of T cells that promote cytotoxic T lymphocyte (CTL) responses led to a significant delay in tumor growth. Importantly, when administered therapeutically, either alone or in combination with a programmed cell death protein 1 (PD-1) immune checkpoint inhibitor, this homogeneous vaccine significantly increased the survival of mice bearing colon adenocarcinoma and pancreatic cancer. The combined advantages of using synthetic non-natural antigens and site-specificity could allow applications to vaccine design strategies with reduced batch-to-batch variation and with significant immunogenicity and therapeutic potential.
Results and Discussion
Synthesis of an Artificial MUC1 Glycopeptide and Disulfide-Specific Bioconjugation to the Carrier Protein CRM197
We started by designing and synthesizing glycopeptide 1’ (Figure S1), which closely resembles the previously utilized derivative 1 used in the development of a nano-vaccine.12 Compound 1 was developed using a structure-guided design approach with the aim to improve the binding affinity toward anti-MUC1 antibodies. This features ensures that the antibodies generated in mice using a non-natural MUC1 derivative can effectively target naturally occurring TA-MUC1 proteins present on the surface of cancer cells.12 The use of non-natural amino acids in 1 also has the potential to increase its immunogenicity, making this non-natural MUC1 derivative more readily recognized and responsive by the immune system than its natural counterpart.12 The new glycopeptide 1’ used in the current study differs from 1 by the absence of the cysteine-histidine (Cys-His) sequence in the N-terminal region. Instead, it features a cyclooctyne handle for disulfide-specific conjugation with the protein carrier CRM197.
Concerning the protein carrier, treatment of the protein CRM197 with an excess of tris(2-carboxyethyl)phosphine (TCEP) at 25 °C for 2 h selectively reduced C186–C201 bond (Figure 2a).18 Subsequent reaction with 20 equiv. of 2 (see Supporting Information for details) in phosphate-buffered saline (PBS) buffer (pH 7.4) at 21 °C for 5 days gave the desired azide-tagged protein upon purification using a Zeba spin column (CRM197-linker-azide). Liquid chromatography–mass spectrometry (LC–MS) analysis revealed a single peak at 58705 Da, corresponding to the modification of one disulfide and subsequent re-bridging to introduce the azido-tag (Figure 2b, see also the obtained SDS-PAGE in Figure S2). Circular dichroism (CD) confirmed that the installation of 2 retained the secondary structure of native CRM197 (Figure 2c).18, 19 The conjugate CRM197-linker-azide was subsequently treated with glycopeptide 1’ (1 : 20 protein/glycopeptide ratio) at 25 °C for 20 h (Figure 2d). The reaction mixture was then purified on a Zeba spin column and analyzed by LC–MS confirming successful conjugation and formation of homogeneous CRM197-linker-1’ (Figure 2e). Molecular dynamics (MD) simulations performed with this conjugate showed that the addition of the glycopeptide has no impact on the 3D structure of the protein (Figure 2f and Figure S3). These calculations suggested that the linker between the carrier and the glycopeptide is flexible, which is reflected in optimal exposure of the glycopeptide (antigen) to the solvent. This exposure is essential for the efficient antigen presentation to the immune system. It is worth noting that although only one copy of glycopeptide 1’ was presented by the protein, up to 4 possible isomers could be expected (Z/E-oxime and 1,4/1,5-triazole adducts). Importantly, the conjugation procedure was amenable to scale up and enabled production of homogeneous vaccine CRM197-linker-1’ for immunization studies in mice. Therefore, we successfully produced a chemically defined homogeneous vaccine by site-specific conjugation of a single copy of an artificial antigen to the CRM197 that preserved the 3D structure of the protein and is ready for mouse immunization studies.
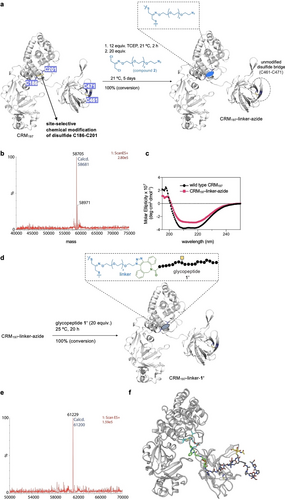
Disulfide-specific CRM197 modification with a non-natural MUC1. a, Optimized conditions used in this work for the selective modification of cysteines 186 and 201 of the CRM197 protein. b, ESI–MS spectrum of CRM197-linker-azide. c, CD spectra of CRM197 and CRM197-linker-azide. d, Optimized conditions used in this work to prepare the vaccine candidate CRM197-linker-1’. e, ESI–MS spectrum of CRM197-linker-1’. f, Representative ensembles obtained from MD simulations. The protein is shown in white ribbons and carbon atoms of the linker, MUC1 and GalNAc are represented as blue, green and orange sticks, respectively. The E-oxime and 1,5-triazole adduct was considered in these calculations.
Specific Antibodies Against the Unnatural MUC1 are Elicited by CRM197-Linker-1’
Once verified that the endotoxin levels in the samples were safe for mouse administration [below 1 endotoxin units per milliliter (EU/mL), Figures S4 and S5],21 we tested the immunogenic potential of CRM197-linker-1’ in mice. For this purpose, a group of five MUC1 transgenic (MUC1.Tg) mice was immunized with an initial subcutaneous (SC) dose followed by three equal booster doses of the vaccine candidate with 21-day intervals, while one control group was treated with unconjugated CRM197 and another with only PBS. Serum from the immunized mice was collected 5 days after each injection to examine the levels of anti-MUC1 antibodies (Figure 3a). For this, an ELISA assay was performed loading the plates with glycopeptide 1’ (Figure 1). As shown in Figure 3b, anti-MUC1 antibodies were detected after the 2nd injection. Antibody titers were higher after both the 3rd and 4th immunizations, which suggests a potential boost effect. Importantly, the antibodies elicited by CRM197-linker-1’ in mice were mainly IgG antibodies (Figure 3b, c), while the levels of IgM antibodies were significantly lower (Figure 4d). This result is indicative of a more mature response that involved class-switch recombination, as the immunoglobulin heavy chain class switching is known to occur rapidly after activation of mature naive B cells, resulting in a switch from expressing IgM to IgG. Thus, having higher levels of IgG in the sera indicate that activation of mature B cells have occurred.7b-7d, 7f, 22 The IgG antibodies were further examined with an additional ELISA assay to determine their isotypes (Figure 3c). While all IgG isotypes were detected, the triggered antibodies were mostly of the IgG2 type, which suggests a triggered Th1 response. These findings differed from our previous studies in which a MUC1 glycopeptide with the same non-natural substitutions (glycopeptide 1, Figure 1) but displayed heterogeneously on the surface of a gold nanoparticle, where we observed elevated levels of IgG1 typical of a Th2 response.12 In mice, high levels of IgG1 are associated with a Th2 response, a humoral immune response type that promotes B cell proliferation and induction of antibodies production. Conversely, high levels of IgG2 are related to a Th1 response, a cellular mediated immune response that stimulates B cells to produce IgM and IgG1 antibodies and promotes the activation of CD8+ CTLs.23 Moreover, antibodies from the IgG2a and IgG2b subclasses are known to activate the complement. Thus, Th1-associated antigens are considered good candidates for vaccine development. In addition, we also detected antibodies against CRM197, which indicated that CRM197 is promoting an immune response as predicted, leading to a stronger effect (Figures S6, S7 and S8).

Vaccine CRM197-linker-1’ triggers specific antibodies that recognize TA-MUC1. a, Vaccine administration Scheme used in this work. b, Total level of IgG antibodies elicited by CRM197-linker-1’, CRM197 and PBS at different stages, as determined by ELISA assay with glycopeptide 1’ coating. c, IgG isotypes detected after the 4th immunization by ELISA assay. d, Total level of IgM antibodies elicited by CRM197-linker-1’, CRM197 and PBS after 4th immunization, as determined by ELISA assay. Flow cytometry analysis to study the binding to e, HEK293T cells (negative control) and f, TD47 cells that express TA-MUC1 on their surface. Black line—serum from mice injected with PBS; Blue line—commercial anti-MUC1 antibody; Pink line—serum from mice immunized with CRM197; Red line—serum from mice immunized with CRM197-linker-1’. g, Confocal microscopy images show that mice antisera after vaccination CRM197-linker-1’ do not stain HEK293T cells (lower panel) as expected because these cells do not express TA-MUC1 on their surface. On the contrary, breast cancer cells T47D, expressing TA-MUC1 are positively stained by mice antisera (upper panel). Blue=Hoechst (nuclei); green=secondary anti-mouse IgG Alexa 488. Statistical significance was determined by an unpaired T-test (* p≤0.05; ** p≤0.01; *** p≤0.001; **** p≤0.0001). Data represents the mean and standard error of the mean (SEM) of five animals per group (N=5).
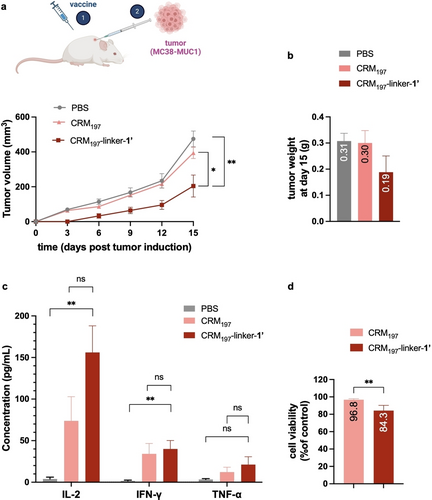
CRM197-linker-1’ delays tumor growth through a Th1 type immune response, when administered prophylactically. a, Tumor volume measured after tumor induction (MC38-MUC1 cells). Mice were first treated with the different formulations before the tumor induction. b, Tumor weight determined at day 15 post tumor induction. Differences not statistically significant. c, Circulating Th1 cytokine levels, after tumor induction, in mice treated with the different conjugates. d, Cytotoxic T Lymphocyte assay. T-cells isolated from the spleens of the immunized mice were co-cultured with MC38-MUC1 cells for 24 h at 90 : 1 ratio (T-cells: MC38-MUC1 cells). A decrease in MC38-MUC1 cells viability was observed. Statistical significance was determined by an unpaired T-test (* p≤0.05; ** p≤0.01). Data represents mean+SEM (N=5).
When comparing total IgG titers of the vaccine candidate CRM197-linker-1’ with those of the previously reported formulation, where unnatural glycopeptide 1 (Figure 1) was conjugated to gold nanoparticles (AuNP-1) in a heterogeneous formulation,12 and using the same immunization protocol, the homogeneous vaccine described here induces statistically higher total IgG titers. This outcome is observed despite differences in vaccine design and the distinct mouse models used for immunization (MUC1.Tg for CRM197-linker-1’ and Balb/c for AuNP-1) (Figure S9).
Next, we checked whether the generated antibodies were able to recognize human TA-MUC1, which is overexpressed in cancer cells. Serum from the immunized mice was incubated with T47D cell line, which displays TA-MUC1 on its surface24 (used as a positive control) and with HEK293T25 (as a negative control), and a binding analysis was performed using flow cytometry. This experiment showed that the antibodies generated by our vaccine in mice were able to bind to T47D cells. In contrast, negligible binding was observed when using HEK293T cells, which is consistent with the absence of TA-MUC1 on their surface (compare Figure 3e and 3f). These results are in good agreement with those obtained from confocal microscopy (Figure 3g) that showed the presence of the MUC1 antigen on the surface of T47D cells (green color) but not on HEK293T cells. Hence, we could clearly demonstrate that CRM197-linker-1’ vaccine successfully triggers the production of antibodies that are able to recognize the natural human TA-MUC1.
Vaccine CRM197-Linker-1’ Vaccine Delays Tumor Growth when Administered Prophylactically
To evaluate the potential of the vaccine-induced antibodies in providing protection against cancer, we induced tumors 6 days after the last injection in immunized mice. We inoculated MC38-MUC1 cells which derive from a murine colon adenocarcinoma engineered to express human MUC1.26 Tumor growth was followed for 15 days after inoculation at which time mice were sacrificed. A delay in tumor growth was observed in animals immunized with CRM197-linker-1’ relative to controls immunized with CRM197 or PBS (Figures 4a, b). The tumor size of the treated mice was decreased by approximately 55 % at day 15 relative to control. Of note, one mouse of the vaccine-treated group did not exhibit detectable tumor growth up to day 15 following the inoculation with cancer cells. Circulating cytokine levels were also analyzed after tumor inoculation. We observed increased levels of Th1 related cytokines, such as IL-2 and IFN-γ and TNF-α (Figure 4c), which correlates with low amounts of Th2 associated cytokines, such as IL-4, IL-10 and IL-13 (Figure S10). This data reinforces a Th1 type response to CRM197-linker-1′ also suggested by the isotype of the IgGs detected in the sera (Figure 3c).
Next, we evaluated the efficacy of T-cells to promote CTL responses. Briefly, CD3+ T-cells (which include CD4+ and CD8+ T-cells) were isolated from the spleens of the mice from both the treatment group and the controls (Figures S11 and S12) and used in a CTL assay. We observed a ~20 % reduction in the viability of MC38-MUC1 cells when they were co-cultured with T-cells isolated from the spleens of treated mice compared to those obtained from the control group (Figure 4d). The experiment suggests that T-cells from the treatment group have the capacity to recognize TA-MUC1 expressed in the MC38-MUC1 cells, which triggers cancer cell death. Hence, CRM197-linker-1’ can retard tumor proliferation, potentially by boosting the activity of cytotoxic T-cells targeting cancer cells that express human TA-MUC1, along with other contributing factors.
CRM197-Linker-1’ Vaccine Delays Tumor Growth and Prolongs Survival when Administered Therapeutically
Next, we evaluated the efficacy of our homogeneous conjugate as potential therapeutic vaccine for reducing tumor growth. For this purpose, we administered MC38-MUC1 or Panc02-MUC1 cancer cells to untreated mice (Figures 5 and 6). As previously mentioned, the former cell line represents a murine colon adenocarcinoma that expresses TA-MUC1 on its surface. The latter cell line corresponds to a murine pancreatic cancer model that expresses the human MUC1 sequence on its surface.27 Once tumors reached an average size of 100 mm3, treatments were initiated with the CRM197-linker-1’ either as sole therapy (Figure 5) or in combination with a checkpoint inhibitor (Figure 6).
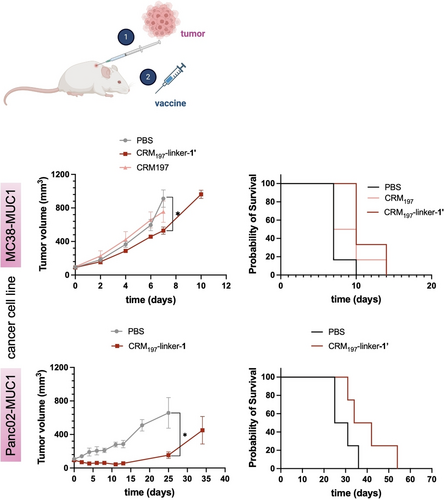
CRM197-linker-1’ delays tumor growth and prolongs survival when administered therapeutically. Upper panel: MC38-MUC1 tumor model, Tumor volume measured after tumor induction with the different formulations (* p=0.0121), together with probability of survival of mice after inducing of a tumor and being treated with the corresponding formulations (* p<0.0265). Lower panel: Panc02-MUC1 tumor model, Tumor volume measured after tumor induction with the different formulations (* p=0.0197), together with probability of survival of mice after inducing of a tumor and being treated with the corresponding formulations (* p=0.1092). Data represents mean+SEM (N=6).
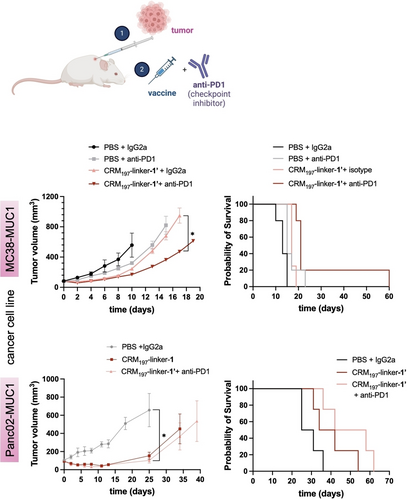
CRM197-linker-1’ delays tumor growth and prolongs survival when administered therapeutically in a combination treatment with a checkpoint inhibitor. Tumor volume measured after tumor induction (MC38-MUC1—upper panel, p=0,0362—or Panc02-MUC1—lower panel, p=0,0201—cell lines) with the different formulations, along with probability of survival of mice under these conditions (MC38-MUC1—upper right, * p=0.1017—or Panc02-MUC1—lower right, * p=0.0163—cell lines). Data represents mean+SEM (N=5).
When using the CRM197-linker-1’ vaccine alone (Figure 5), four identical SC doses as in the previous experiments were administered 2 days apart. In the MC38-MUC1 cancer model, tumor size in treated mice decreased by approximately 40 % by day 7, while in the Panc02-MUC1 model it decreased by approximately 75 % by day 25, days when the first control animals reached the endpoint (Figure 5, upper and lower panels, respectively). In terms of survival probability, all vaccine-treated mice in the MC38-MUC1 model survived beyond day 10, in contrast to the non-treated mice, whose tumors reached 1000 mm3 by day 10. The survival probability of the treated mice was about 40 % until day 15 (Figure 5, upper panel). Although tumors generally grow more slowly in the Panc02-MUC1 model, similar results were observed, with delayed tumor growth and prolonged survival in the treated animals compared to the controls. In this case, the survival probability of treated mice on day 54 was 25 % (Figure 5, lower panel). These results indicate that our homogeneous vaccine has a potential therapeutic effect on tumor growth, as evidenced by the prolonged survival time.
Immune checkpoint inhibitors employed in immune checkpoint blockade therapies have shown great results and revolutionized the field of cancer treatment.28 Therefore, we chose to administer our homogeneous vaccine in combination with checkpoint inhibitors to study the combined effect of both treatments in one therapeutic formulation. Typically, antibodies targeting the PD-1/PD-L1 axis or CTLA-4 have demonstrated remarkable effectiveness in generating anti-tumor responses across a spectrum of cancers, characterized by durable outcomes and wide-ranging biological effects.29 As a PD-1 inhibitor, we chose an anti PD-1 antibody. PD-1, also known as CD279, is expressed on activated T-cells and interacts with one of its two ligands, PD-L1 or PD-L2, which are often located on tumor cells. This interaction often leads to T cell dysfunction and allows the tumor to evade the immune system.30 This innovative approach to immunotherapy has recently been shown to be effective in enhancing the immune response in mice.31 Moreover, anti-PD1 immunotherapy has shown a great success in treating cancer patients. However, it is notable that only a minority of patients, approximately 20 %, exhibit significant responses to these therapies likely due to tumor heterogeneity and others immune evasion mechanisms employed by tumors.32 Therefore, we chose to administer our homogeneous vaccine in combination with checkpoint inhibitors to study the combined effect of both treatments in one therapeutic formulation.
In this combined therapeutic approach, four identical SC doses of the vaccine were administered 2 days apart, regardless of the cancer cell line used (Figure 6), while the anti-PD1 antibody was administered via three intraperitoneal (IP) injections 3 days apart, with an IgG2a isotype antibody used as a control. The mice were closely monitored and sacrificed once tumors reached a size of 1000 mm3. Of note, this therapy resulted in a significant reduction in tumor size. In the MC38-MUC1 cancer model, there was an approximately 70 % reduction in tumor size in the treated mice group compared to the PBS-treated mice by day 10 (when the first control mouse reached the endpoint). Additionally, mice treated with CRM197-linker-1’ and anti-PD1 showed a survival rate of approximately 80 % by day 20. Remarkably, one mouse showed a complete regression of the tumor at day 65 when it was euthanized (Figure 6, upper panel). Similar results were obtained with the pancreatic cancer model. In this case, tumor growth was reduced approximately 84 % by day 25 (day of death of the first control animal) in mice treated with the combination therapy, and more than 20 % of the mice survived at day 60 (Figure 6, lower panel).
Together our data demonstrates that the homogeneous conjugate CRM197-linker-1’ is a promising vaccine candidate for cancer treatment. This candidate elicits a robust Th1 immune response and generates antibodies that selectively recognize tumor-associated antigens. Furthermore, it significantly delays tumor growth and enhances survival in mouse models of both colon adenocarcinoma and pancreatic cancer. Importantly, its efficacy is further amplified when combined with the PD-1 immune checkpoint inhibitor, suggesting a synergistic potential that leads to improved survival rates in murine models.
Conclusion
In summary, our research represents a significant milestone as we present an innovative homogeneous and chemically defined vaccine based on an artificial MUC1 glycopeptide, which was previously developed by our group following a structure-guided design. This breakthrough vaccine was carefully engineered through a selective conjugation process with the carrier protein CRM197, achieved by selective reduction and re-bridging of one of the disulfide bridges presents in CRM197. Our conformational analysis, which include techniques such as circular dichroism and molecular modeling, shows that this site-selective modification does not alter the structural preferences of the protein. Furthermore, our preclinical studies in mice clearly underscore the efficacy of the vaccine and provide compelling evidence of its ability to elicit a robust immune response. Despite the differences in vaccine design and mouse models used for immunization, which prevent a direct and fair comparison, the homogeneous vaccine CRM197-linker-1’ induces significantly higher total IgG titers compared to our previously reported results using the same MUC1 antigen and conjugated to AuNPs. In addition, the antibodies, raised in mice, exhibit specificity for human cells carrying natural tumor-associated MUC1 on their surface. Our vaccine shows efficacy in both prevention and therapeutic treatments, whether administered as a stand-alone treatment or in combination with a checkpoint inhibitor. Specifically, using colon adenocarcinoma and pancreatic cancer models, our vaccine demonstrates the ability to inhibit the growth of these type of tumors in mice, resulting in a significant increase in survival time of treated animals. In addition to these promising therapeutic results, our approach holds the potential to standardize vaccine production and reduce batch-to-batch variability associated with the manufacturing process. This advance could help to streamline the production of chemically defined glycopeptide-based cancer vaccines with clinical potential. Finally, and despite a prevailing dogma wherein glycoconjugate vaccines require the display of multiple copies of antigens on a protein carrier, our work demonstrates that the use a single copy of a non-natural, potent antigen precisely displayed on a protein carrier can induce strong immune responses in vivo.
Supporting Information
Synthesis and characterization of glycopeptide 1’ and the different conjugates, mass spectrometry analysis, SDS-PAGE, CD spectroscopy, MD simulations, MUC1.Tg mice genotyping, in vivo studies, antibody titers, antibody isotypes, tumor induction, cytokine and chemokine levels determination, flow cytometry analysis, and cytotoxic T-lymphocyte assay.
Acknowledgments
We acknowledge the Agencia Estatal de Investigación (AEI, PID2021-127622OB-I00 to F. C. and P. O.), Asociación Española contra el Cáncer, FCT Portugal (Ph.D. studentship, SFRH/BD/115932/2016 to A. G.) and Basinnov Lifesciences (sponsored research agreement to G. J. L. B.). This Project has received funding from the European Union's Horizon 2020 research and innovation program under grant agreement N° 852985 (SIMICA) and N° 956544 (DIRNANO) and N° 101034288 (an innovation programme under the Marie Skłodowska-Curie COFUND grant agreement).
Conflict of Interests
A. G., I. C., F. C. and G. J. L. B. are co-inventors on a patent application that describes non-natural glycopeptide-based vaccines. G. J. L. B. is a scientific advisor of Basinnov LifeSciences.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.