Donor-Acceptor Type Supra-Carbon-Dots with Long Lifetime Photogenerated Radicals Boosting Tumor Photodynamic Therapy
Graphical Abstract
A donor-acceptor type supra-CDs has been fabricated by using red emissive CDs as donor and electron-withdrawing molecule as acceptor. This peculiar donor-acceptor configuration facilitates the efficient charge separation in supra-CDs, thereby generating more superoxide radicals and additional holes-related radicals, which collectively boosts type-I tumor photodynamic therapy.
Abstract
Carbon dots (CDs) have gained significant interest because of their potential in biomedical applications. Nevertheless, developing CDs with efficient photoinduced charge separation for tumor photodynamic therapy (PDT) remains a challenge. This study presents a novel class of supra-carbon-dots (supra-CDs) developed by fusing red emissive CDs with 2,3-dicyanohydroquinone (DCHQ) via post-solvothermal treatment. In supra-CDs, the core, acting as electron donors, is formed by assembled CDs with substantial sp2 domains, the fused interface originating from DCHQ with electron-withdrawing groups functions as the electron acceptor. This configuration creates the unique donor-acceptor nanostructure. Upon white light irradiation, the excited electrons from the assembled CDs were transferred to the electron-withdrawing interface, whereas the photogenerated holes were retained within the assembled CDs as radicals, leading to effective photoinduced charge separation. The separated photogenerated electrons then react with oxygen to generate superoxide radicals. Simultaneously, the photogenerated holes undergo oxidation of crucial cellular substrates. This dual action underscores the exceptional cell-killing efficacy of supra-CDs. Moreover, the increased particle sizes (~20 nm) ensure supra-CDs to exhibit a notable capacity for tumor accumulation via the improved permeability and retention effect, thereby achieving satisfactory anti-tumor PDT efficacy in a mouse subcutaneous tumor model.
Introduction
Photodynamic therapy (PDT) is a potent approach for the treatment of solid tumors, with minimal side effects and drug resistance.1 PDT processes can be classified into two types based on the mechanisms of reactive oxygen species (ROS) generation: In the type-I PDT process, hydrogen peroxide (H2O2), superoxide radicals (O2⋅−), or hydroxyl radicals (⋅OH) are generated through electron and/or proton transfer between excited photosensitizers (PSs) and adjacent substrates or oxygen (O2).2 In the Type-II PDT process, singlet oxygen (1O2) is generated through energy transfer from the excited photosensitizers to O2.3 As a crucial kind of ROS, O2⋅− plays a significant role in DNA strand breaks, membrane damage, and oxidative phosphorylation in mitochondria. Furthermore, it serves as the primary precursor for generating other essential ROS.4 Compared with type-II PDT, the O2⋅− generated in the type-I PDT process can undergo disproportionation or Haber-Weiss reactions, compensating for O2 consumption and making the process less dependent on O2.5 Given the typically hypoxic nature of the tumor microenvironment, there is an urgent need to exploit novel PDT agents capable of efficiently generating O2⋅− to enhance the efficacy of tumor PDT.
Boosting the photoinduced charge separation within PSs and facilitating the electron transfer process between PSs and O2 are recognized methods for enhancing the generation of O2⋅−.6 Constructing donor-acceptor (D−A) type PSs is an effective strategy for achieving efficient photoinduced electron transfer.7 Due to the improved electron push-pull effect between the D and A units, molecules with D−A architectures demonstrate enhanced efficiency in charge separation.8 Incorporating suitable D or A units has been shown to enhance the photoinduced generation of O2⋅− in various molecular PSs with D−A architectures.9 Nevertheless, molecular PSs often exhibit poor water solubility, necessitating the use of organic solvents, such as dimethylsulfoxid, or requiring modification to enhance their water solubility for broader biomedical applications.
Carbon dots (CDs) represent a class of ultrasmall-sized carbon-based nanomaterials that are water-soluble, boosting notable advantages such as exceptional biocompatibility, adjustable fluorescence characteristics, strong photostability, and low production cost.10 Up to now, CDs have been extensively investigated in various domains, including biosensing, bioimaging, disease diagnosis, drug delivery, anti-inflammatory treatment, and antibacterial application.11 Furthermore, the diverse photophysical and photochemical characteristics of CDs exhibit potential for converting absorbed light into ROS for tumor PDT.12 However, to the best of our knowledge, there have been few reports of pure CDs that can efficiently generate O2⋅−. Because of the abundant surface functional groups, such as hydroxyl, amino, and carboxyl, CDs can aggregate into supra-CDs through covalent or non-covalent bonds, resulting in an expanded array of photophysical properties.13 The cores of CDs, which are rich in sp2 domain, possess an abundance of electrons. Therefore, combining CDs with compounds abundant in electron-withdrawing groups could offer a promising approach to construct water-soluble D−A-type supra-CDs, thereby enhancing the photoinduced generation of O2⋅−.
Herein, we developed a new approach for constructing D−A-type supra-CDs. This involves the incorporation of 2,3-dicyanohydroquinone (DCHQ) as an electron-withdrawing segment, which is then co-fused with red emissive CDs through post-solvothermal treatment. The supra-CDs exhibited the core composed of assembled CDs acted as the D-type component, and the interface derived from the fused DCHQ served as the A-type component. The D−A-type nanostructure facilitates the transfer of excited electrons from the assembled CDs to the electron-withdrawing interface upon exposure to white light irradiation. Meanwhile, the photogenerated holes were stabilized in the assembled CDs with long lifetime as radicals. The efficient photoinduced charge separation facilitates the reaction of photoexcited electrons with O2, leading to the efficient generation of O2⋅−. Interestingly, the photogenerated radicals in supra-CDs could oxidize biological substrates, such as tetrahydrobiopterin (BH4) and nicotinamide adenine dinucleotide (NADH), resulting in enhanced photoinduced cell-killing ability. Furthermore, the increased size of supra-CDs (~20 nm) enhances their tumor accumulation capacity leveraging the improved permeability and retention (EPR) effect, thereby achieving satisfactory tumor PDT in a mouse subcutaneous tumor model.
Results and Discussion
Synthesis and Characterization of Supra-CDs
The fabrication of supra-CDs involved the assembly of CDs with DCHQ via a post-solvothermal treatment process, as shown in Figure 1a. The raw CDs were synthesized using the methods detailed in our previous study.14 Transmission electron microscopy (TEM) was employed to analyze the morphology and structure of both the raw CDs and supra-CDs. Figure 1b displays the spherical morphology and uniform dispersion of the raw CDs, which measured approximately 2.5 nm. High-resolution transmission electron microscopy (HRTEM) images of the CDs revealed an intercrystalline spacing of 0.21 nm, corresponding to the [100] plane of graphene. Figure 1c illustrates the well-dispersed nature of the supra-CDs, which is accompanied by an obvious increase in particle sizes. In Figure 1d, the HRTEM image reveals that the supra-CDs comprise assemblies of numerous CDs. Dynamic light scattering (DLS) analysis, as shown in Figure 1e, indicates that the size of the supra-CDs was approximately 20 nm, which was significantly greater than that of the raw CDs.
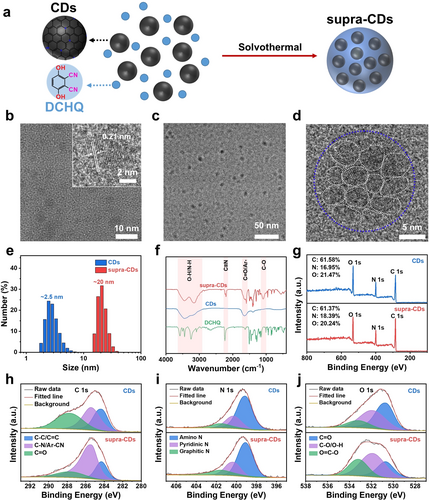
Synthesis and characterization of supra-CDs. (a) Diagram of the synthesis of supra-CDs. (b) Transmission electron microscopy (TEM) image of raw CDs (Inset: High-resolution transmission electron microscopy (HRTEM) of raw CDs). TEM (c) and HRTEM (d) images of supra-CDs. (e) Dynamic light scattering (DLS) of raw CDs and supra-CDs. (f) FTIR spectra of DCHQ, raw CDs, and supra-CDs. (g) XPS surveys of raw CDs and supra-CDs. XPS C 1s (h), N 1s (i) and O 1s spectra (j) of raw CDs and supra-CDs.
Chemical Structure of Supra-CDs
Fourier transform infrared (FTIR) spectroscopy and X-ray photoelectron spectroscopy (XPS) were employed to analyze the chemical structure of supra-CDs. Figure 1f illustrates the FTIR spectra of the raw CDs, DCHQ, and supra-CDs. The broad peak spanning from 3270 to 3640 cm−1 is likely attributed to -NH2 and -OH groups, whereas the peak at approximately 1640 cm−1 confirms the presence of C=O/Ar- bonds. Compared with raw CDs, a distinct characteristic peak corresponding to -CN at approximately 2196 cm−1 was observed in the supra-CDs, signifying the successful incorporation of DCHQ. Figure 1g and Table S1 present the XPS findings, indicating peaks corresponding to C at 285 eV, N at 400 eV, and O at 532 eV. This confirms that C, N, and O are the primary constituent elements of both raw CDs and supra-CDs. In contrast, supra-CDs exhibited a higher N content of 18.39 % than raw CDs, which had an N content of 16.95 %. This increase can be attributed to the incorporation of the cyanide group from DCHQ. Figure 1h illustrates the C 1s spectrum, which can be classified into three distinct regions: C−C/C=C at 284.3 eV, C−N/Ar-CN at 285.4 eV, and C=O at 287.7 eV. The N 1s spectrum shown in Figure 1i comprises amino N at 399.1 eV, pyridinic N at 400.3 eV, and graphitic N at 401.5 eV. Figure 1j shows the O 1s spectrum, which displays three peaks at 530.7, 531.9, and 533.2 eV, indicating the presence of C=O, C−O/O−H, and O=C−O, respectively. Table S1 presents the proportions of each component. Because of the incorporation of the cyanide group from DCHQ, the content of -C−N/-CN in supra-CDs increased greatly from 35.62 % to 62.68 % compared with that in raw CDs. The O=C−O content rose from 14.42 % in CDs to 26.48 % in supra-CDs, suggesting that the -COOH groups on the raw CDs reacted with the -OH groups of DCHQ through an esterification reaction. These findings show that the raw CDs were assembled with DCHQ through covalent bonding. This bonding is facilitated by reactions between the -COOH groups on the surface of raw CDs and the -OH groups in DCHQ under solvothermal conditions, resulting in the formation of supra-CDs.
Optical Properties of Supra-CDs
The optical properties of DCHQ, raw CDs, and supra-CDs were compared by measuring their ultraviolet-visible (UV/Vis) and fluorescence spectra. Figure 2a illustrates that the raw CDs exhibited two absorption peaks at 552 and 416 nm, whereas DCHQ displayed a characteristic absorption peak at 346 nm. The absorption peaks of raw CDs at 552 nm and 416 nm can be attributed to the surface-related state and core state, respectively. In the aqueous solution containing both raw CDs and DCHQ, the absorption bands for each component were distinguishable, suggesting that there were no significant interactions between the raw CDs and DCHQ in the mixture. In contrast, for supra-CDs, since the reaction between DCHQ and the surface functional groups of raw CDs, the surface-related absorption of raw CDs at 552 nm decreased dramatically. In addition, the distinctive absorption peak of DCHQ vanished and a new strong absorption band appeared between 350 and 450 nm. The appearance of this absorption band can be attributed to the interface formed by merging DCHQ among the assembled CDs in the supra-CDs. Figure S1a reveals that in excitation-emission maps, the raw CDs exhibited two fluorescence centers at approximately 550 and 650 nm under 425 and 550 nm excitations, respectively. Figure 2b illustrates that the supra-CDs exhibit a noticeably weakened fluorescence center at approximately 650 nm. In addition, Figure S1b displays a new strong blue fluorescence center at approximately 450 nm under 390 nm excitation in the supra-CDs. The blue emission center within the supra-CDs resembles the fluorescence of DCHQ, which typically exhibits a peak of approximately 450 nm under excitation at 346 nm. However, Figure S1d demonstrates that simply mixing DCHQ and raw CDs did not alter their fluorescence properties, indicating supra-CDs were not simple mixture of raw CDs and DCHQ. Given the strong absorption band at 390 nm and the blue fluorescence, it can be concluded that the blue emission in the supra-CDs comes from the fused DCHQ at the interface of the assembled CDs. Figure 2c shows that the average lifetime of red fluorescence from the supra-CDs was 2.68 ns, compared with 1.12 ns from the raw CDs. The reduction in red fluorescence accompanied by a longer lifetime suggests that the photoexcited electrons from the assembled CDs were transferred to other states with an extended electron-hole recombination time, signifying effective charge separation in the supra-CDs.15

Optical properties of raw CDs and supra-CDs. (a) Normalized ultraviolet-visible (UV/Vis) spectra of raw CDs, supra-CDs, DCHQ, and Mixed CDs and DCHQ aqueous solutions. (b) Fluorescence spectra of raw CDs and supra-CDs with the same absorption intensity under 550 nm excitation. (c) Fluorescence decay curves of raw CDs and supra-CDs aqueous solutions under 510 nm excitation at room temperature (IRF: instrument response function). Typical femtosecond transient absorption (fs-TA) spectra at various delay times (d), species-associated spectra (e), and the corresponding kinetic traces (f) of raw CDs. Typical fs-TA spectra at different delay times (g), species-associated spectra (h), and the corresponding kinetic traces (i) of supra-CDs. (CAS1: CDs-associated species 1; CAS2: CDs-associated species 2; CSS: charge-separated state).
Charge Transfer Dynamics in Supra-CDs
Transient absorption (TA) spectra were measured using 330 nm pump pulses to reveal the charge transfer dynamics of supra-CDs. Upon photoexcitation, the raw CDs exhibited extensive ground state bleaching (GSB) signals from two characteristic species spanning 450–600 nm, as shown in Figure 2d. Figures 2e–f show that the species at 520 nm, designated as CDs-associated species 1 (CAS1), was initially populated. The CDs-associated species 2 (CAS2) was subsequently populated through the energy transfer process at a rate of 2.6×10−11 s−1. The CDs recovered to the ground state at approximately 2.1 ns (Figures 2e and 2f). For supra-CDs, CDs-related GSB signals remained from the beginning (Figure 2g). Differently, a GSB signal appeared successively from 400 nm to 440 nm, which might belong to the electron transfer to the interface excited state. By tracing the kinetics from the global analysis, a charge separation rate of 4.6×10−9 s−1 and a dramatically increased lifetime of 27.1 ns corresponding to the charge-separated state (CSS) were derived, as shown in Figures 2h and 2i. These findings confirm that the electron-withdrawing interface of supra-CDs promoted photoinduced electron transfer from the assembled CDs to the interface region. The prolonged charge-separation process enables the photoinduced electrons to potentially react with the surrounding O2.
Photodynamic Properties of Supra-CDs
The photodynamic properties of supra-CDs were evaluated when subjected to white light irradiation. Firstly, the total ROS generation was assessed using the fluorescence probe 2’,7’-dichlorodihydrofluorescein (DCFH), which undergoes oxidation by ROS to form DCF, exhibiting increased green fluorescence.9 Figure S2 illustrates that in the presence of raw CDs or supra-CDs, the fluorescence of DCFH increased steadily with prolonged white light irradiation time, demonstrating their capacity to generate ROS. The quantitative data of fluorescence intensities at 525 nm in Figure 3a reveals that I/I0 of DCFH in the presence of supra-CDs reached 150 after 5 min of white light irradiation, significantly exceeding that of raw CDs, which stood at 56. This indicates a considerably greater ROS-generating capacity of supra-CDs. Subsequently, further investigation delved into the type of ROS generated by supra-CDs. 9,10-anthracenediyl-bis(methylene)dimalonic acid (ABDA) was employed to identify type-II ROS, 1O2, which selectively reacts with 1O2 to generate corresponding internal peroxides with diminished absorption.16 The findings in Figures 3b and S3 reveal that under white light irradiation, the raw CDs generated a minimal amount of 1O2, as indicated by a slight decrease in ABDA absorption. Conversely, almost no 1O2 signal was detected in supra-CDs. Subsequently, type-I ROS, including O2•− and •OH, were identified using the fluorescence probes dihydrorhodamine 123 (DHR123) and hydroxyphenyl fluorescein (HPF), respectively.17 The findings depicted in Figures 3c and S4 reveal that the fluorescence of DHR123 increased with prolonged white light irradiation time in the presence of either raw CDs or supra-CDs, suggesting the generation of O2⋅−. After 5 min of white light irradiation, the I/I0 ratio of DHR123 at 528 nm in the presence of supra-CDs reached 40, which was much higher than the value of 22 observed with raw CDs. This indicates that supra-CDs have a greater ability to generate O2⋅−. Figure S5 demonstrates that both raw CDs and supra-CDs did not generate ⋅OH under white light irradiation. In addition, the capability of both raw CDs and supra-CDs to generate O2⋅− was determined using electron paramagnetic resonance (EPR) spectra. Figure 3d displays four signals of equal intensity ratio (1 : 1 : 1 : 1) were observed following white light irradiation. These signals are attributed to the O2•− captured by 5,5-dimethyl-1-pyrroline N-oxide (DMPO) in the DMSO solution.18 In Figure 3d–f, it was observed that the EPR signal intensity rose to 125 a.u. after 8 min of white light irradiation in the presence of raw CDs. Conversely, in the presence of supra-CDs, this intensity increased notably to 462 a.u. Notably, in supra-CDs DMSO solution, a distinct EPR signal with the peak at 337.58 mT emerged upon exposure to white light irradiation, as illustrated in Figure 3e. For an in-depth analysis of this signal, the EPR spectra of supra-CDs in DMSO were measured without the addition of DMPO. In Figures 3g and h, a distinct EPR signal, centered at 337.58 mT, significantly intensified in the supra-CDs under white light irradiation, peaking at an intensity of 3617 a.u. after 8 min of exposure. Conversely, Figure S7 shows the EPR signal induced by white light in raw CDs was almost undetectable. Furthermore, the EPR signal generated by white light irradiation of supra-CDs exhibited remarkable persistence. Even after 5 min of irradiation followed by 40 min of dark storage, the attenuation of this EPR signal was less than 20 %, suggesting the presence of stable photogenerated radicals within the supra-CDs, as illustrated in Figures 3i and S8. Considering the aforementioned findings, one can conclude that supra-CDs exhibit efficient photoinduced charge separation, wherein photoexcited electrons from the assembled CDs are effectively transferred to the interface area and effectively react with O2 to generate O2⋅−, while the photogenerated holes are stabilized in the assembled CDs.
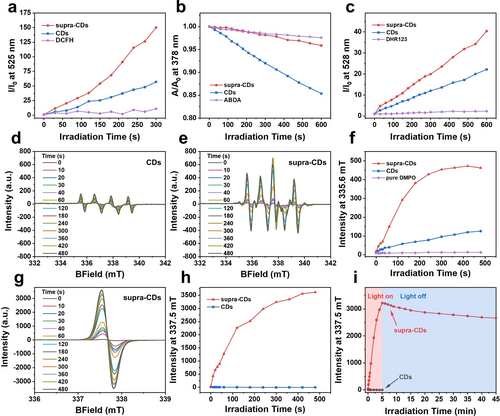
The generation of ROS by supra-CDs and associated photogenerated radicals under white light irradiation. a) Total ROS, b) 1O2, and c) O2⋅−generation of raw CDs and supra-CDs identified by DCFH, ABDA, and DHR123, respectively, under white light irradiation (60 mW cm−2). EPR spectra of raw CDs (d) and supra-CDs (e) in DMSO using DMPO as the spin trap agent under white light irradiation (60 mW cm−2) for various durations. (f) The EPR intensity curves at 335.6 mT for raw CDs and supra-CDs in DMSO with DMPO under white light irradiation (60 mW cm−2) for various periods. (g) EPR spectra of supra-CDs in DMSO without DMPO under white light irradiation (60 mW cm−2) for various durations. (h) EPR intensity curves at 335.6 mT for raw CDs and supra-CDs in DMSO without DMPO under white light irradiation (60 mW cm−2) for various periods. (i) EPR intensity curves at 335.6 mT for supra-CDs after 5 min of white light irradiation (60 mW cm−2) and kept in the dark for various durations.
Although no ⋅OH signals were identified in the supra-CDs following white light irradiation, it is plausible that the photogenerated holes within the supra-CDs can oxidize biological substrates. Tetrahydrobiopterin (BH4) serves as a cofactor for numerous enzymes, such as nitric oxide synthase (NOS), thereby promoting tumor growth through NOS-mediated nitrosylation of latent TGF-β binding proteins.19 Nicotinamide adenine dinucleotide (NADH) serves as a biological form of hydrogen, contributing to the production of ATP, an energy molecule crucial for tumor cells.20 Therefore, the inhibition of tumor cell growth can occur through the oxidation of BH4 and NADH, which was investigated by assessing their absorption under conditions involving supra-CDs and white light irradiation, as documented in previous literature.21 As illustrated in Figure 4a, upon white light irradiation, the absorption at approximately 300 nm decreased and the absorption at approximately 280 nm increased, indicating the oxidation of BH4. Figure 4b demonstrates that the absorption of NADH around 340 nm decreased, while the absorption of its oxidization product around 260 nm increased after white light irradiation, indicating the oxidation of NADH. Based on the aforementioned results, it can be concluded that the efficient photoinduced charge separation in supra-CDs enhances both the oxidation capacity of the photogenerated holes and the transform from excited electrons to O2 to generate O2⋅−.
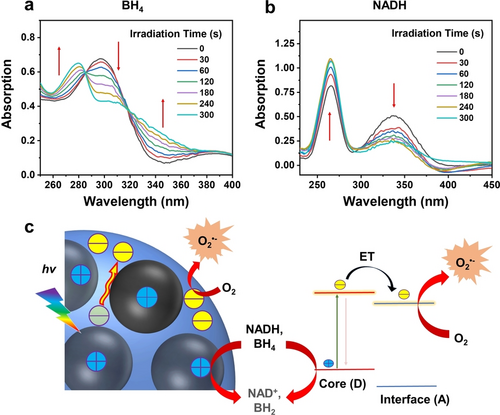
Absorption spectral changes of tetrahydrobiopterin (BH4) aqueous solution (100 μM) by adding supra-CDs under white light irradiation (60 mW cm−2) at various intervals. (b) Changes in the absorption spectral of nicotinamide adenine dinucleotide (NADH) aqueous solution (100 μM) by adding supra-CDs under white light irradiation (60 mW cm−2) at various intervals. (c) Diagram depicting potential photodynamic mechanisms in supra-CDs (ET: electron transfer; hv: white light irradiation).
Mechanism for Charge Separation and Radicals Generation in Supra-CDs
A possible mechanism for the photophysical processes in supra-CDs was proposed based on the aforementioned findings (Figure 4c). The composition of the supra-CDs comprises electron-rich assembled CDs and electron-withdrawing interface originating from DCHQ configured in a D−A-type nanostructure. Upon white light irradiation, the push-pull effect between the D and A regions enabled effective photoinduced charge separation within supra-CDs. This process involved the transfer of photoexcited electrons from the D-type assembled CDs to the A-type interface, where they reacted with surrounding O2 to generate O2⋅−. Simultaneously, the photogenerated holes were stabilized within the assembled CDs as radicals, exhibiting significant oxidation ability to oxidize biological substrates. These distinctive photophysical processes suggest that supra-CDs possess a promising capacity for photoinduced cell killing.
Photoinduced Cell Killing Capacity of Supra-CDs
The cell counting kit-8 (CCK-8) assay was performed to evaluate the cytotoxicity of supra-CDs. Figures 5a, 5b and S9 demonstrate that both raw CDs and supra-CDs exhibited low cytotoxicity to murine breast cancer cells (4T1) under dark condition, with cell viability exceeding 80 % at the concentration of 500 μg mL−1. Confocal laser scanning microscopy (CLSM) and flow cytometry were employed to examine the cellular uptake of supra-CDs. As illustrated in Figures 5c, S10 and S11, after 24 h of incubation with supra-CDs at a concentration of 500 μg mL−1, green fluorescence was observed in the cytoplasm and nucleus of 4T1 cells, similar to previous work that carbon-based nanomaterials (including CDs, carbon nanotubes, and graphene) are distributed in both cytoplasm and nucleus.22 These results indicate the effective uptake of supra-CDs by cancer cells. Figures S12 and S13 demonstrate the uptake of supra-CDs by 4T1 cells in both time-dependent and concentration-dependent manners. Further investigation was conducted into the photocytotoxicity of both raw CDs and supra-CDs. Figure 5a shows a notable decrease in cell viability to 36 % in cells incubated with raw CDs (500 μg mL−1) following 10 min of white light irradiation (60 mW cm−2). Figure 5b depicts only a 4 % remain in cell viability observed in cells incubated with supra-CDs (500 μg mL−1) following the same white light irradiation, highlighting a stronger photoinduced cell-killing ability compared with raw CDs. The cytotoxicity of supra-CDs under white light irradiation was then evaluated by live/dead staining. Figure S14 shows that supra-CDs had no toxicity to 4T1 cells in the absence of light. When exposed to white light under the same condition, supra-CDs can effectively kill cancer cells. The generation of ROS in vitro were then investigated in the CDs and supra-CDs incubated 4T1 cells by employing DCFH-DA and DHR123 as probes, respectively. Figures 5d and supplementary Figures S15 and S16 depict weak fluorescence signals observed in CDs-incubated cells following white light irradiation (CDs+L). In contrast, much stronger green fluorescence signals were observed in supra-CDs-incubated cells under the same conditions (supra-CDs+L), indicating higher generation of total ROS and O2⋅− by supra-CDs than by raw CDs. This observation aligns well with the ROS measurements conducted in their solutions, as shown in Figure 3. The specific mechanism of cell death is crucial for understanding the efficacy of supra-CDs. Therefore, we then investigated whether supra-CDs could induce apoptosis of tumor cells by the annexin V/PI staining experiment. The results in Figure S17 display that the proportion of 4T1 cells undergoing apoptosis increased significantly after incubating with supra-CDs followed by white light irradiation, among which early apoptosis accounted for 47.9 % and late apoptosis accounted for 51.8 %. These results confirm that ROS generated by supra-CDs under white light induced the apoptosis of cancer cells.
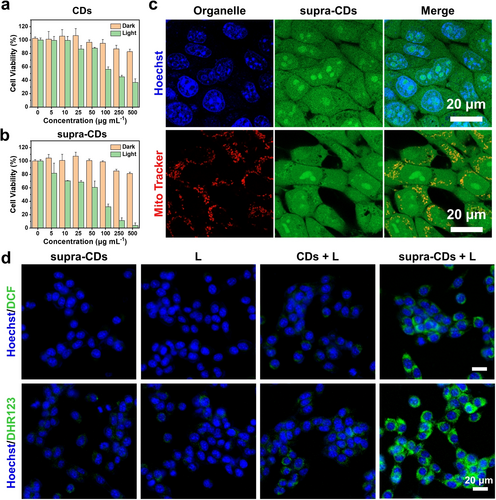
The dark and photo-induced cytotoxicity of raw CDs (a) and supra-CDs (b) detected by CCK-8 assay (n=3). (c) CLSM) image of 4T1 cells incubated with supra-CDs at 500 μg mL−1 (Ex: 488 nm; Em: 500–600 nm) and stained with Hoechst 33342 or Mito Tracker. Scale bar: 20 μm. (d) Merged CLSM images of total ROS and O2⋅− generated by raw CDs or supra-CDs under white light irradiation (60 mW cm−2) using DCFH-DA or DHR123 as the fluorescence probes, respectively (Ex: 488 nm; Em: 500–600 nm; L: white light irradiation for 10 min). Scale bar: 20 μm.
Tumor Photodynamic Therapy Based on Supra-CDs
Given the excellent photoinduced cell-killing efficacy of supra-CDs, their antitumor PDT efficacy was evaluated in a subcutaneous tumor model. The tumor accumulation potential and biodistribution of supra-CDs was assessed by ex vivo fluorescence imaging of tumors and major organs at various intervals following intravenous administration of supra-CDs (10 mg kg−1). Figure S18 demonstrates the bright fluorescence emitted by supra-CDs under 480 nm excitation. Figure S19 illustrates the rapid distribution of supra-CDs within the liver in an hour, followed by their gradual metabolism and excretion via the hepatic (bile to faeces) pathway over the subsequent 24 h period. Figures 6b and S19 indicate that supra-CDs could accumulate in tumors, displaying a maximum fluorescence signal after 12 h of injection.
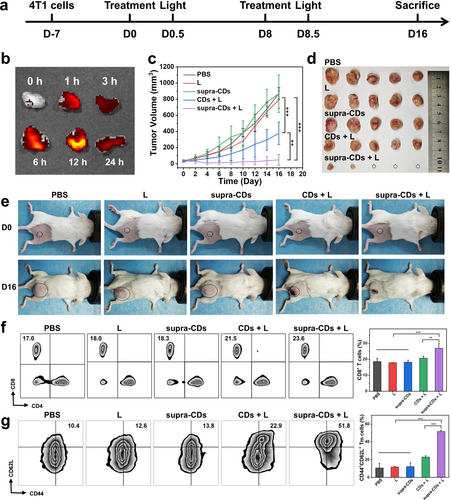
In vivo antitumor therapeutic efficacy and antitumor immune response of supra-CDs. (a) Schematic diagram depicting the establishment of 4T1 tumor-bearing BALB/c mice and the schedule for antitumor treatment. (b) Ex vivo fluorescence imaging of tumors and major organs following intravenous injection of supra-CDs at 10 mg kg−1 at various intervals (Ex: 480 nm; Em: 570 nm). (c) Tumor volumes curve during the treatment (n=5). (d) Tumor images of 4T1 tumor-bearing mice after treatment in different groups. (e) Representative images of mice pre- and post-treatments. (f) Flow cytometric analysis of CD4+ and CD8+ T cell populations in blood samples from various groups and corresponding quantitative statistical analysis (n=3). (g) Flow cytometric analysis of CD44+CD62L+ memory T cell populations in spleen samples from various groups and corresponding quantitative statistical analysis (n=3). (L: white light irradiation for 10 min).
Subsequently, the potential of supra-CDs for in vivo tumor PDT was examined. A total of 25 4T1 tumor-bearing mice were randomly assigned to five groups (n=5 for each group) and treated with PBS, light only (L), supra-CDs, CDs+L, and supra-CDs+L. The mice received intravenous injections of PBS, raw CDs, or supra-CDs and were kept in darkness or subjected to white light irradiation. Figure 6a illustrates the treatment schedule. In the PBS-treated group, tumors grew rapidly, with volumes reaching 872±72 mm3 after 16 days of observation. In the group treated with light (L) only and the group treated with supra-CDs without light exposure, tumor volumes reached 793±109 mm3 and 867±233 mm3, respectively. This suggests that neither the light source nor the supra-CDs in the absence of light exposure demonstrated any tumor suppressive effects. After 16 days of observation, the tumor volume decreased to 377±140 mm3 in the group treated with raw CDs followed by 10 min of white light irradiation (120 mW cm−2). In contrast, the tumor volume was reduced to just 48±67 mm3 in the group treated with supra-CDs and the same white light irradiation (120 mW cm−2). After 16 days of observation, the mice were sacrificed, and the tumors were dissected, weighed, and photographed. In Figure 6d, it is evident that tumors in the group treated with supra-CDs followed by 10 min of white light irradiation were significantly smaller than those in the other groups. While raw CDs with light also exhibited tumor growth inhibition, their antitumor effect was inferior to that of supra-CDs. In Figure S20, the tumor weights were recorded as follows: 987.5±339.5 mg, 948.0±244.3 mg, 755.7±262.3 mg, 495.6±124.7 mg, and 38.2±63.1 mg. The comparison of representative photos before and after treatment in Figure 6e also shows that supra-CDs with light effectively inhibited tumor growth. After the treatment, blood samples were collected to analyze T cell populations, and spleen samples were collected to analyze memory T cell populations. In Figure 6f, a significant increase in CD8+ T cell populations was observed in the supra-CDs+L group, whereas in Figure 6g, a significant increase was observed in CD44+CD62L+ memory T cell populations in the same group, suggesting activation of the immune response. The analysis of tumor section staining in Figure S21 reveals extensive regions of apoptosis and necrosis within the tumors. Moreover, the highest cellular apoptotic rate induced by supra-CDs mediated PDT was reflected in the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining as compared with other groups. These findings indicate the significant antitumor effect and successful activation of the antitumor immune response of supra-CDs under white light irradiation.
In addition, the in vivo biosafety of supra-CDs was assessed. Figure S22 indicates that the body weights of mice in the supra-CDs treated groups remained constant throughout the treatment, suggesting that the systemic cytotoxicity from supra-CDs was minimal. After different treatments, mice serum was collected for blood biochemical analysis, and sections of major organs were subjected to H&E staining for analysis. The findings presented in Figure S23 reveal that supra-CDs did not exert a notable impact on the key indices of liver and kidney function. Figure S24 shows that major organs exhibited no signs of organic lesions or inflammatory reactions following the various treatments. These findings indicate that supra-CDs exhibit favorable biocompatibility.
Conclusion
In conclusion, novel supra-CDs were successfully developed through the fusion of red emissive CDs with DCHQ via post-solvothermal treatment and demonstrated effective photoinduced charge separation for high-performance tumor PDT efficacy. Within the supra-CDs, the electron-rich assembled CDs with substantial sp2 domains constituted the core state and the fused DCHQ with electron-withdrawing groups constituted the interface state, forming D−A-type nanostructure. The peculiar D−A configuration facilitated the efficient transfer of excited electrons from the core to the interface when exposed to white light irradiation, resulting in efficient photoinduced charge separation. Subsequently, the excited electrons were directed toward the surrounding O2, leading to the generation of O2⋅−. Meanwhile, the photogenerated radicals were stabilized in the assembled CDs, which can further oxidize crucial intracellular substrates such as BH4 and NADH. Benefit from the outstanding properties, improved photoinduced cell killing capacity of supra-CDs was achieved. In addition, the increased particle sizes of supra-CDs, reaching approximately 20 nm, resulted in a notable enhancement of tumor accumulation capacity because of the improved EPR effect, leading to satisfactory tumor PDT efficacy in a subcutaneous tumor model in mice. It is anticipated that this construction strategy for supra-CDs could open a new avenue for the design of functional nanomaterials derived from CDs, showcasing attractive photophysical characteristics for different biomedical applications.
Acknowledgments
The authors are grateful to the National Natural Science Foundation of China (U21A2097, 61935017, 62175268, 62288102), Science and Technology Development Fund of Macau SAR (0139/2022/A3, 0007/2021/AKP, 006/2022/ALC), and Department of Science and Technology of Guangdong Province (2019ZT08Y191, 2019QN01Y640, 2022B1212010003) for financial support. The authors also acknowledge the SUSTech Core Research Facilities for technical support, and the SUSTech Laboratory Animal Center for in vivo studies. All in vivo procedures have been approved by the Animal Ethics Committee of the Center for Experimental Animal Research of SUSTech, China.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.