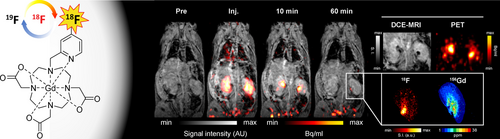
A Macrocyclic Hybrid PET/MRI Probe for Quantitative Perfusion Imaging In Vivo
Graphical Abstract
This work demonstrates that the integration of PET and MRI in molecular imaging has the potential to improve diagnostic accuracy and patient care. We have developed a hybrid PET/MRI probe, [18F][Gd(FL1)], which is exceptionally stable, rapidly synthesized, efficiently radiolabeled, and allows simultaneous PET/MR imaging. The potential for in vivo application is demonstrated by quantitative characterization of tissue and renal clearance in mice.
Abstract
Perfusion dynamics play a vital role in delivering essential nutrients and oxygen to tissues while removing metabolic waste products. Imaging techniques such as magnetic resonance imaging (MRI), computed tomography (CT), and positron emission tomography (PET) use contrast agents to visualize perfusion and clearance patterns; however, each technique has specific limitations. Hybrid PET/MRI combines the quantitative power and sensitivity of PET with the high functional and anatomical detail of MRI and holds great promise for precision in molecular imaging. However, the development of dual PET/MRI probes has been hampered by challenging synthesis and radiolabeling. Here, we present a novel PET/MRI probe, [18F][Gd(FL1)], which exhibits excellent stability comparable to macrocyclic MRI contrast agents used in clinical practice. The unique molecular design of [18F][Gd(FL1)] allows selective and expeditious radiolabeling of the gadolinium chelate in the final synthetic step. Leveraging the strengths of MRI and PET signals, the probe enables quantitative in vivo mapping of perfusion and excretion dynamics through an innovative voxel-based analysis. The diagnostic capabilities of [18F][Gd(FL1)] were demonstrated in a pilot study on healthy mice, successfully detecting early cases of unilateral renal dysfunction, a condition that is typically challenging to diagnose. This study introduces a new approach for PET/MRI and emphasizes a streamlined probe design for practical synthesis and improved diagnostic accuracy.
Introduction
Early detection of disease significantly improves treatment outcomes and patient prognosis. Pathological changes often begin at the molecular and cellular level, altering the tissue microenvironment long before discernible symptoms are revealed. Such changes may impact the natural flow of molecules through the vasculature and extracellular space, thereby affecting tissue perfusion and excretion, key biomarkers for the assessment of cancer, stroke, and renal diseases. Medical imaging technologies provide the crucial capability to non-invasively detect, localize, and visualize these disease biomarkers, potentially revealing collateral incidental findings of unsuspected conditions, further benefiting the patient. Combining Positron Emission Tomography (PET) and Magnetic Resonance Imaging (MRI) into a hybrid PET/MRI instrument represents a significant technological advance in current medical imaging, uniting the capabilities of two leading diagnostic technologies. This integration combines the quantitative precision and high sensitivity of PET radionuclide detection with MRI unparalleled temporal and spatial resolution, differentiating soft tissues.1, 2 Both techniques depend on externally administered agents to enhance imaging accuracy and extract relevant clinical parameters. PET utilizes radioactive tracers that, upon positron-electron annihilation, emit gamma photons for detection.3 Similarly, MRI often leverages gadolinium-based contrast agents (GBCAs) to differentiate anatomical tissue features, notably those altered by conditions affecting perfusion and extravasation, such as stroke or tumors.4 The agents used for each modality differ both chemically and in the amount of administered dose. While highly sensitive PET requires only trace amounts of radioactive substances, contrast-enhanced MRI typically requires about 1 g of gadolinium for an adult.4 This reflects a disparity of 6–9 orders of magnitude in molar concentrations.
In current clinical practice, PET tracers and MRI contrast agents are distinguished as separate classes of compounds. However, the advent of PET/MRI technology prompted efforts to synthesize unified hybrid PET/MRI contrast agents. These efforts have focused primarily on responsive or targeted probes for specific molecular markers, leveraging the inherent complementarity of the two techniques. For instance, the PET signal can act as a beacon, pinpointing areas of interest for high-resolution MRI inspection. Moreover, PET allows for agent quantification—a critical attribute for responsive MRI probes5, 6—enabling differentiation between molecular responses to stimuli and probe concentration variations, both affecting the MRI signal. However, these probes face significant hurdles. While hybrid probes anchored to superparamagnetic iron oxide nanoparticles (SPIOs) are straightforward to prepare,7 these probes lack reproducibility and poor pharmacokinetic properties. Small-molecule PET/MRI agents are less common, which underscores the challenge of integrating the two imaging modalities into a single compound. To address this, various strategies for combining PET isotopes and paramagnetic metals have been explored, including a pH-responsive (18F/Gd),8 temperature-responsive (68Ga/Gd),9 fibrin-targeting (64Cu/Gd)10 and integrin-targeting (68Ga/Gd)11 molecular probes, and (52gMn/55Mn) based probes.12-14 Although innovative, these prototypes involve complex synthesis methods, dual metal chelation management, and the use of less common isotopes. Additionally, the image quality of these isotopes is notably inferior to that of isotopes with shorter positron ranges, such as 18F.
GBCAs enhance the MRI signal by shortening the T1 relaxation time (accelerating relaxation rate R1=1/T1) of nearby water protons.4 The contrast agent efficacy is described with its r1 relaxivity (r1=R1/c, where c is the concentration in mM). A major limitation of MRI is its inability to determine the in vivo concentration of the contrast agent accurately. The possibility of quantifying MRI contrast agents using PET could overcome this long-standing limitation. This is particularly relevant for renal imaging, as the kidneys experience the most dynamic GBCA concentration fluctuations of any organ, both temporally and spatially.
The standard clinical method for assessing kidney function involves measuring serum creatinine concentration to calculate the estimated glomerular filtration rate (eGFR).15 However, this blood test has significant limitations as it can only detect abnormal function when both kidneys are severely impaired, lacking the ability to provide insights into the status of individual kidneys.16 In contrast, medical imaging techniques can potentially map the function of each kidney in great detail. From a chemical perspective, GBCAs are ideal for assessing renal function (i.e., perfusion, filtration, and excretion) because GBCAs are primarily cleared through the kidneys, freely filtered by the glomerulus, and neither secreted nor reabsorbed by the nephrons. Millions of contrast-enhanced MRI exams are performed annually for various diagnostic purposes.4 However, the potential to detect early renal disease is often overlooked due to the poor quantification of GBCAs in the kidneys during MRI scans.
While GBCAs offer valuable diagnostic insights, acyclic GBCAs have been associated with Nephrogenic Systemic Fibrosis (NSF), a rare but severe condition observed in patients with impaired kidney function.17 There are also concerns about the long-term retention of gadolinium in the body.18, 19 Yet, these issues have not been associated with macrocyclic GBCAs, known for their robust kinetic inertness that limits the release of harmful Gd3+ ions.20, 21 To carefully address all the discussed hybrid PET/MRI agent preparation hurdles, we have developed a structurally uncomplicated and easy-to-prepare agent with properties similar to established, clinically safe, and approved macrocyclic GBCAs. This unique hybrid PET/MRI agent [Gd(FL1)] has been designed to allow rapid and selective radiolabeling with 18F via an innovative metal chelate-directed isotopic exchange reaction. [18F][Gd(FL1)] enables accurate monitoring of fundamental biological processes transversal to all tissues—perfusion, extravasation, and excretion dynamics. The advantages of using non-specific PET/MRI contrast agents for precision imaging are demonstrated in vivo by detecting unexpected cases of unilateral renal dysfunction.
Results and Discussion
Design and Synthesis of Gd(III) Chelates
An optimal design for a versatile PET/MRI contrast agent should aim to closely replicate the properties of clinically approved macrocyclic MRI contrast agents, such as high kinetic inertness, high relaxivity, low molecular weight, and fast renal clearance. Equally important is carefully selecting the positron-emitting radionuclide to be incorporated into the molecule for optimal PET detection. Among viable positron-emitting radionuclides, 18F emerges as the most suitable candidate because (i) it offers minimal molecular alteration, (ii) it has a β+ decay half-life of 110 minutes, which is in line with the pharmacokinetics of standard GBCAs, (iii) it has low positron energy, which is optimal for high PET resolution, and (iv) it is widely available and can be produced efficiently and cost-effectively in medical cyclotrons worldwide.22 Finally, the radionuclide should be incorporated into the chelator molecule during the final synthetic step to minimize losses due to decay. This requires direct radiolabeling of a gadolinium chelate, which differs significantly from the conventional organic substrates typically used in PET imaging.
To meet all these requirements in a single molecular design, we hypothesized that a pyridine moiety, known for its strong donor properties, could replace one of the acetate arms in DOTA.23 This modification would provide an ideal platform for subsequent derivatization, as the pyridine ring is amenable to nucleophilic aromatic substitution,24, 25 allowing the substitution of appropriate leaving groups by fluoride anions. Although these reactions have been described for purely organic substrates, their adaptation for successful radiolabeling gadolinium chelates required extensive optimization of structural design and reaction conditions. Firstly, choosing suitable leaving groups was narrowed down to nitro- and fluoro-derivatives. While substitution of the nitro-group by fluoride anion has been reported,26 we explored the possibility of 19F−18F isotopic exchange for the first time in gadolinium chelates (Figure 1). Secondly, the coordinated pyridine arm provides multiple positions for placing these substituents, with their reactivity influenced by both first-order factors (electronic activation/deactivation) and second-order effects (coordination/steric hindrance). By thoroughly exploring all positional isomers, we found that only the para-position enables efficient radiolabeling through a combination of strong activation and favorable steric accessibility (Table 1, Table S5, Figures S11, S13). Although the ortho-position is expected to be highly activated, radiolabeling did not occur due to steric hindrance caused by coordination (Table S5, Figure S16).
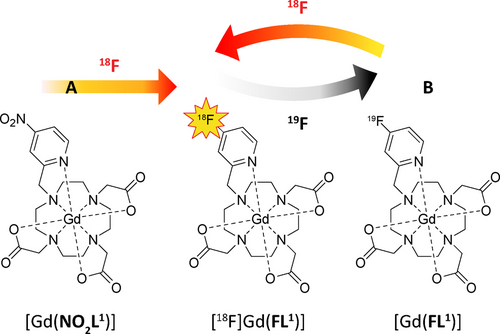
Two routes leading to the hybrid PET/MRI probe [18F][Gd(FL1)]. a) Nucleophilic substitution of the para-nitro group by 18F− on the precursor [(Gd(NO2L1)]. b) 18F-for-19F isotopic exchange with radioactive 18F on the precursor [Gd(FL1)].
Precursor |
TBAF |
RCY (%)[a] |
Purity [b] |
|---|---|---|---|
[Gd(NO2L1)] |
3 equiv. |
16.8±2.9 |
76.8±4.3 |
[Gd(FL1)] |
3 equiv. |
17.6±1.3 |
86.3±4.3 |
[Gd(FL1)] |
No TBAF |
6.6±0.9 |
90.5±1.8 |
FL1 |
3 equiv. |
0 |
75.0±4.2 |
- [a] The radiochemical yield is calculated as the peak area of the product compared to the area of all peaks in the gamma detector measured by HPLC (mean±SD; n=3). [b] The reaction purity is calculated as the peak area of the product compared to the area of all peaks at 254 nm measured by HPLC (mean±SD; n=3).
The synthetic pathways leading to Gd(III) chelates of the best-working para-nitro- and para-fluoro-derivatives are outlined in Scheme 1. The synthesis of the other tested isomers is described in the Supporting Information. For [(Gd(NO2L1)], the synthesis begins with the commercially available 2-methyl-4-nitropyridine 1-oxide (1), which was derivatized into the hydroxomethyl-derivative 2 via the Boekelheide rearrangement in trifluoroacetic acid anhydrate (TFAA).27 This compound was then converted to the chloromethyl-derivative 3 in SOCl2. Following this, 3 was used for the alkylation of tBu3DO3A, generating the protected chelator 4. Deprotection in trifluoroacetic acid (TFA) yielded NO2L1. The subsequent complexation of GdCl3 in the MOPS buffer, followed by purification through RP-HPLC, generated [Gd(NO2L1)] with an overall yield of 45 % relative to tBu3DO3A (Scheme 1a). The synthesis of [Gd(FL1)] started with the reduction of methyl 4-fluoro picolinate (5) using NaBH4, producing alcohol 6. This was then converted into the corresponding mesylate 7. The subsequent steps mirrored those of the nitro-derivative, providing the [Gd(FL1)] product with an overall yield of 34 % relative to tBu3DO3A (Scheme 1b). Importantly, it should be noted that the chelator FL1 exhibits only moderate stability in acidic aqueous solutions; therefore, we used pH-neutral stock solutions for the subsequent steps.
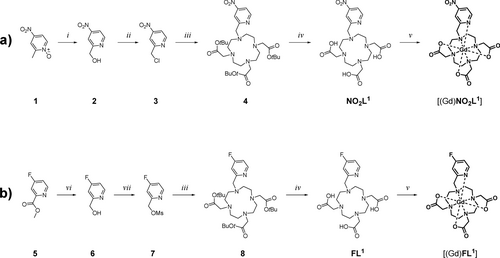
Synthesis of Gd(III) chelates used in this work. a) Synthesis of [(Gd(NO2L1)]. b) Synthesis of [Gd(FL1)]. Reaction conditions: (i) TFAA, DCM, RT; (ii) SOCl2, DMF, DCM, RT (iii) tBu-DO3A*HBr (hydrobromide salt of DO3A-tert-butyl ester), K2CO3, MeCN, RT; (iv) TFA (trifluoroacetic acid), RT; (v) GdCl3, aq. MOPS (3-(N-morpholino)propanesulfonic acid)/NaOH (pH 7.0), RT; (vi) NaBH4, MeOH, RT; (vii) MsCl, Et3N, DCM, 0 °C to RT. Intermediates 4 and 8 were not isolated.
Radiolabeling with 18F
The reaction conditions for the nitro-to-fluoro substitution were initially tested using the non-radioactive tetrabutylammonium fluoride (TBAF), adopting conditions previously used for purely organic pyridine substrates,26 modified to be compatible with the chelate [(Gd(NO2L1)]. Specifically, we used DMSO as a solvent to guarantee complete solubility of the Gd(III) chelate and excess TBAF to drive the reaction to completeness. The fact that the conversion to [Gd(FL1)] can be easily observed on HPLC (Figure S9) has facilitated this work. Next, we explored and optimized two methods for preparing fresh [18F]TBAF, a radiolabeling reagent whose quality proved crucial for successful radiolabeling. Both methods start from [18F]HF, the primary product of the cyclotron production of the 18F isotope. In Method A, the [18F]HF solution was loaded onto a QMA (quaternary methyl ammonium) SPE (solid-phase extraction) cartridge preconditioned with NaHCO3. The aforementioned [18F]TBAF was generated from eluting the immobilized [18F]fluoride from the QMA SPE with a solution of tetrabutylammonium bicarbonate (TBAHCO3), diluted with an equal volume of acetonitrile. The solution containing [18F]TBAF was then evaporated to dryness under a stream of argon. For Method B, the QMA SPE cartridge was pre-treated with KOTf28 (potassium trifluoromethylsulfonate), loading the [18F]HF solution, washing the resin with dry MeOH, and drying it under an argon stream for 3 minutes. Elution of [18F]TBAF was conducted with TBAOTf (tetrabutylammonium trifluoromethylsulfonate) in dry MeOH, followed by drying at 100 °C for 5 minutes under an argon stream. Reaction conditions for the conversion of [(Gd(NO2L1)] to [18F][Gd(FL1)] were tested manually using aliquots of thoroughly dried [18F]TBAF and small solvent volumes. Extensive screening (Table S3) found an optimum at 90 °C and a reaction time of 5 minutes with 3 eq. of [19F]TBAF. Notably, [19F]TBAF serves the dual purpose of enhancing the chemical conversion and generating the non-radioactive product required for the PET/MRI formulation applications—high specific activity is not necessary in this instance.
The [18F]TBAF produced through Method B demonstrated superior results, with a radiochemical yield (RCY) of 17 % and a purity of 87 %, surpassing the results obtained by Method A, which yielded an RCY of 11 % with a purity of 74 % (Table S3). Interestingly, using [Gd(FL1)] as the substrate in the isotopic exchange 18F/19F reaction under identical conditions resulted in further purity improvement (Table 1). This isotopic exchange has been previously reported for simple fluoro-pyridine compounds26, 29, 30 but never for metal chelates. Furthermore, it is worth noting that our attempts to label the free ligand FL1 with [18F]TBAF were unsuccessful (Figure S17, Table S5). This could be attributed to the interaction of the [18F]F− anion with the protonated tertiary amines of the macrocyclic ligand, rendering the anion unavailable for reaction. Data suggest that the presence of the chelated metal ion in [Gd(FL1)] is crucial for successful radiolabeling (Table 1).
Scaling up the [18F][Gd(FL1)] synthesis to higher activities for imaging experiments required the use of a custom-built automated synthesis module (GE). Starting from 16 GBq of [18F]HF, the automated synthesis yielded 1340 MBq of the final product (8 % RCY decay uncorrected) in 32 minutes, exhibiting 97 % chemical and 100 % radiochemical purity (Figure 2). For in vitro and in vivo PET/MRI experiments, the product was further combined with non-radioactive [Gd(FL1)] to adjust the activity/Gd ratio to levels compatible with both modalities. The concerted efforts in rational design, precursor screening, and radiolabeling optimization provided scalable synthesis as a prerequisite to the practical applicability of the hybrid PET/MRI agent. Notably, this first reported case of directly labeling a gadolinium chelate with 18F emphasizes the active role of the chelated metal in enabling radiolabeling and directing regioselectivity.
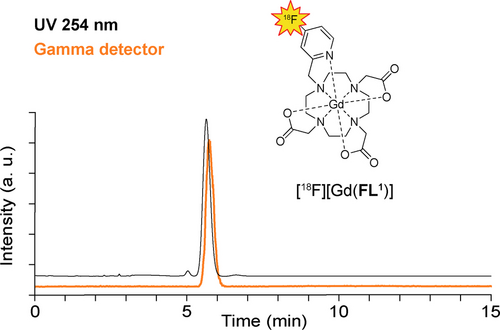
Radioactive and UV HPLC chromatograms of [18F][Gd(FL1)] sample produced on the automated radiosynthesis module. Only a single product peak (100 %) is visible in the gamma detection (orange), while the UV detection at 254 nm demonstrates purity of >97 % (black).
In Vitro Physicochemical Measurements
The relaxivity of GBCAs (r1) depends on several physicochemical parameters.31, 32 The number of bound water ligands (hydration, q) was assessed using the 17O NMR lanthanide-induced shift method with Dy(III) complexes.33 This approach confirmed the coordination of a single water molecule in solution (Figure S1. The exchange rate (kex) of the bound water with the bulk in solution was determined by temperature-dependent 17O NMR relaxometric studies (Figure S2, 2). A value of kex=1.7×106 s−1 was found, approximately 2.4 times slower than that of [Gd(DOTA)].34 This observation is consistent with the slightly lower longitudinal relaxivity of 3.43 mM−1 s−1 (0.47 T, 37 °C) for [Gd(FL1)]. However, it should be noted that this value falls well within the typical range of 3–4 mM−1 s−1 observed in clinically approved MRI contrast agents (Table 2).35
Complex |
r1 (mM−1 s−1) |
q |
kex (s−1) |
t1/2 (h) |
|---|---|---|---|---|
[Gd(FL1)] |
3.4±0.1 |
1.0±0.2 |
1.7×106±0.1 |
45 |
[Gd(DOTA)] |
3.9[a] |
1[c] |
4.1×106[b] |
44 |
Another critical factor for potential clinical use is the kinetic inertness of the metal chelate. Macrocyclic Gd(III) chelates, particularly those of the DOTA type, exhibit significantly higher inertness when compared to their acyclic counterparts.32, 37, 38 We have used an acid-assisted decomplexation method in 0.1 M HCl at 37 °C to monitor the kinetic inertness of [Gd(FL1)] in solution.39 Under these conditions, the chelate breaks down, releasing a fully hydrated Gd(III) ion into the solution, which has a higher r1 relaxivity. The overall mono-exponential increase in relaxation rate (R1) can be described by the half-life (t1/2, Figure S3) as a measure of the dechelation rate. We observed that while the acyclic clinical MRI contrast agent [Gd(DTPA)] dissociated rapidly (t1/2<6 s), [Gd(FL1)] showed a remarkable kinetic inertness with a dissociation half-life of 45 hours, comparable to the clinically approved [Gd(DOTA)] (Table 2).
In Vitro PET/MRI Phantom Imaging
Following the successful radiolabeling and comprehensive in vitro analysis of [Gd(FL1)], we designed a phantom imaging study for PET/MRI examination. We prepared a formulation containing [18F][Gd(FL1)] from automated radiosynthesis and a 0.5 mM stock solution of [Gd(FL1)] in PBS, to provide a reference solution of known molar activity and chemical concentration. This mixture was aliquoted into test tubes to create an array of samples with concentrations ranging from 0.048 to 0.489 mM (quantified by ICP-OES analysis) (Figure 3a). The quantification of PET/MR images acquired in a 7T preclinical scanner exhibited a remarkable linear correlation between PET-measured radioactivity and signal derived from T1-weighted MR images (R2=0.99, Figure 3b). This correlation was mirrored by the relaxation rate derived from MRI T1-maps (R2=0.99, Figure 3c). The relationship was further validated by quantifying 18F activity using gamma counting and Gd(III) via ICP-OES (R2=0.99, Figure S21). These findings show the potential of [18F][Gd(FL1)] to provide quantitative PET/MRI.
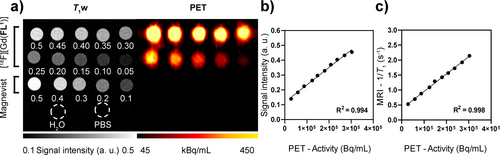
Simultaneous PET/MRI imaging of a phantom containing titrations of [18F][Gd(FL1)]. The concentration in each tube is given in mM. Magnevist titration, H2O and PBS were included as references for MR images and negative controls for PET images. b), c) Correlation plots between radioactivity measured with PET imaging and MRI T1-weighted (T1-w) signal (b) or MRI relaxation rates (c). Solid lines represent linear regression analysis.
In Vivo PET/MRI Studies in Mice
The in vivo performance of [Gd(FL1)] as a hybrid PET/MRI contrast agent was investigated in healthy C57BL/6 mice (n=6). A formulation of [Gd(FL1)] and its radiolabeled counterpart [18F][Gd(FL1)] in saline was administered to anesthetized mice via a single tail vein bolus injection. The administered dose was 0.09±0.01 mmol/kg of Gd, equivalent to 0.91±0.07 MBq. Two cohorts of three mice each underwent different imaging protocols (Scheme S1). In the first group, we conducted a continuous 62-minute session of simultaneous dynamic contrast-enhanced (DCE) MRI and dynamic PET scanning. The second group underwent 11 sequential T1 maps acquired over 55 minutes, followed by a 4.6-minute static PET scan. After completing the imaging procedures, all animals were euthanized 62 minutes post-injection, and organs were collected for ex vivo analysis. The [18F][Gd(FL1)] agent exhibited a consistent pharmacokinetic profile in both MRI (Figure 4a) and PET (Figure 4b) modalities, demonstrating swift systemic distribution and rapid blood clearance through the kidneys into the bladder—a common pathway for most non-targeted Gd-based agents. The agent exhibited minimal liver uptake and blood retention, indicating negligible hepatobiliary excretion (Figure 4c).
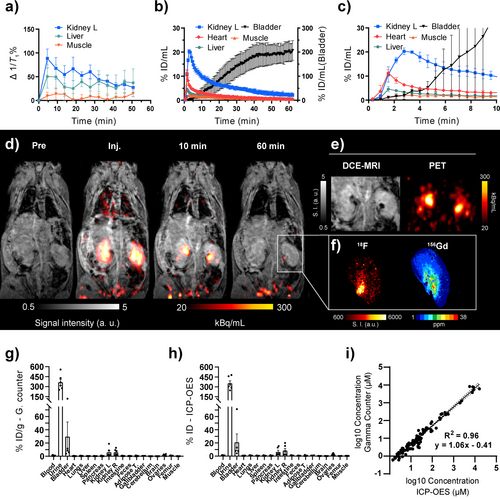
In vivo biodistribution of [18F][Gd(FL1)]. a) 1/T1 change over time after i. v. injection of [18F][Gd(FL1)] measured with dynamic T1 mapping (n=3), with the kidneys showing the highest T1 change. b, c) PET-based pharmacokinetics (n=3) expressed as %ID/mL showing low liver and muscle uptake and rapid accumulation in the heart followed by accumulation and clearance from the kidneys into the bladder over the entire PET/MR scan (b) or the first 10 minutes post-injection (c). d) Representative DCE-MRI and PET overlays before and after [18F][Gd(FL1)] injection. e) Comparison of the same kidney images in DCE-MRI and PET at 10 minutes post-injection showing accumulation of [18F][Gd(FL1)] in the renal pelvis as hypo-intense spots in MRI and high-intensity spots in PET. f) Autoradiography (left) and LA-ICP-MS (right) images of a kidney showing co-localization of 18F and Gd. g, h) Ex vivo biodistribution analysis 62 minutes post-injection based on gamma counting (g) and ICP-OES (h) (n=6); uptake of [18F][Gd(FL1)] is expressed as percentage of injected dose per gram of wet tissue. i) Linear regression analysis between tissue concentrations of [18F][Gd(FL1)] calculated from gamma counter activity and concentration measured by ICP-OES on a logarithmic scale shows excellent correlation (R2=0.96). Solid line: linear regression; dotted lines: 95 % confidence interval. a,b,c,g,h) Data are presented as mean±SEM.
The representative PET/MRI images (Figure 4d) clearly showed immediate enhancement of the renal cortex and medulla at the time of injection, followed by a rapid transit of the contrast agent to the renal pelvis within 10 minutes and its almost complete elimination to the bladder within 60 minutes (Figure 4b). PET/MRI showed no tissue retention of [18F][Gd(FL1)]. While PET and MRI signal enhancement generally correlated perfectly, an intriguing discrepancy was noted in the pelvis of healthy kidneys at 10 minutes post-injection (Figure 4e). Here, the freshly formed urine had a high local concentration of the contrast agent, resulting in hypo-intense regions on the MRI due to potent T2 and T2* relaxation mechanisms that quenched the signal in T1-weighted contrast.40 This highlights the complex non-linear relationship between GBCA concentration and its impact on the MRI signal, making it challenging to quantify in vivo MRI-based GBCA reliably. However, the hybrid [18F][Gd(FL1)] allows an independent and quantitative evaluation of the local concentration of the contrast agent through the PET signal. This was particularly evident as the hypo-intense areas observed on the MRI correlated perfectly with the regions of high PET signal (Figure 4e).
Kidney tissue from the healthy mice was sectioned and further analyzed by autoradiography (for 18F) and laser ablation ICP-MS (for Gd). As previously reported, the combination of laser ablation-inductively coupled plasma mass spectrometry (LA-ICP-MS) and autoradiography provides complementary measurements, offering detailed insights into both metal distributions and radioactivity.41 Both methods confirmed the observed in vivo distribution of [Gd(FL1)], showing the lowest concentration of the contrast agent in the cortex and the highest concentration in the pelvis (Figure 4f). Furthermore, all tissue samples from euthanized animals were analyzed to quantify radioactivity using a gamma counter and the gadolinium content using ICP-OES (Figure 4g–h). The biodistribution pattern remained consistent across both methods, revealing the highest concentration of [Gd(FL1)] in the urine and bladder, followed by the kidneys. These biodistribution patterns are consistent with the study by Le Fur et al. where [86Y][Y(DOTA)] was used to track [Gd(DOTA)] biodistribution.42 All other tissues had significantly lower [Gd(FL1)] contents. Importantly, radioactivity in the femur was low (0.86±0.12 %ID/g), suggesting no significant release of the bone-seeking [18F]F− anion from [18F][Gd(FL1)].43 Tissue concentrations determined by gamma counting and ICP-OES covered several orders of magnitude. A back-to-back comparison on a logarithmic scale revealed an excellent correlation (R2=0.96), underlining the robustness and stability of the 18F/Gd ratio across various concentrations and tissue types (Figure 4i). Furthermore, we performed an LC–MS analysis of a urine sample, which confirmed that the agent was excreted intact into the bladder, indicating an optimal in vivo pharmacokinetic and pharmacodynamic profile (Figure S8).
PET/MRI Renography
The renal system has a robust compensatory mechanism that effectively compensates one kidney for the dysfunction of the other kidney. However, detecting kidney disease early is challenging. The standard diagnostic test, which measures serum creatinine concentration, only indicates a significant stage of renal dysfunction44 and is not sensitive to unilateral or partial kidney disease.45, 40 Imaging techniques provide more nuanced information but present specific limitations. Planar SPECT renography is obscured by organ overlap, while SPECT/CT and PET/CT have significant exposure to ionizing radiation.44 Thus, MRI using renally excreted GBCAs emerges as an attractive alternative despite its limitations in quantification.40 While imaging is not typically employed for early diagnosis, it enables radiologists to identify unforeseen conditions when conducted for any other primary reason. Hence, PET/MRI using [18F][Gd(FL1)] emerges as a potent and versatile tool for studying renal function due to its quantitative nature.
During the in vivo studies, we identified abnormal renal filtration profiles in two mice, M1 and M6, that underwent two different PET/MRI protocols (details in Scheme S1). Both mice presented unilateral retention of [18F][Gd(FL1)] in the right kidney. For mouse M6, this was evident on the static PET image at 55 min post-injection, revealing a strong and persistent signal in the right renal pelvis, whereas no probe accumulation was observed in the left kidney (Figure 5a). The whole PET signal in the right kidney was approximately 10-fold higher than in the left (Figure S24c). Conversely, T1-weighted MR images were less conclusive, showing hypo-intense regions in the right kidney due to the strong T2 and T2* effects (Figure 5a, left image), but no substantial difference between the kidneys in the mean relaxation rate (Figure S24d). Thus, relying solely on MRI data might result in overlooking evidence of abnormal function in the right kidney. However, by leaveraging PET as a reporter, we were able to quantify the signal and perform a detailed ex vivo analysis of tissue sections with autoradiography (18F) and laser ablation ICP-MS (Gd). This approach confirmed the heterogeneous accumulation of [18F][Gd(FL1)] in the right kidney compared to the left (Figure 5b–c). Histology revealed dilated tubules and luminal proteinaceous material in the right kidney, confirming the renal pathological dysfunction (Figure 5d).
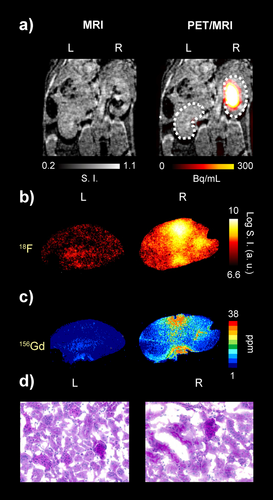
Sequential [18F][Gd(FL1)] PET/MRI imaging of mouse kidneys (mouse M6) showing normal and impaired renal excretion. a) T1w MR and PET/MR overlay after injection of the hybrid probe obtained with sequential PET/MRI protocol Scheme (MRI: 50 minutes; PET: 55 minutes) b) Autoradiography images of the left (L) and right (R) kidney sections. c) 156Gd concentration images of the left and right kidney sections obtained by LA-ICP-MS imaging. d) PAS staining of the left and right kidney sections.
In the second detected abnormal case (mouse M1), the imaging protocol included a 62-minute simultaneous PET/MRI acquisition (Figure 6), allowing us to follow the dynamic behavior of the probe and evaluate the renal function in detail. The left kidney and the kidneys of two other mice from the same cohort (M2, M3) served as references for normal renal function. We used an innovative voxel-based analysis of co-registered images from both modalities to calculate probe wash-out parameters (Figure 6b–e). The resulting parametric maps derived independently from the MRI and PET data showed heterogeneous clearance patterns within the abnormal right kidney, which correlated well with the hot-spot areas of impaired function (Figure 6d–e). While these areas showed signal loss on MRI, incorrectly suggesting rapid probe wash-out (Figure 6b–d), the areas showed substantial probe accumulation in the PET imaging (Figure 6c–e). In a further voxel-based analysis, we calculated the area under the curve (AUC) and compared two regions of interest (ROI) in the impaired kidney, one with a highly abnormal excretion pattern and the other with a near-normal excretion pattern. The near-normal region was characterized by a narrow distribution of the AUC values, whereas the abnormal ROI showed a broad distribution in both MRI- and PET-derived data (Figure 6f–g). Compared to the abnormal case, all healthy kidneys generally showed relatively homogeneous excretion patterns in both modalities (Figures S26–27).
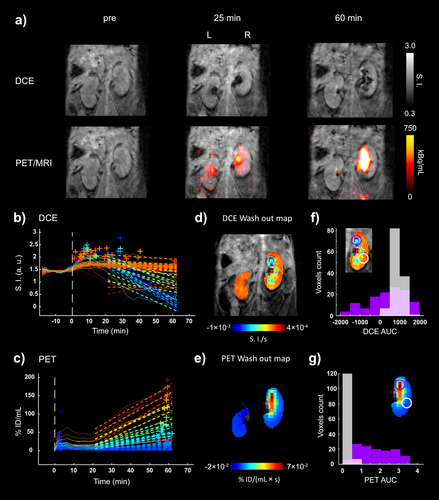
Simultaneous dynamic PET/MRI imaging of C57BL/6 mouse kidneys with [18F][Gd(FL1)] showing normal and impaired renal function. a) PET images (mouse M1) overlaid on T1w images before, after 25 and 60 minutes post-injection of [18F][Gd(FL1)] show remarkable differences in both PET and MRI signal between the left and right kidneys. The MRI signal shows a peculiar accumulation pattern in the right kidney compared to the left one, with a major change in the medulla and only becoming to be visible in the pelvis area after 25 min post-injection. The uptake (kBq/mL) in the right kidney is significantly higher than in left kidney and it increases over time. b,c) Representative DCE signal intensity (SI) and PET uptake (%ID/ml) vs time curves on a voxel basis of the right and left kidneys. Dashed lines describe the linear regression used to calculate the wash out parametric maps. +, highest intensity (DCE) and uptake (PET) peaks for each curve. d,e) DCE and PET wash out parametric maps obtained from dynamic curves. The MRI shows a homogeneous signal in the left kidney, while the excretion impairment of the right kidney is marked by a peculiar ring structure. f,g) Histograms of DCE and PET area under the curve (AUC) determined in two different subregions (purple and white circles) appear notably different in terms of voxel distribution.
Overall, PET/MRI with the hybrid contrast agent [18F][Gd(FL1)] offers significant advantages for the assessment of renal function, with both modalities providing crucial diagnostic value. The PET channel quantifies the agent independently of the wide filtration dynamics in the renal tissue, allowing for the precise detection of abnormal excretion patterns. On the other hand, MRI provides anatomical context and detailed spatial localization. As shown in Figure 6, MRI alone can be confounding due to negative contrast enhancement, whereas PET alone may lack the resolution to discern individual hot spots of impaired tissue function. To the best of our knowledge, this is the first report using such PET/MRI analysis obtained with a hybrid PET/MRI probe.
Conclusion
In this work, we present a novel hybrid PET/MRI contrast agent [18F][Gd(FL1)]. Designed to retain the favorable properties of clinical macrocyclic GBCAs, such as high r1 relaxivity, excellent kinetic inertness, and rapid renal excretion, this agent also enables reliable quantification through the PET signal. A rapid and scalable synthesis is achieved through an innovative 19F to 18F isotopic exchange performed directly on a gadolinium chelate for the first time. A pilot PET/MRI study on healthy mice demonstrated the capability of [18F][Gd(FL1)] to detect unexpected cases of unilateral renal dysfunction, a condition notoriously difficult to detect due to the compensatory mechanism of the renal system. In combination with a voxel-based image analysis, PET/MRI with [18F][Gd(FL1)] provides detailed anatomical, functional, and quantitative information on perfusion and molecular excretion dynamics that cannot be achieved with MRI or PET alone. This hybrid agent thus maximizes the synergistic potential of PET/MRI technology. Accurate mapping of perfusion and excretion dynamics is vital for diagnosing diseases such as stroke and cancer. The incidental findings of renal conditions reported here are particularly pertinent for monitoring tumor-targeted radiotherapies, where renal damage is a potential side effect. Further studies with appropriate, well-established animal models of kidney diseases will explore the full potential of our dual-modality approach. Moreover, PET/MRI with [18F][Gd(FL1)] may represent a reliable ground truth for modeling compartmentalized microvascular parameters, particularly in cases where estimating the concentration of GBCAs is challenging.46, 47
Supporting Information
The authors have cited additional references within the Supporting Information.[47–53]
Acknowledgments
We acknowledge the support from the National Institute for Research of Metabolic and Cardiovascular Diseases (Programme EXCELES, ID Project No. LX22NPO5104) - Funded by the European Union - NextGenerationEU; the Charles University project GAUK 1608218; the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—456007791, 516238665 and 390900677. This work was supported by the Cluster of Excellence iFIT (EXC 2180) “Image-Guided and Functionally Instructed Tumor Therapies,” University of Tuebingen, Germany, the Werner Siemens Foundation, the Alexander von Humboldt Foundation within the framework of the Sofja Kovalevskaja Award (to AFM), and the DKTK German Cancer Consortium Innovation program “HYPERBOLIC.” We gratefully acknowledge the help of Astrid Küppers in compiling the LA-ICP-MS images. We thank Dr. Julia Mannheim for the valuable input in imaging data interpretation and Dr. Sabrina Hoffmann for the assistance in MRI acquisitions and data analysis. Open Access funding enabled and organized by Projekt DEAL.
Conflict of Interests
M.P. and J.K. are co-inventors on a pending patent application no. EP22212471 filed in the name of applicant Ustav Organicke Chemie a Biochemie AV CR V.V.I. The application covers some of the compounds and their use discussed in this work.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.