Divergent Synthesis of Sulfur-Containing Bridged Cyclobutanes by Lewis Acid Catalyzed Formal Cycloadditions of Pyridinium 1,4-Zwitterionic Thiolates and Bicyclobutanes
Yuanjiu Xiao
State Key Laboratory of Chemo/Biosensing and Chemometrics, Advanced Catalytic Engineering Research Center of the Ministry of Education, College of Chemistry and Chemical Engineering, Hunan University, Changsha, Hunan, 410082 P. R. China
Search for more papers by this authorFeng Wu
State Key Laboratory of Chemo/Biosensing and Chemometrics, Advanced Catalytic Engineering Research Center of the Ministry of Education, College of Chemistry and Chemical Engineering, Hunan University, Changsha, Hunan, 410082 P. R. China
Search for more papers by this authorLei Tang
State Key Laboratory of Chemo/Biosensing and Chemometrics, Advanced Catalytic Engineering Research Center of the Ministry of Education, College of Chemistry and Chemical Engineering, Hunan University, Changsha, Hunan, 410082 P. R. China
Search for more papers by this authorDr. Xu Zhang
School of Chemistry & Chemical Engineering, Yangzhou University, Yangzhou, 225002 P.R. China
Search for more papers by this authorMengran Wei
Institute of Theoretical and Computational Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210093 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Guoqiang Wang
Institute of Theoretical and Computational Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210093 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Jian-Jun Feng
State Key Laboratory of Chemo/Biosensing and Chemometrics, Advanced Catalytic Engineering Research Center of the Ministry of Education, College of Chemistry and Chemical Engineering, Hunan University, Changsha, Hunan, 410082 P. R. China
Search for more papers by this authorYuanjiu Xiao
State Key Laboratory of Chemo/Biosensing and Chemometrics, Advanced Catalytic Engineering Research Center of the Ministry of Education, College of Chemistry and Chemical Engineering, Hunan University, Changsha, Hunan, 410082 P. R. China
Search for more papers by this authorFeng Wu
State Key Laboratory of Chemo/Biosensing and Chemometrics, Advanced Catalytic Engineering Research Center of the Ministry of Education, College of Chemistry and Chemical Engineering, Hunan University, Changsha, Hunan, 410082 P. R. China
Search for more papers by this authorLei Tang
State Key Laboratory of Chemo/Biosensing and Chemometrics, Advanced Catalytic Engineering Research Center of the Ministry of Education, College of Chemistry and Chemical Engineering, Hunan University, Changsha, Hunan, 410082 P. R. China
Search for more papers by this authorDr. Xu Zhang
School of Chemistry & Chemical Engineering, Yangzhou University, Yangzhou, 225002 P.R. China
Search for more papers by this authorMengran Wei
Institute of Theoretical and Computational Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210093 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Guoqiang Wang
Institute of Theoretical and Computational Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210093 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Jian-Jun Feng
State Key Laboratory of Chemo/Biosensing and Chemometrics, Advanced Catalytic Engineering Research Center of the Ministry of Education, College of Chemistry and Chemical Engineering, Hunan University, Changsha, Hunan, 410082 P. R. China
Search for more papers by this authorGraphical Abstract
Abstract
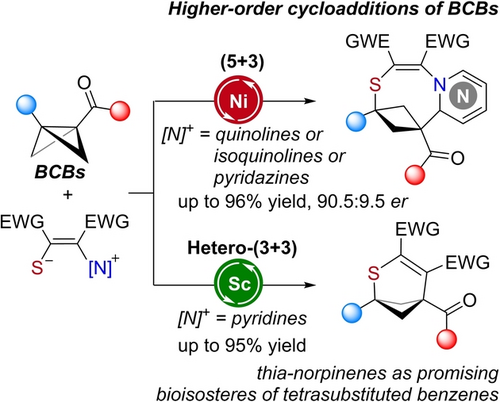
Bridged cyclobutanes and sulfur heterocycles are currently under intense investigation as building blocks for pharmaceutical drug design. Two formal cycloaddition modes involving bicyclobutanes (BCBs) and pyridinium 1,4-zwitterionic thiolate derivatives were described to rapidly expand the chemical space of sulfur-containing bridged cyclobutanes. By using Ni(ClO4)2 as the catalyst, an uncommon higher-order (5+3) cycloaddition of BCBs with quinolinium 1,4-zwitterionic thiolate was achieved with broad substrate scope under mild reaction conditions. Furthermore, the first Lewis acid-catalyzed asymmetric polar (5+3) cycloaddition of BCB with pyridazinium 1,4-zwitterionic thiolate was accomplished. In contrast, pyridinium 1,4-zwitterionic thiolates undergo an Sc(OTf)3-catalyzed formal (3+3) reaction with BCBs to generate thia-norpinene products, which represent the initial instance of synthesizing 2-thiabicyclo[3.1.1]heptanes (thia-BCHeps) from BCBs. Moreover, we have successfully used this (3+3) protocol to rapidly prepare thia-BCHeps-substituted analogues of the bioactive molecule Pitofenone. Density functional theory (DFT) computations imply that kinetic factors govern the (5+3) cycloaddition reaction between BCB and quinolinium 1,4-zwitterionic thiolate, whereas the (3+3) reaction involving pyridinium 1,4-zwitterionic thiolates is under thermodynamic control.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202408578-sup-0001-misc_information.pdf5.6 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aS. L. Schreiber, Science 2000, 287, 1964–1969;
- 1bM. D. Burke, S. L. Schreiber, Angew. Chem. Int. Ed. 2004, 43, 46–58;
- 1cL. Li, Z. Chen, X. Zhang, Y. Jia, Chem. Rev. 2018, 118, 3752–3832.
- 2
- 2aH. Liu, X. Jiang, Chem. Asian J. 2013, 8, 2546–2563;
- 2bM. Feng, B. Tang, S. H. Liang, X. Jiang, Curr. Top. Med. Chem. 2016, 16, 1200–1216;
- 2cS. Pathania, R. K. Narang, R. K. Rawal, Eur. J. Med. Chem. 2019, 180, 486–508;
- 2dN. R. Candeias, A. Efimov, Compr. Heterocycl. Chem. IV 2022, 7, 512–670.
- 3
- 3aL. Min, X. Liu, C.-C. Li, Acc. Chem. Res. 2020, 53, 703–718;
- 3bL. Min, Y.-J. Hu, J.-H. Fan, W. Zhang, C.-C. Li, Chem. Soc. Rev. 2020, 49, 7015–7043;
- 3cT. Wu, W. Tang, Chem. Eur. J. 2021, 27, 3944–3956;
- 3dZ. Zhang, H. Han, L. Wang, Z. Bu, Y. Xie, Q. Wang, Org. Biomol. Chem. 2021, 19, 3960–3982;
- 3eM. Presset, Y. Coquerel, J. Rodriguez, Chem. Rev. 2013, 113, 525–595.
- 4
- 4aB. F. Abdel-Wahab, S. Shaaban, G. A. El-Hiti, Mol. Diversity 2018, 22, 517–542;
- 4bE. A. Ilardi, J. T. Njardarson, J. Org. Chem. 2013, 78, 9533–9540;
- 4cN. Wang, P. Saidhareddy, X. Jiang, Nat. Prod. Rep. 2020, 37, 246–275, and references therein.
- 5B. Cheng, Y. Li, T. Wang, X. Zhang, H. Li, Y. Li, H. Zhai, Chem. Commun. 2019, 55, 14606–14608.
- 6
- 6aY. He, P. Wu, X. Zhang, T. Wang, Q. Tao, K. Zhou, Z. Ouyang, H. Zhai, D.-J. Cheng, B. Cheng, Org. Chem. Front. 2022, 9, 4612–4618;
- 6bB. Cheng, Y. Li, X. Zhang, S. Duan, H. Li, Y. He, Y. Li, T. Wang, H. Zhai, Org. Lett. 2020, 22, 5817–5821;
- 6cB. Cheng, X. Zhang, Y. Li, H. Li, Y. He, Y. Li, T. Wang, H. Zhai, Chem. Commun. 2020, 56, 8396–8399;
- 6dS. Duan, C. Chen, Y. Chen, Y. Jie, H. Luo, Z.-F. Xu, B. Cheng, C.-Y. Li, Org. Chem. Front. 2021, 8, 6962–6967;
- 6eC.-C. Wang, Y.-T. Yang, Q.-L. Wang, X.-H. Liu, Y.-J. Chen, Org. Chem. Front. 2022, 9, 4271–4276;
- 6fS. Sun, Y. Wei, J. Xu, Chem. Commun. 2023, 59, 239–242;
- 6gS. Sun, Y. Wei, J. Xu, Org. Lett. 2022, 24, 6024–6030;
- 6hY.-C. Luo, Y. Wang, R. Shi, X.-G. Zhang, H.-H. Zhang, P.-F. Xu, Org. Lett. 2023, 25, 6105–6109; For reviews, see:
- 6iJ. Huang, L. Zhang, X. Meng, Org. Chem. Front. 2023, 10, 2813–2829;
- 6jS. Das, SynOpen 2022, 6, 86–109;
- 6kZ.-H. Wang, Y. You, J. -Qi Zhao, Y.-P. Zhang, J.-Q. Yin, W.-C. Yuan, Molecules 2023, 28, 3059.
- 7Yuan attempted to synthesize bridged rings from pyridinium1,4-zwitterionic thiolates, but only a fused polycyclic structure was obtained. T.-J. Sun, X.-S. Peng, W. Sun, Y.-P. Zhang, X.-M. Ma, J.-Q. Zhao, Z.-H. Wang, Y. You, M.-Q. Zhou, J.-Q. Yin, W.-C. Yuan, Org. Lett. 2023, 25, 9191–9196.
- 8For reviews on BCPs synthesis, see:
- 8aB. R. Shire, E. A. Anderson, JACS Au 2023, 3, 1539–1553;
- 8bJ. M. Anderson, N. D. Measom, J. A. Murphy, D. L. Poole, Angew. Chem. Int. Ed. 2021, 60, 24754–24769;
- 8cM. M. D. Pramanik, H. Qian, W.-J. Xiao, J.-R. Chen, Org. Chem. Front. 2020, 7, 2531–2537;
- 8dX. Ma, L. N. Pham, Asian J. Org. Chem. 2020, 9, 8–22;
- 8eF.-S. He, S. Xie, Y. Yao, J. Wu, Chin. Chem. Lett. 2020, 31, 3065–3072;
- 8fJ. Kanazawa, M. Uchiyama, Synlett 2019, 30, 1–11.
- 9For (3+1) cycloadditions of BCBs with dihalocarbenes, see:
- 9aD. E. Applequist, J. W. Wheeler, Tetrahedron Lett. 1977, 18, 3411–3412;
10.1016/S0040-4039(01)83253-6 Google Scholar
- 9bR. Bychek, P. K. Mykhailiuk, Angew. Chem. Int. Ed. 2022, 61, e202205103;
- 9cR. M. Bychek, V. Hutskalova, Y. P. Bas, O. A. Zaporozhets, S. Zozulya, V. V. Levterov, P. K. Mykhailiuk, J. Org. Chem. 2019, 84, 15106–15117;
- 9dX. Ma, D. L. Sloman, Y. Han, D. J. Bennett, Org. Lett. 2019, 21, 7199–7203;
- 9eX. Ma, W. Pinto, L. N. Pham, D. L. Sloman, Y. Han, Eur. J. Org. Chem. 2020, 4581–4605;
- 9fR. E. McNamee, A. L. Thompson, E. A. Anderson, J. Am. Chem. Soc. 2021, 143, 21246–21251.
- 10Representative examples for synthesis of BCHs, see:
- 10aA. Cairncross, E. P. Blanchard Jr, J. Am. Chem. Soc. 1966, 88, 496–504;
- 10bA. de Meijere, H. Wenck, F. Seyed-Mahdavi, H. G. Viehe, V. Gallez, I. Erden, Tetrahedron 1986, 42, 1291–1297;
- 10cP. Wipf, M. A. A. Walczak, Angew. Chem. Int. Ed. 2006, 45, 4172–4175; Angew. Chem. 2006, 118, 4278–4281;
- 10dR. Kleinmans, T. Pinkert, S. Dutta, T. O. Paulisch, H. Keum, C. G. Daniliuc, F. Glorius, Nature 2022, 605, 477–482;
- 10eR. Kleinmans, S. Dutta, K. Ozols, H. Shao, F. Schäfer, R. E. Thielemann, H. T. Chan, C. G. Daniliuc, K. N. Houk, F. Glorius, J. Am. Chem. Soc. 2023, 145, 12324–12332;
- 10fS. Dutta, D. Lee, K. Ozols, C. G. Daniliuc, R. Shintani, F. Glorius, J. Am. Chem. Soc. 2024, 146, 2789–2797;
- 10gR. Guo, Y.-C. Chang, L. Herter, C. Salome, S. E. Braley, T. C. Fessard, M. K. Brown, J. Am. Chem. Soc. 2022, 144, 7988–7994;
- 10hS. Agasti, F. Beltran, E. Pye, N. Kaltsoyannis, G. E. M. Crisenza, D. J. Procter, Nat. Chem. 2023, 15, 535–541;
- 10iM. Xu, Z. Wang, Z. Sun, Y. Ouyang, Z. Ding, T. Yu, L. Xu, P. Li, Angew. Chem. Int. Ed. 2022, 61, e202214507; Angew. Chem. 2022, 134, e202214507;
- 10jY. Liu, S. Lin, Y. Li, J.-H. Xue, Q. Li, H. Wang, ACS Catal. 2023, 13, 5096–5103;
- 10kN. Radhoff, C. G. Daniliuc, A. Studer, Angew. Chem. Int. Ed. 2023, 62, e202304771; Angew. Chem. 2023, 135, e202304;
- 10lH. Yan, Y. Liu, X. Feng, L. Shi, Org. Lett. 2023, 25, 8116–8120;
- 10mH. Ren, T. Li, J. Xing, Z. Li, Y. Zhang, X. Yu, J. Zheng, Org. Lett. 2024, 26, 1745–1750;
- 10nD. Ni, S. Hu, X. Tan, Y. Yu, Z. Li, L. Deng, Angew. Chem. Int. Ed. 2023, e202308606; Angew. Chem. 2023, e202308606;
- 10oL. Tang, Y. Xiao, F. Wu, J.-L. Zhou, T.-T. Xu, J.-J. Feng, Angew. Chem. Int. Ed. 2023, 62, e202310066;
- 10pA. Denisenko, P. Garbuz, S. V. Shishkina, N. M. Voloshchuk, P. K. Mykhailiuk, Angew. Chem. Int. Ed. 2020, 59, 20515–20521; Angew. Chem. 2020, 132, 20696–20702;
- 10qK. Takao, H. Kai, A. Yamada, Y. Fukushima, D. Komatsu, A. Ogura, K. Yoshida, Angew. Chem. Int. Ed. 2019, 58, 9851–9855; Angew. Chem. 2019, 131, 9956–9960;
- 10rM. Reinhold, J. Steinebach, C. Golz, J. C. L. Walker, Chem. Sci. 2023, 14, 9885–9891;
- 10sJ. M. Posz, N. Sharma, P. A. Royalty, Y. Liu, C. Salome, T. C. Fessard, M. K. Brown, J. Am. Chem. Soc. 2024, 146, 10142–10149.
- 11For pioneering examples of synthesizing BCHeps through ring-opening reactions of [3.1.1]propellanes, see:
- 11aN. Frank, J. Nugent, B. R. Shire, H. D. Pickford, P. Rabe, A. J. Sterling, T. Zarganes-Tzitzikas, T. Grimes, A. L. Thompson, R. C. Smith, C. J. Schofield, P. E. Brennan, F. Duarte, E. A. Anderson, Nature 2022, 611, 721–726;
- 11bT. Iida, J. Kanazawa, T. Matsunaga, K. Miyamoto, K. Hirano, M. Uchiyama, J. Am. Chem. Soc. 2022, 144, 21848–21852. For the first successful synthesis of BCHeps via (4+2) reactions, see:
- 11cA. S. Harmata, T. E. Spiller, M. J. Sowden, C. R. J. Stephenson, J. Am. Chem. Soc. 2021, 143, 21223–21228.
- 12For reviews on BCBs, see:
- 12aM. Golfmann, J. C. L. Walker, Commun. Chem. 2023, 6, 9;
- 12bC. B. Kelly, J. A. Milligan, L. J. Tilley, T. M. Sodano, Chem. Sci. 2022, 13, 11721–11737;
- 12cA. Fawcett, Pure Appl. Chem. 2020, 92, 751–765;
- 12dJ. Turkowska, J. Durka, D. Gryko, Chem. Commun. 2020, 56, 5718–5734;
- 12eM. A. A. Walczak, T. Krainz, P. Wipf, Acc. Chem. Res. 2015, 48, 1149–1158;
- 12fP. Bellotti, F. Glorius, J. Am. Chem. Soc. 2023, 145, 20716–20732;
- 12gS. J. Sujansky, X. Ma, Asian J. Org. Chem. 2024, e202400045.
- 13
- 13aK. Dhake, K. J. Woelk, J. Becica, A. Un, S. E. Jenny, D. C. Leitch, Angew. Chem. Int. Ed. 2022, 61, e202204719; Angew. Chem. 2022, 134, e202204719;
- 13bY. Liang, F. Paulus, C. G. Daniliuc, F. Glorius, Angew. Chem. Int. Ed. 2023, 62, e202305043; Angew. Chem. 2023, 135, e202305043;
- 13cY. Liang, R. Kleinmans, C. G. Daniliuc, F. Glorius, J. Am. Chem. Soc. 2022, 144, 20207–20213;
- 13dB. D. Schwartz, A. P. Smyth, P. E. Nashar, M. G. Gardiner, L. R. Malins, Org. Lett. 2022, 24, 1268–1273;
- 13eM. Wang, Y. Huang, C. Li, P. Lu, Org. Chem. Front. 2022, 9, 2149–2153.
- 14
- 14aY. Zhang, W. Huang, R. K. Dhungana, A. Granados, S. Keess, M. Makvandi, G. A. Molander, J. Am. Chem. Soc. 2022, 144, 23685–23690;
- 14bT. Yu, J. Yang, Z. Wang, Z. Ding, M. Xu, J. Wen, L. Xu, P. Li, J. Am. Chem. Soc. 2023, 145, 4304–4310;
- 14cT. V. T. Nguyen, A. Bossonnet, M. D. Wodrich, J. Waser, J. Am. Chem. Soc. 2023, 145, 25411–25421; For aza-BCHeps, see:
- 14dJ. Zhang, J.-Y. Su, H. Zheng, H. Li, W.-P. Deng, Angew. Chem. Int. Ed. 2024, e202318476; Angew. Chem. 2024, e202318476;
- 14eY. Liang, R. Nematswerani, C. G. Daniliuc, F. Glorius, Angew. Chem. Int. Ed. 2024, e202402730; Angew. Chem. 2024, e202402730.
- 15M. Mustafa, J.-Y. Winum, Expert Opin. Drug Discovery 2022, 17, 501–512.
- 16
- 16aD. Dibchak, M. Snisarenko, A. Mishuk, O. Shablykin, L. Bortnichuk, O. Klymenko-Ulianov, Y. Kheylik, I. V. Sadkova, H. S. Rzepa, P. K. Mykhailiuk, Angew. Chem. Int. Ed. 2023, e202304246; Angew. Chem. 2023, e202304246;
- 16bV. V. Levterov, Y. Panasyuk, V. O. Pivnytska, P. K. Mykhailiuk, Angew. Chem. Int. Ed. 2020, 59, 7161–7167; Angew. Chem. 2020, 132, 7228–7234;
- 16cA. Denisenko, P. Garbuz, N. M. Voloshchuk, Y. Holota, G. Al-Maali, P. Borysko, P. K. Mykhailiuk, Nat. Chem. 2023, 15, 1155–1163.
- 17S. Kosuge, N. Hamanaka, M. Hayashi, Tetrahedron Lett. 1981, 22, 1345–1348.
- 18
- 18aL.-N. Wang, Z.-X. Yu, Chin. J. Org. Chem. 2020, 40, 3536–3558;
- 18bL. Yet, Chem. Rev. 2000, 100, 2963–3007;
- 18cG. A. Molander, Acc. Chem. Res. 1998, 31, 603–609;
- 18dN. De, E. J. Yoo, ACS Catal. 2018, 8, 48–58;
- 18eM.-M. Zhang, B.-L. Qu, B. Shi, W.-J. Xiao, L.-Q. Lu, Chem. Soc. Rev. 2022, 51, 4146–4174;
- 18fD. J. Lee, D. Ko, E. J. Yoo, Angew. Chem. Int. Ed. 2015, 54, 13715–13718; Angew. Chem. 2015, 127, 13919–13922;
- 18gS.-y. Baek, J. Y. Lee, D. Ko, M.-H. Baik, E. J. Yoo, ACS Catal. 2018, 8, 6353–6361;
- 18hJ. Y. Lee, R. K. Varshnaya, E. J. Yoo, Org. Lett. 2022, 24, 3731–3735;
- 18iC. Yuan, Y. Wu, D. Wang, Z. Zhang, C. Wang, L. Zhou, C. Zhang, B. Song, H. Guo, Adv. Synth. Catal. 2018, 360, 652–658;
- 18jQ.-Z. Li, Y.-L. Guan, Q.-W. Huang, T. Qi, P. Xiang, X. Zhang, H.-J. Leng, J.-L. Li, ACS Catal. 2023, 13, 1164–1172;
- 18kL.-C. Yang, Y.-N. Wang, R. Liu, Y. Luo, X. Q. Ng, B. Yang, Z.-Q. Rong, Y. Lan, Z. Shao, Y. Zhao, Nat. Chem. 2020, 12, 860–868;
- 18lB. Niu, X.-Y. Wu, Y. Wei, M. Shi, Org. Lett. 2019, 21, 4859–4863.
- 19V. V. Semeno, V. O. Vasylchenko, I. M. Fesun, L. Yu Ruzhylo, M. O. Kipriianov, K. P. Melnykov, A. Skreminskyi, R. Iminov, P. Mykhailiuk, B. V. Vashchenko, O. O. Grygorenko, Chem. Eur. J. 2023, e202303859.
- 20H. Wang, H. Shao, A. Das, S. Dutta, H. T. Chan, C. Daniliuc, K. N. Houk, F. Glorius, Science 2023, 381, 75–81.
- 21L. Moafi, S. Ahadi, H. R. Khavasi, A. Bazgir, Synthesis 2011, 1399–1402.
- 22Deposition Numbers 2323631 (for 3 aa) and 2323636 (for 5 ab) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 23
- 23aK. W. Quasdorf, L. E. Overman, Nature. 2014, 516, 181–191;
- 23bF. Zhou, L. Zhu, B.-W. Pan, Y. Shi, Y.-L. Liu, J. Zhou, Chem. Sci. 2020, 11, 9341–9365.
- 24M. de Robichon, T. Kratz, F. Beyer, J. Zuber, C. Merten, T. Bach, J. Am. Chem. Soc. 2023, 145, 24466–24470.
- 25Q. Fu, S. Cao, J. Wang, X. Lv, H. Wang, X. Zhao, Z. Jiang, J. Am. Chem. Soc. 2024, 146, 8372–8380.
- 26
- 26aS.-B. Wu, C. Long, E. J. Kennelly, Nat. Prod. Rep. 2014, 31, 1158–1174;
- 26bK. Surana, B. Chaudhary, M. Diwaker, S. Sharma, MedChemComm 2018, 9, 1803–1817;
- 26cU. M. Acuña, N. Jancovski, E. J. Kennelly, Curr. Top. Med. Chem. 2009, 9, 1560–1580;
- 26dM. Chen, Y. Cui, X. Chen, R. Shang, X. Zhang, Nat. Commun. 2024, 15, 419.
- 27
- 27aC. Lee, W. Yang, R. G. Parr, Matter Mater. Phys. 1988, 37, 785–789;
- 27bA. D. Becke, J. Chem. Phys. 1993, 98, 5648–5652.
- 28
- 28aS. Grimme, J. Antony, S. Ehrlich, H. Krieg, J. Chem. Phys. 2010, 132, 154104;
- 28bS. Grimme, S. Ehrlich, L. Goerigk, J. Comput. Chem. 2011, 32, 1456–1465.
- 29When we subjected 3 aa to Conditions B, we indeed observed the decomposition of the (5+3) cycloadduct (see Scheme S4). Although yielding some unidentified by-products, This experiment verifies that cycloadduct 3 aa is kinetically reversible, which is consistent with computed mild heat release for the reaction of 1 a+2 a→3 aa.