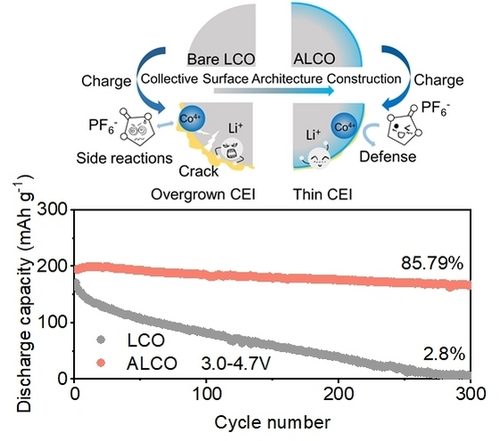
Scalable Precise Nanofilm Coating and Gradient Al Doping Enable Stable Battery Cycling of LiCoO2 at 4.7 V
Graphical Abstract
The overgrowth of surface CEI and the irreversible phase transition from H1–3 to O1 are the primary causes of LiCoO2 cathode degradation at 4.7 V. These phenomena can be significantly suppressed by employing an ultrathin, dense LiAlO2 surface coating and Al gradient doping, which enables a stable cycling of LiCoO2 at 4.7 V.
Abstract
The quest for smart electronics with higher energy densities has intensified the development of high-voltage LiCoO2 (LCO). Despite their potential, LCO materials operating at 4.7 V faces critical challenges, including interface degradation and structural collapse. Herein, we propose a collective surface architecture through precise nanofilm coating and doping that combines an ultra-thin LiAlO2 coating layer and gradient doping of Al. This architecture not only mitigates side reactions, but also improves the Li+ migration kinetics on the LCO surface. Meanwhile, gradient doping of Al inhibited the severe lattice distortion caused by the irreversible phase transition of O3−H1−3−O1, thereby enhanced the electrochemical stability of LCO during 4.7 V cycling. DFT calculations further revealed that our approach significantly boosts the electronic conductivity. As a result, the modified LCO exhibited an outstanding reversible capacity of 230 mAh g−1 at 4.7 V, which is approximately 28 % higher than the conventional capacity at 4.5 V. To demonstrate their practical application, our cathode structure shows improved stability in full pouch cell configuration under high operating voltage. LCO exhibited an excellent cycling stability, retaining 82.33 % after 1000 cycles at 4.5 V. This multifunctional surface modification strategy offers a viable pathway for the practical application of LCO materials, setting a new standard for the development of high-energy-density and long-lasting electrode materials.
Introduction
Lithium cobalt oxide (LCO) is esteemed for its high volumetric energy density, positioning it as an indispensable cathode material for portable and smart electronics.1 Despite its widespread use, the specific capacity of commercial LCO is limited to 150–180 mAh g−1 due to the low charging cut-off voltage (4.3–4.5 V vs. Li/Li+). This limitation starkly contrasts with its theoretical specific capacity of 274 mAh g−1, thereby restricting its energy density and broader application potential.2 Elevating the charging cut-off voltage at 4.7 V to extract more Li+ presents a promising approach to bolster the specific capacity for LCO cathode, however, this approach is fraught with tremendous challenges.3 Firstly, at high states of delithiation, the oxidized Co4+ reacts with the carbonate electrolyte solvent, causing a significant increase in the interface resistance and polarizations of the battery.4 Secondly, charging beyond 4.55 V triggers a phase transition in LCO from the O3 phase to the hybridized H1–3 hexagonal phase, rendering the surface lattice vulnerable to structural collapse and crystal fragmentation due to a lithium-ion concentration gradient.5 Last but not least, at approximately 4.65 V, LCO undergoes its final phase transition from H1–3 to O1, a series of side reactions at the interface are exacerbated owing to the very low stability of the O3 phase.6 To address these issues, strategies that limit interfacial side reactions at high voltage and fortify the lattice structure of the surface have emerged as pivotal for improving surface integrity of LCO at 4.7 V.
Surface design and construction have been proven effective in enhancing the stability of interface between LCO and electrolyte at elevated charging cut-off voltage at 4.7 V.7 Optimal surface coatings are envisaged to be ultra-thin yet integral, facilitating rapid lithium-ion diffusion while ensuring the electrochemical window‘s stability, thereby providing sustained physical isolation and mechanical protection to the cathode material.8 Conventional coating techniques, including dry and wet coating methods, possess inherent limitations that may result in thicker or uneven coatings, ultimately impacting the capacity release of LCO. Atomic layer deposition (ALD), a technique capable of fabricating highly conformal and pinhole-free coatings with sub-nanometer dimensions, has been introduced and utilized by battery researchers.9 Teranishi et al. deposited Al2O3 on LCO by ALD, and the modified material possessed good cycling stability.10 However, surface coating is insufficient to prevent the H1−3−O1 phase transition in LCO at 4.65 V.11 Therefore, a mere coating cannot significantly improve the electrochemical performance of LCO under high voltages exceeding 4.65 V. Surface gradient doping has emerged as an effective strategy to optimize the stability of the H1–3 phase on the LCO surface. This approach stabilizes the lattice structure of the surface at 4.7 V by creating a surface that consists of highly active centers and stable regions, thus exhibiting superior cycling stability while retaining high capacity.12 Zhang et al. proposed a facile surface gradient Mg doping method using dry mixing and calcination, which stabilized the electrochemical performance of LCO at 4.7 V.13 Although significant research efforts to enhance the stability and robustness of LCO surfaces, there remains a notable gap in the development of strategies that offer multifunctional modifications. These include simultaneously improving surface stability, stabilizing the lattice structure, and maintaining high lithium-ion migration kinetics. Current methodologies fall short of addressing all these aspects in a unified manner. Therefore, it is crucial to design new approaches that not only ensure high-energy-density and prolonged cycle stability for LCO but also enable its operation beyond the critical threshold of 4.65 V.
In this work, we introduce a versatile surface architecture for LCO, named ALCO, which integrates an ultra-thin LiAlO2 coating layer with Al gradient doping, employing precise nanofilm coating and doping (PNCD) bases on scalable powder Atomic Layered Deposition (ALD). This ultra-thin LiAlO2 coating layer not only serves as a robust a physical barrier against adverse side reactions between LCO and electrolyte but also acts as a Li+ conductor, significantly enhancing Li+ diffusion kinetic. The strategic incorporation of Al gradient doping further stabilizes the LCO by restricting the H1–3 to O1 phase transition via the suppression of crystal lattice fluctuation and enhances the Li+ diffusion kinetics by regulating the Li+ diffusion pathways. As a result, ALCO displays a superior reversible capacity of 230 mAh g−1 at ultrahigh cut-off voltage up to 4.7 V, marking a 28 % increase compared to the capacity achieved when charged to 4.5 V. Furthermore, this modified ALCO exhibits an unprecedented capacity retention of 85.79 % after 300 cycles at 4.7 V in the half cell. This work contributes a viable and effective solution to the longstanding challenge of improving the performance of LCO cathodes beyond conventional voltage limits, offering promising avenues for future energy storage technologies.
Results and Discussion
Characterizations of bare LCO and ALCO
The schematic of ALCO preparation by ALD is shown in Figure 1a. The scanning electron microscope (SEM) were employed to examine the morphology of the samples (Figure 1b, c). The morphology of cathode particles remained almost unchanged before and after modification, indicating that the implementation of a collective surface architecture does not adversely impact the surface morphology of the materials. The crystal structures of the two samples in Figure S1 can be assigned to the typical layered hexagonal structure characterized by X-ray diffraction (XRD).14 In addition, the discernible peak separation of (006)/(102) and (108)/(110) can be observed, which indicates planes underscore a highly ordered layered architecture within both the bare LCO and ALCO samples.15 This structural order is pivotal for the electrochemical performance of lithium cobalt oxide, as it facilitates efficient lithium-ion migration, a fundamental determinant of battery capacity and stability. Furthermore, the crystallographic parameters and atomic occupancies of bare LCO and ALCO were obtained by XRD Rietveld refinement (Figure 1d and Figure S2, Table S1 and S2), which exhibited the lattice parameters (a=2.8211 Å and c=14.0861 Å) of ALCO were slightly reduced compared with the bare LCO (a=2.8677 Å and c=14.2232 Å) with a trace of Al doping in the matrix of ALCO, indicating that Al element was successfully introduced in the crystal lattice of LiCoO2 after collective surface architecture construction. Further elucidation of the impact of this surface architecture on the materials was achieved through transmission electron microscopy (TEM). The TEM images (Figure 1e) showed a flat and smooth surface of the unmodified LCO. Additionally, the corresponding image of selected area electron diffraction (SAED) displayed in Figure 1e, which was demonstrated that bare LCO possess a highly ordered layered structure. Contrastingly, High-Resolution TEM (HRTEM) imaging of ALCO (Figure 1f) unveiled the successful integration of LiAlO2 with distinct (202) planes post-modification, evidencing the precise engineering at the nanoscale enabled by the collective surface architecture. In addition, selected area electron diffraction (SAED) image shown in Figure 1f also displayed a typical tetragonal type structure of LiAlO2 on the surface region of ALCO, despite this LiAlO2 coating layer being less apparent visually. To further confirm the formation of LiAlO2, sintered samples with 200 cycles of ALD and the heated at same temperature were analyzed by XRD, and the formation of LiAlO2 was found as shown in Figure S3.

a) The schematic of the construction process of the collective surface architecture construction of ALCO. The scanning electron microscopy (SEM) images of b) bare LCO and c) ALCO. d) Rietveld refinement of the XRD patterns for ALCO. Transmission electron microscope (TEM) and Selected area electron diffraction (SAED) of e) bare LCO and f) ALCO. X-ray photoelectron spectroscopy (XPS) spectra of g) Al 2p, h) Li 1s. XPS spectra of Al with different depths in i) bare LCO and j) ALCO.
X-ray photoelectron spectroscopy (XPS) was employed to analyze the surface chemical constitution of both bare LCO and ALCO. The 50–80 eV region included the Al 2p, Co 3p, and Li 1s core levels. ALCO showed Al 2p peaks at 74.0 eV and 73.1 eV (Figure 1g). These peaks were indicative of LiAlO2 and LiCoxAl1−xO2, signifying the incorporation of aluminum into the LCO matrix and the formation of LiAlO2 on the surface.16 From the Li 1s XPS spectrum (Figure 1h) and O 1s XSP spectrum (Figure S4), the enhancement of the peak at 54.7 eV in Li 1s and 531 eV in O 1s was attributed to the generation of LiAlO2 in ALCO after collective surface architecture construction, which was consistent with the result of Al 2p spectrum.17 In addition, the Co 2p spectrum of ALCO shown a higher Co3+/Co2+ ratio compared with bare LCO, while the weakly absorbed oxygen at 532 eV in the O 1s spectrum of ALCO occurs reduced (Figure S4), which was attributed to the reduction of Co(OH)2 on the surface of bare LCO after collective surface architecture construction.18 Furthermore, XPS etching conducted by increasing the etching depth (0–120 nm) of the bare LCO and ALCO particles was exhibited in Figure 1 i, j. The Al 2p spectrum of bare LCO and ALCO exhibited a decrease in the Al 2p peak intensity with depth, which vanished at 120 nm. This gradient demonstrates the successful doping of aluminum into the ALCO surface lattice, with the doping depth was approximately 120 nm. The result confirms that the collective surface architecture with LiAlO2 coating and Al concentration gradient doping was successfully constructed by PNCD technique.
Electrochemical Performance
To analyze the effect of collective surface architecture on the electrochemical behaviors of bare LCO and ALCO, the electrochemical performance of bare LCO and ALCO was first systemically evaluated in half cells within a voltage window of 3.0 to 4.7 V. The galvanostatic charge–discharge capacity curves at 0.1 C, presented in Figure 2a, b, reveal distinct differences between the two materials. Bare LCO displayed a high capacity of 233.1 mAh g−1, then rapidly decreased to 213.2 mAh g−1 after only 5 cycles, indicating a poor stability of bare LCO at an operational voltage of 4.7 V. In comparison with bare LCO, ALCO not only possessed a capacity of 230.2 mAh g−1, which represented 28 % enhancement compared to capacity of LCO charged to 4.5 V (180 mAh g−1), and still obtained 230.1 mAh g−1 after 5 cycles, but also showing higher cycling stability due to the electrochemical inertness of the ultrathin and homogeneous LiAlO2. This enhanced performance was attributed to the electrochemical inertness afforded by the ultrathin and homogeneous LiAlO2 layer, which effectively inhibited the interfacial side reactions. It was worth noting that the initial coulombic efficiency (CE) of ALCO was observed at 92.34 %, surpassing the 91.13 % recorded for bare LCO. The improvement in the coulombic efficiency of ALCO was attributed to the ultra-thin and dense LiAlO2 layer in the collective surface architecture, which not only improved the stability of the CEI film, but also isolated the cathode from the electrolyte, thereby inhibiting side reactions.

The charge/discharge curves for a) bare LCO and b) ALCO of different cycle numbers at 0.1 C. c) The rate performance of bare LCO and ALCO at 4.7 V. d) The cyclic performance of bare LCO and ALCO within 3.0–4.7 V at 1 C. e) The charge/discharge curves of bare LCO and ALCO at different cycles during cycling at 1 C. f) Comparison of ALCO cycling performance with previous representative reports. g) Cyclic performance of bare LCO‖graphite and ALCO‖graphite full-cells cycling in the range of 3.0–4.5 V at 0.5 C. Inset: photo of an assembled pouch cell.
To further investigate the effect of collective surface architecture on the reversibility of LCO at 4.7 V, we analyzed the cyclic voltammetry (CV) curves of bare LCO and ALCO within a voltage range of 3.0–4.7 V (Figure S5). The redox peaks of the bare LCO sample was gradually diminished with the increasing scanning number. In contrast, the peaks of the ALCO were enhanced at different cycles, which indicated that the enhanced structural stability of the ALCO at 4.7 V. Furthermore, at the 5th cycle, the potential difference between the oxidation and reduction peaks for the two O3 phase transitions of ALCO was only 0.21 V, which was significantly lower than the 0.40 V observed in bare LCO. The smaller potential gap suggested a lower degree of polarization in ALCO, demonstrating that the conductivity of ALCO was improved. The LiAlO2 layer enhances Li+ migration efficiency across the ALCO surface, thereby optimizing electrochemical reversibility and reducing polarization effects.19
The rate performance of bare LCO and ALCO at 4.7 V were shown in Figure 2c. At 0.1 C, 0.2 C, 0.5 C, 1 C, 2 C, 5 C and 10 C, the discharge capacity of bare LCO was 235.6, 196.3, 163.3, 130.9, 92.7, 25.3 and 6.9 mAh g−1, respectively. Notably, the discharge capacity of bare LCO only paetially recovered to 162.4 mAh g−1 when the current density returned to 0.2 C, revealing a significant capacity fading of 33.9 mAh g−1 from its initial performance at 0.2 C. In contrast, ALCO showed a higher rate capability across all current density, with discharge capacity of 229.3, 224.4, 211.7, 198.2, 181.9, 157.3 and 128.3 mAh g−1, respectively. Crucially, upon returning the current density to 0.2 C, ALCO′s capacity was impressively sustained at 217.7 mAh g−1, exhibiting lower capacity fading than that of bare LCO. The enhanced rate performance of is a direct testament to the efficacy of the ultra-thin LiAlO2 coating layer implemented through the collective surface architecture, especially at a high charge cut-off voltage of 4.7 V. The ultra-thin LiAlO2 coating layer not only significantly reduced the thickness of the cathode electrolyte interphase (CEI) film, thereby reducing the resistance to electron migration across the ALCO surface, by also enhanced the Li+ migration kinetics due to its properties as a Li+ conductor. The synergistic effect of these two factors significantly enhanced the conductivity of ALCO, ultimately boosting its rate performance.
Furthermore, the polarization and Li+ diffusion coefficients of bare LCO and ALCO were analyzed between 3.0 and 4.7 V by Galvanostatic Intermittent Titration Technique (GITT) as shown in Figure S6. The Li+ diffusion coefficients of bare LCO and ALCO were comparable during the first charging and discharging process, indicating that the ultra-thin collective surface architecture constructed based on ALD technology possessed inconspicuous effect on LCO. However, a significant difference in performance emerged after 100 cycles. The Li+ diffusion coefficient of bare LCO exhibited a substantial decrease, whereas that of ALCO showed minimal reduction under the same testing condition. Additionally, the results of the EIS test revealed that the Rct values of ALCO, both after 24 hours of resting and after 5 cycles at 0.1 C, were consistently lower than those of bare LCO (Figure S7). The results of GITT and EIS were indicated that the presence of an ultrathin LiAlO2 layer within the collective surface architecture functioned as an efficient Li+ conductor, thereby notably enhancing the Li+ transportation on the ALCO surface and simultaneously mitigating the excessive growth of the CEI layer throughout the cycling process. Meanwhile, the Li+ migration channels near the ALCO surface was regulated by Al gradient doping. Therefore, the strategic construction of the collective surface architecture effectively facilitated Li+ diffusion and optimized the redox kinetics of ALCO, ultimately leading to the enhancement of both its capacity and rate capability at 4.7 V.20
The long-cycle stability of LCO at high voltages is a serious challenge at present.21 Figure 2d shown the long-cycle performance of bare LCO and ALCO at a cut-off voltage of 4.7 V under current density of 1 C (1 C=270 mAh g−1). Compared with bare LCO, ALCO displayed an excellent cycling performance at 4.7 V cut-off voltage, with high capacity retention of 95.45 %, 90.75 %, and 85.79 % after 100, 200, and 300 cycles, respectively. In addition, the capacity of ALCO was 166.1 mAh g−1, while the capacity of bare LCO was only 4.7 mAh g−1 after 300 cycles under the same test condition. Figure 2e exhibited the charge/discharge curves for bare LCO and ALCO with different cycles at 4.7 V under 1 C, which displayed that the polarization of ALCO was much lower than that of bare LCO during the charge/discharge process. Notably, it was worth noting that ALCO exhibited an excellent cycling performance at an ultra-high cut-off voltage of 4.75 V simultaneously. Figure S8 displayed the cycling performance of bare LCO and ALCO at 4.75 V, which displayed that the remarkable capacity retention of ALCO was 82.08 % with the excellent capacity of 157.6 mAh g−1 after 200 cycles at 1 C current density, while that of bare LCO was only 17.54 % with a low capacity of 30 mAh g−1. The incredible cycle performance mentioned above was mainly attributed to the collaborative optimization of the LiAlO2 coating layer and the gradient doping of Al in the collective surface architecture. The LiAlO2 coating ensured the formation of a stable CEI on ALCO during the electrochemical process, isolated the electrolyte from direct contact with ALCO, inhibiting the occurrence of side reactions, thus improved the surface stability of LCO at 4.7 V. Meanwhile, the gradient doping of Al effectively restricted the irreversible lattice degradation during the O3−H1−3−O1 phase transition process, thereby improving the lattice stability of ALCO bulk during cycling at 4.7 V. Significantly, compared with the previous and representative works reported on enhancing the cycling performance of LCO not less than 4.7 V by material modification, ALCO in this work exhibited an unprecedented comprehensive electrochemical properties especially cycling stability, and even at 4.75 V, the capacity retention of ALCO still had an advantages, as shown in Figure 2f and Table S3.13, 18, 22
The practical application potential of ALCO was evaluated in commercial pouch cells, configured with capacities of 150 mAh and 1 Ah, to test its performance at cut-off voltages of 4.5 V and 4.45 V, respectively. These cells utilized a bare LCO or ALCO cathode, paired with a standard graphite anode and commercial liquid electrolyte, as depicted in Figure 2g and Figure S9. The cycling performance outcomes underscore the superior cycling stability of ALCO, specifically, the 150 mAh pouch cells with ALCO showed an excellent capacity retention of 82.33 % with remaining capacity of 120.2 mAh after 1000 cycles. This performance starkly contrasts with that of bare LCO, which exhibited a capacity retention of merely 46.64 % with 72.8 mAh remaining under identical conditions. Furthermore, commercial 1 Ah pouch full cell tested at a slightly reduced cut-off voltage of 4.45 V (Figure S9), ALCO continued to demonstrate its robustness, preserving 91.55 % of its capacity after 700 cycles.
The collective surface architecture of ALCO, characterized by its ultra-thin and dense LiAlO2 layer, offers multiple crucial functions for this enhanced performance. Firstly, this layer provides a stable CEI film that effectively isolating the direct contact between the electrolyte and ALCO to suppress side reactions and stabilize the ALCO during electrochemical cycling. Secondly, the LiAlO2 layer, serving as a Li+ conductor, effectively improves the diffusion kinetics of Li+ on the surface of ALCO. The ultra-thinness of LiAlO2 layer further reduces the thickness of the CEI film, facilitating faster electron migration and thereby augmenting the electronic conductivity of LCO. Furthermore, the gradient doping of Al within the collective surface architecture effectively inhibited irreversible phase transitions during the O3−H1−3−O1 phase change process, which further improved the stability of LCO during cycling. Therefore, the synergistic interaction between the ultra-thin and dense LiAlO2 layer and the gradient doping of Al within the collective surface architecture comprehensively enhanced the electrochemical performance of ALCO, especially under high voltage conditions.
To further explore the impact of collective surface architecture construction on the structural stability of LCO at 4.7 V, in situ XRD characterization was employed to investigate the structural evolution during the first charge/discharge between 3.0 V and 4.7 V at 0.1 C, as shown in Figure 3a,b. In general, the position changes of the (003) peak indicated the variation of c value, while (101) peak reflects the variation of a and b values in LCO lattice structure.15 In addition, the c value changed significantly when Li+ were extracted from LCO while a value changed slightly.23 During the initial charging process, with the gradual delithiation at early stages, the (003) peak of bare LCO and ALCO shifted to the lower angles due to the consequence of the repulsion between the neighboring oxygen layers, when charging to high voltage, the c-axis contracted because of the insufficient support force from the remaining Li atoms, the (003) peak rapidly shift back to the higher angle.24 Figures 3a,b depicted the evolution of the (003) diffraction peak and the corresponding charge/discharge curves of bare LCO and ALCO within the voltage range of 3.0–4.7 V, revealing that the (003) peak variation for ALCO was 1.447°, which was less pronounced compared to that of bare LCO exhibited a variation of 1.748°. In addition, the variation of the lattice c-value parameter during charge/discharge after the conversion via Bragg's law were shown in Figure S10. The ▵c of bare LCO during charge/discharge process was 1.24 Å, which displayed more drastic than that of ALCO (1.04 Å). Furthermore, because of the degree of change in the c-axis during charging and discharging was related to the residual stress experienced by electrode materials with layered structure during charge–discharge process, the residual stress experienced by ALCO after cycling is lower than that of LCO. Therefore, the layered structure of ALCO exhibited better reversibility and lower residual stress during deep de-lithiation process compared to bare LCO, which primarily due to the enhanced stability of lattice structure resulting from the gradient doping of Al within the collective surface architecture.13 Significantly, the deconvoluted (003) reflections of Bare LCO and ALCO charge to 4.7 V were shown in Figure 3c. The occurrence of two conspicuous peaks was apparent, wherein one peak represented H1–3/O1 mixed phase while the other exclusively denoted the O1 phase, indicating that the H1–3/O1 mixed phase and O1 phases coexisted when the two samples were charged to 4.7 V.6 It should be noticed that both peaks of ALCO were observed to shift towards a lower angle in comparison to bare LCO, which was ascribed to the constraint imposed on lattice alteration by collective surface architecture. From the position of the peaks representing the of H1–3/O1 mixed phase, the peak of ALCO shifted towards lower angle compared to that of bare LCO, indicating that the proportion of H1–3 phase in the H1–3/O1 mixed phase in ALCO charged to 4.7 V was greater than in bare LCO charged to 4.7 V. In addition, based on the calculated results of the deconvoluted peak regions, the proportion of the O1 phase in ALCO charged to 4.7 V was 35.48%, which was significantly smaller than that in bare LCO (43.85%) charged to the same voltage (Figure S11). The aforementioned findings suggested that when charged to 4.7 V, the content of O1 phase in ALCO was lower than that in bare LCO, indicating that the irreversible phase transition from H1–3 to O1 was significantly mitigated in ALCO. It was mainly attributed to the change of the electron cloud density between Co−O−Li in LCO due to Al doping, which led to the change of the Co−O bond length. The results of XRD refinement indicated that the average bond length of the Co−O bond was shortened by the intervention of Al atoms (Figure S12), which enriched the bond energy of Co−O bond, thus mitigate the irreversible phase transition during the cycling process.
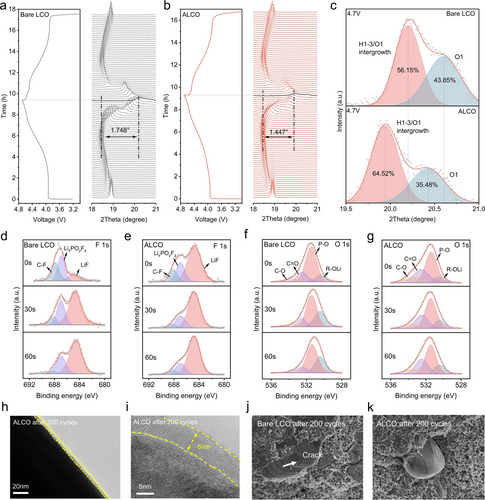
In situ XRD patterns and corresponding charge–discharge curves of the a) bare LCO and b) ALCO during the first cycle at 0.1 C. c) XRD spectra of bare LCO and ALCO at 4.7 V. d)–g) XPS spectra of F 1s and O 1s for bare LCO and ALCO after 200 cycles. h)–i) Transmission electron microscopy (TEM) images of ALCO cathodes after 200 cycles. The scanning electron microscopy (SEM) images of j) bare LCO and k) ALCO electrode after 200 cycles at 4.7 V.
The post-cycling morphology and chemical composition analysis of the CEI formed on both bare LCO and ALCO surfaces after 200 cycles at 4.7 V were revealed by XPS etching and TEM. Figure 3d–g displayed the F 1s and O 1s spectra of bare LCO and ALCO after 200 cycles. Analysis of the F 1s spectra reveals that the content of LixPFyOz on bare LCO is significantly higher than that on the ALCO as shown in Figure 3d,e and Figure S13a. The LixPFyOz byproducts are mainly attributed to the decomposition of LiPF6, which is detrimental to Li+ transport.25 Meanwhile, the content of inert components LiF in ALCO is higher than that in bare LCO. The inert LiF could contribute to prevent the reaction between the material and the electrolyte during the electrochemical process at 4.7 V, thereby improving the stability of ALCO surface during long term cycling26. In addition, the O 1s spectra reveal that bare LCO possessed a higher content of P−O bonds than ALCO from Figure 3f,g and Figure S13b. The P−O bond was also attributed to the decomposition product of LixPFyOz from LiPF6, indicating that the collective surface structure on ALCO surface suppresses electrolyte decomposition at 4.7 V. In addition, the morphology of CEI was observed by TEM (Figure S14 and Figure 3h, i). The surface of bare LCO not only becomes notably rough after cycling and shows an uneven CEI layer, mainly due to the adverse side reactions, including decomposition of the electrolyte and the reactions between electrolyte and bare LCO. These reactions compromise the conductivity and stability of bare LCO, leading to deminished capacity and cycle life. In contrast, a layer of approximately 6 nm thick and uniform CEI film was delicately formed on the ALCO surface by the reaction between ultra-thin and uniform LiAlO2 coating layer and electrolyte at 4.7 V as shown in Figure 3i. This layer not only effectively prevents direct contact between ALCO and the electrolyte, thereby inhibited undesirable side reactions, but also minimized the impact of CEI on the Li+ and electrons transportation across the surface of ALCO. Morphological analysis after 200 cycles (Figure 3j, k) exhibited the protective effects of the collective surface architecture. The bare LCO particles significant cracks due to uneven stress distribution caused by the irreversible phase transition from O3−H1−3−O1 and side reactions between bare LCO and electrolyte during electrochemical process. In contrast, the particles of the ALCO electrodes remained intact and smooth, demonstrating that the stress distribution was uniform during cycling on the surface of ALCO by effective suppression of irreversible phase transition and side reactions through the synergistic effects of gradient doping of Al and LiAlO2 coating layer. These insights collectively affirm the efficacy of the collective surface architecture in bolstering the structural and chemical stability of LCO at elevated voltages at 4.7 V.
To further illustrate the effect of concentration gradient surface architecture construction on the electrochemical of LCO at 4.7 V, we employed first-principles calculations based on density functional theory (DFT). Due to the close correlation between the electrochemical properties of LCO and the energy band gap, we compared the projected density of states (PDOS) of bare LCO, Al−LCO (LCO doped with Al), LiAlO2−LCO (LCO with LiAlO2 as the coating layer) and ALCO. Initial modeling of these structures (Figure 4a and Figure S15a) set the stage for a comprehensive analysis. Our calculations revealed a band gap of ALCO (0.611 eV) notably lower than that of Al−LCO (0.678 eV), LiAlO2−LCO (0.642 eV) and bare LCO (0.716 eV). The enhanced electrical conductivity observed in the Al−LCO can be primarily attributed to the effect of Al doping on the electronic structure of the material. Specifically, Al doping weakens the p-d hybridization between Co and O atoms, leading to more delocalized electrons and thereby improving the conductivity of Al−LCO compared to the undoped LCO. Additionally, the band gaps of bare LCO and LiAlO2 are 0.716 eV and 4.57 eV, respectively (Figure 4c and Figure S16). The significant difference in band gaps facilitates the formation of a built-in electric field at the heterointerface in the LiAlO2−LCO, thus enhancing the electrical conductivity of LiAlO2−LCO. This observation is supported by XPS results, which show an enhancement in surface oxygen deficiencies (Odef) in the modified material (Figure S4). Therefore, ALCO with both Al doping and LiAlO2 coating on the collective surface structure possessed higher electrical conductivity. In addition, the charging state models of bare L0.25CO, Al−L0.25CO, LiAlO2−L0.25CO and AL0.25CO were constructed (Figure 4b and Figure S15b). Analysis of these models (Figures 5e, f, and Figure S15d,f) demonstrated that delithiation, characterized by the formation of lithium vacancies, leads to a reduction in the band gap.22a Notably, the calculated band gap of AL0.25CO (0.364 eV) was found to be lower than that of Al−L0.25CO (0.432 eV), LiAlO2−L0.25CO (0.406 eV) and bare L0.25CO (0.471 eV), highlighting the potential of ALCO to reduce the polarization and to improve the reversibility at 4.7 V.27 These DFT calculations verify that the electrical conductivity of ALCO during delithiation process is effectively elevated by the application of a collective surface architecture construction. This enhancement not only optimizes the cycling stability but also the rate capability of LCO when operated at elevated voltages.
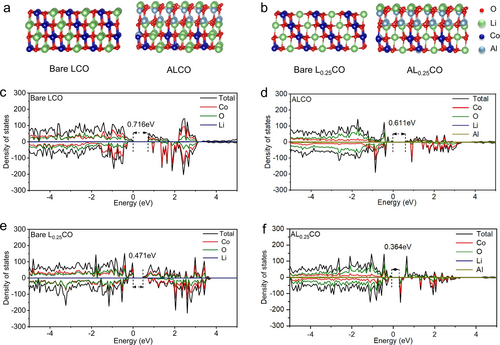
Optimized atomic structure in surface models of a) bare LCO, ALCO, b) bare Li0.25CO and AL0.25CO. PDOS values of selected elements and total elements of c) bare LCO, d) ALCO, e) bare Li0.25CO, and f) AL0.25CO.
Schematic illustration of the degradation mechanism of bare LCO and ALCO.
Conclusion
In this study, we developed a collective surface architecture for LCO by employing PNCD bases on powder ALD. This architecture integrates an ultra-thin LiAlO2 surface coating layer with gradient Al doping, achieving a multifaceted enhancement of LCO′s electrochemical performance at high voltages at 4.7 V. The ultra-thin and dense LiAlO2 surface coating layer not only establishes rapid Li+ migration pathways to facilitate the Li+ transmission on the surface of LCO, but also forms thin and stable CEI that curtails interfacial side reactions at elevated voltage. Concurrently, Al gradient doping effectively suppresses the unfavorable phase transition from H1–3 phase to O1 phase to improve structural stability. DFT calculations further corroborate that the electrical conductivity of LCO is significantly improved through this synergistic surface modification, leading to notable electrochemical performance enhancements. As a result, the optimized LCO (denoted as ALCO) exhibited a remarkable reversible capacity of 230 mAh g−1 at 4.7 V, which represented a substantial 28 % enhancement compared to capacity achieved 4.5 V. Moreover, ALCO showed unprecedented capacity retention, maintaining 85.79 % after 300 cycles of 1 C within a voltage range of 3.0–4.7 V and 82.08 % after 200 cycles of 1 C at a high charge cut-off voltage of 4.75 V. Impressively, in practical pouch cell applications, ALCO achieved a capacity retention of 82.33 % after 1000 cycles at 4.5 V. This work elucidates a viable surface optimization strategy for enhancing the longevity and performance of LCO under high-voltage conditions. The success of this collective surface architecture paves the way for its application to other cathode materials, potentially revolutionizing the development of high-voltage, high-energy-density lithium-ion batteries.
Supporting Information
The authors have cited additional references within the Supporting Information.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (52302258), Wuhan Knowledge Innovation Special project (Grant No. 2022010801010369), the Key Research and Development Program of Wuhan (2023010402010601), Natural Science Foundation of Hubei Province (2024AFB1003), University-Level Research Project Funding Program of JHUN (2022XKZX01) and Excellent Discipline Cultivation Project by JHUN (2023XKZ011). Dr. Jian Peng thanks the Banting Postdoctoral Fellowship supported by the Natural Sciences and Engineering Research Council of Canada (NSERC). Open Access publishing facilitated by University of Wollongong, as part of the Wiley - University of Wollongong agreement via the Council of Australian University Librarians.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.