Azaacene Diradicals Based on Non-Kekulé Meta-Quinodimethane with Large Two-Photon Cross-Sections in the Infrared Spectral Region
Graphical Abstract
Exchanging a sulfur atom against a methine group in a series of azaacene based diradicals furnishes non-Kekulé, meta-quinodimethane based diradicals with singlet ground states and small singlet-triplet gaps. Zwitterionic contributions are ruled out experimentally and quantum-chemically, and the diradicals display huge two-photon cross sections in the infrared spectral region.
Abstract
Non-Kekulé quinoidal azaacences m-A (1 a,b) were synthesized and compared to their para- and ortho-quinodimethane analogues. m-A display high diradical characters (1 b: y0 = 0.88) due to their meta-quinodimethane (m-QDM) topology. Electron paramagnetic, nuclear magnetic resonance spectroscopies and supraquantum interference device measurements in combination with quantum-chemical calculations revealed singlet ground states for m-A with singlet-triplet gaps ΔEST (0.13–0.25 kcal mol−1) and thermally populated triplet states. These non-Kekulé structures are over all void of zwitterionic character and possess record high two-photon absorption cross sections over a broad spectral range in the near-infrared.
Introduction
Delocalized diradicals with, in contrast to biradicals, non-negligible electron exchange integrals between the radical centers1 possess fascinating optoelectronic and magnetic properties. In some but not all diradical ensembles, the triplet manifold can be thermally populated as a consequence of a small singlet-triplet gap. Diradicals, inherently reactive, need steric or electronic stabilization at the positions of highest spin density and/or increased delocalization through extension of the conjugated π-system.2 Species with high diradical character y0 (y0=0: closed-shell molecule; y0=<1, diradical; y0=1, (triplet) biradical)3 form from closed-shell quinoidal/zwitterionic species through aromaticity gain (pro-aromaticity) if the number of Clar sextets is higher in their diradical than in their closed shell forms.4 Conjugated diradicals are of the Kekulé- (one or more closed shell resonance forms, e. g. ortho- and para-quinodimethane (QDM), Figure 1a) or non-Kekulé-type, void of any closed shell resonance structure.1 Examples of Kekulé diradicals include Tchichibabin's hydrocarbon,5 Wu's zethrenes,6 Kubo's bisphenalenyls,7 and Haley's indenofluorenes,8 whereas some triangulenes9 (including Clar's hydrocarbon),10 1,14 : 11,12-dibenzopentacene11 and extended tribenzo[f,k,m]tetraphenes12 represent non-Kekulé diradicals.13 Substitution and/or regioisomerism can tailor diradical character and singlet-triplet gaps (ΔEST). Haley et al. synthesized syn- and anti-IIDBT in which the position of the sulfur atom has a marginal but measurable effect on y0 (y0=0.61–0.66 and ΔEST=8.77–8.06 kcal mol−1), as it leaves the conjugation pathway unchanged.16
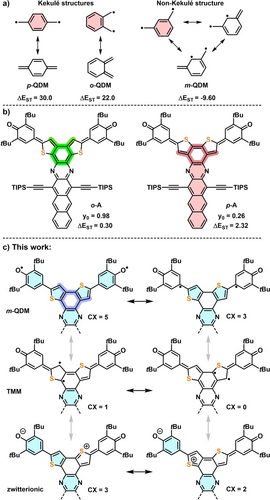
a) Quinodimethane isomers with resonance structures. b) Enhancing the diradical character of acaazenes through isomerism. c) This work: Selected resonance structures of non-Kekulé diradicals m-A with number of Clar sextets (CX, shaded light blue) and highlighted m-QDM subunit (dark blue). Singlet-triplet gaps ΔEST (ΔEST=ET-ES) [kcal mol−1]: p-QDM, o-QDM (calculated) from ref. [14], m-QDM (experimental) from ref. [14]; o- and p-A (experimental) from ref. [15].
We recently reported o-A and p-A (Figure 1b). p-A, a p-QDM, is closed-shell with y0=0.26; o-A is an open-shell, aromatic diradical (y0=0.98). The o-QDM fully integrates the π-electron system, but in the p-QDM p-A the quinone and azaacene are cross-conjugated.15 The difference in diradical character of p-A and o-A is qualitatively estimated by simply tallying the Clar sextets (CX) of the closed shell (cs) and diradical (open shell, os) resonance forms of both compounds: CX(p-Acs)=1, CX(p-Aos)=5 and for CX(o-Acs)=0, CX(o-Aos)=5 (see SI, Scheme S2). This simple analysis ascribes the increased diradical character of o-A to the lack of aromatic stabilization in its closed shell form (note that we only compare the two Kékule systems with respect to extent of diradical character). Besides o-A and p-A the third regioisomer, m-A, can be formulated as an os m-QDM. Alternatively, m-A could possess a trimethylenemethane (TMM), or a cs zwitterionic structure, found in oligo(N-annulated perylene)quinodimethanes,17 S-containing 2,6-naphthoquinodimethane18 or asymmetric diradicals with Blatter and phenoxyl moieties.19 Counting the CX of m-A, the m-QDM form scores with 5 CX, the zwitterionic with 3 CX and the TMM form with 1 CX, suggesting that m-A will be a m-QDM with a high y0, rather than a zwitterion.
Results and Discussion
Synthesis of 1 a,b starts with the condensation of 2 a,b with diketone 3 into 4 a,b (Scheme 1). Suzuki coupling of 4 a,b with boronic acid 5 leads to 6 a,b in 95–99 %. Oxidation of 6 a,b with K3Fe(CN)6 furnishes diradicals 1 a,b (m-A) as deep brown and green solids.
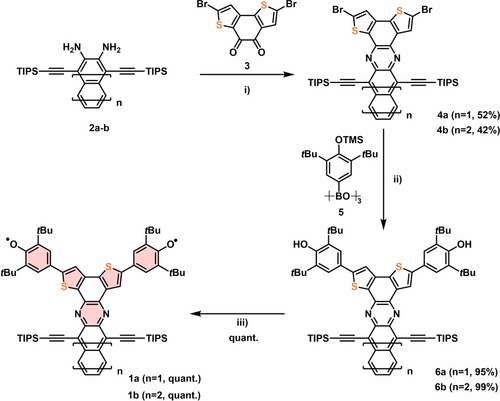
Synthesis of 1 a,b i) AcOH, 120 °C, reflux; ii) Pd(PPh3)4, K2CO3, THF/H2O (10 : 1, v/v) 95 °C; iii) [K3Fe(CN)6], KOH, THF/H2O (1 : 1, v : v), rt.
The absorption spectra of 1 b/6 b are red-shifted with respect to those of 1 a/6 a, testament to the larger acene subunit, also found in the spectra of their direct precursors. Under ambient conditions (dilute toluene solution) the half-life (τ1/2) of 1 a is 6 h and 9.6 h for 1 b (see Figures S2–3), less than that of isomeric o-A (t1/2=19 h) or p-A (t1/2=104 h).15 In the solid-state (Ar, −40 °C) 1 a,b are stable for at least one week. The absorption spectra of 1 b, p-A and o-A (Figure 2) are fairly similar to each other with that of 1 b resembling that of o-A more than that of p-A (note that we refrain our comparative discussion to these regioisomers to rule out any influence of the acene length). The UV/Vis spectra of 1 b in solvents of different polarity (Taft scale20) display only small spectral shifts—the lack of solvatochromism suggests a negligible contribution of zwitterionic resonance forms (see Figure S4).
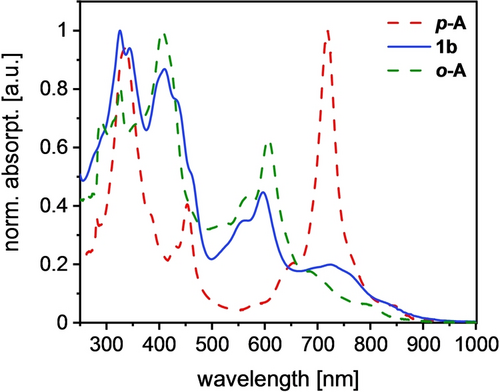
UV/Vis absorption spectra of the regioisomers o-A, 1 b and p-A in chloroform.
Single crystals were grown by slow diffusion of acetonitrile into a dichloromethane solution under nitrogen atmosphere (Figure 3). In 1 a,b the packing is dominated by the π–π interaction of the azaacenes, without appreciable intermolecular radical-radical interactions. 1 b forms dimers in the solid-state with a stacking distance of 3.32 Å. These pairs are repeatedly shifted relative to each other and create a wave-like pattern (Figure 3b). In 1 a the thiophene moieties are disordered. A ‘quasi C2-symmetry’ results, precluding analysis of the bond lengths of the bithienyl units (see Figure S7). 1 a is arranged in one-dimensional stacks with two alternating molecules rotated ~76° relative to each other (Figure 3d).
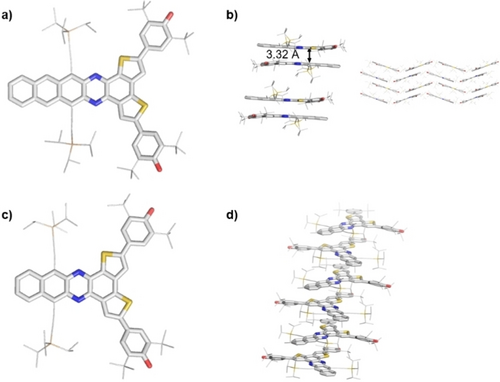
a) X-ray crystal structures of 1 b and b) its solid-state packing (dimer formation). The stacking distance was calculated via average levels of all atoms of the azaacene backbone in Å. c) Crystal structure of 1 a (disorder removed) and d) its solid-state packing. H atoms omitted and silylalkynyl and tert-butyl substituents reduced in size for clarity.
The (measured) bond length alternation (BLA) of 1 b is compared to o-A and p-A with their corresponding bithienophenoxyl cutouts (Figure 4). For p-A a single/double BLA was observed testament to the quinone-moieties. In o-A the BLA is less pronounced, supporting a diradical form.15 For 1 b the BLA-form is asymmetric due to the position of the sulfur atoms. On the side with the sulfur facing the azaacene the bonds a-h are on average elongated. The other side displays a pronounced bond length alternation (j–q, Table 1) The CO bond length of o-A (1.28 Å) indicates a C−O single bond, whereas the CO bond length of p-A (1.22 Å) suggests double bond character.15 The CO bond length of 1 b amounts to 1.25 Å, exactly in between those of its regioisomers, indicating a minor contribution of zwitterionic resonance structures, if packing effects could be ruled out. Comparing the CC bonds e/m, g/k and i (see Table 1) in 1 b and o-A, these bonds are longer CC bonds and belong to the ‘maxima’ in Figure 4. Overall, 1 b is structurally similar to o-A, supporting an open-shell form.
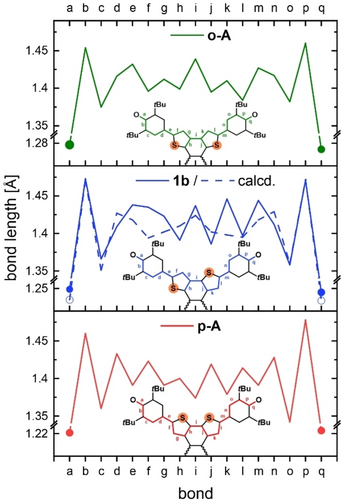
bond |
o-A |
1 b |
p-A |
|---|---|---|---|
a/q (C−O) |
1.28 |
1.25 |
1.22 |
e/m |
1.43 |
1.44 |
1.39 |
g/k |
1.41 |
1.42/1.45 |
1.39 |
i |
1.44 |
1.44 |
1.37 |
We have compared the IR spectra of 1 b, o-A and p-A (Figure 5, for 1 a see Figure S5). The intense C=O band at 1572 cm−1 for p-A suggests a quinone. For 1 b this vibration band is less pronounced and for o-A it is almost absent, in line with the BLA.
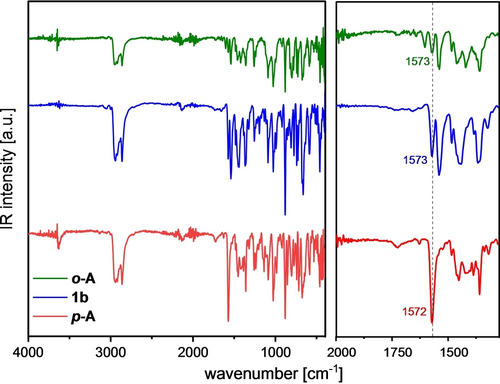
Full IR spectra of all three regioisomers (left). Zoomed in region from 2000–1300 cm−1 with highlighted CO bond vibration (right).
1 a and 1 b (see Figure S6) exhibit half-wave reduction potentials (cyclic voltammetry) at −0.57 V and −0.55 V (vs. Fc+/Fc) respectively, assigned to the radical anion and dianion of the bithienophenoxyl segment. Both compounds show two additional reversible half waves attributed to the reduction of the azaacene backbone at −1.22 V/−1.70 V for 1 b and −1.41 V/−1.89 V for 1 a.
Even at −90 °C, the 1H nuclear magnetic resonance (NMR) spectra of 1 a/1 b only display broad resonances at ~1.25 ppm (see Figure S8) from their tert-butyl and TIPS-groups, testament to the delocalization of the electron spins over the conjugated system. The electron paramagnetic resonance (EPR) spectrum of 1 b (g-value=2.0045, see Figure 6; S9 for 1 a) is structured with hyperfine coupling constants of aH=5.8 G, 4.7 G, and 3.4 G. Variable temperature (VT) solid-state EPR of 1 a and 1 b revealed after fitting the VT-EPR (T∫∫IEPR-T) data with the Bleaney–Bowers equation singlet-triplet gaps ΔEST of 0.13 kcal mol−1 for 1 b and 0.25 kcal mol−1 for 1 a. Both species exist in an open shell singlet ground state with a thermally accessible triplet state. Superconducting quantum interference device (SQUID) measurements (Figures S10–11) support this interpretation. The χT-T plot for 1 a,b show an increasing signal from 4 K until 300 K as expected for a singlet ground state. The χT-values of 1.35 cm3 K mol−1 for 1 b (0.85 cm3 K mol−1 for 1 a) are in accordance with the spin-only equation21 confirming the presence of two unpaired electrons.
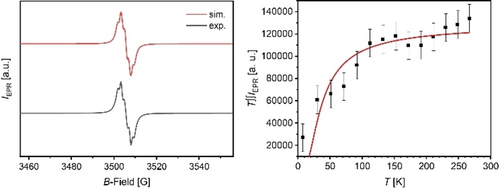
Experimental and simulated EPR spectra of 1 b in dry benzene (under argon atmosphere) at room temperature (left) and VT-EPR plot (double integral intensity*temperature; T∫∫IEPR vs T) of solid-state sample with Bleaney–Bowers fit (right).
y0 of 1 a,b was calculated by population analysis of natural orbitals (NOs) at the DFT/B3LYP/6-311G(d,p) level of theory (see Table 2 and SI). Spin-flip time-dependent density functional theory (SF-TDDFT) calculations suggest open-shell singlet ground states for 1 a and 1 b with small singlet-triplet energy gaps of 0.25 kcal/mol for 1 a and 0.62 kcal/mol for 1 b. In comparison to the experimental values (see Table 2) the calculated values are a bit higher for all three regio-isomers but show the same trend: The singlet-triplet gap is largest for the para-isomer, followed by the ortho-isomer, and is smallest for the meta-isomer [ΔEST(p-A)>ΔEST(o-A)>ΔEST(1 b)].
Figure 7 displays the spin-density distribution of the triplet state, nucleus-independent chemical shift (NICS) (1)zz values,22 anisotropy of the induced current density (ACID) plot and electrostatic potential maps of 1 b. The spin density is distributed over the whole azaacene backbone with alternating α and ß coefficients (Figure 7) explaining the absence of NMR signals, due to the contribution of the m-QDM diradical resonance form. NICS(1)zz calculations (B3LYP/6-311+G(d,p)) for the triplet state of 1 b indicate negative, aromatic chemical shifts for the acene backbone and the thiophene rings as expected for the diradical form. The m-QDM unit connecting the thiophenes is almost non-aromatic, which is also the case for o-A and contrasts with the slight antiaromaticity for p-A. The phenoxy groups are non-aromatic—a finding that all three regioisomers (1 b, o-A and p-A) have in common and is in accordance with literature and attributed to the large residing spin density on the oxygen atoms and the neighboring rings (Figure 7).23 The ACID24 plot of 1 b supports the antiaromaticity of the m-QDM unit even further while the electrostatic potential maps of the triplet state of 1 b dispel the contribution of a zwitterionic resonance form.
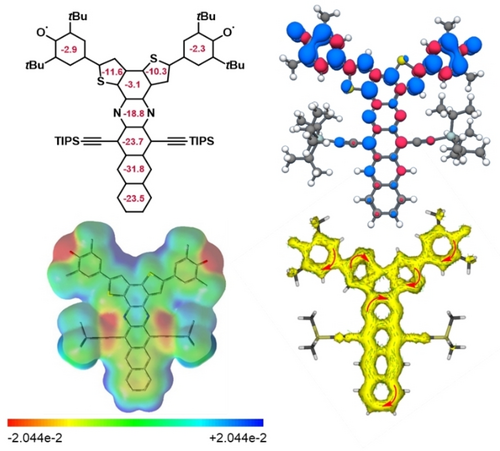
Calculation of NICS(1)zz values calculated at B3LYP/6-311+G(d,p) level (top left) and spin distribution for the triplet state (top right). Electrostatic potential map of the triplet state (−2.044 ⋅ 10−2 bis +2.044 ⋅ 10−2 (red to blue, bottom left) and π-only ACID plot (bottom right; optimization limit: 0.02, isovalue: 0.02). For the calculations Me groups were used instead of tert-butyl and iso-propyl groups.
Organic diradicals promise unique physical properties, such as enhanced third-order nonlinear optical (NLO) responses and unusually large two-photon absorption (2PA) cross sections.25 The 2PA cross-section (σ2) of 1 a and 1 b (Z-scan technique, from 800 to 1700 nm, see SI, Figure 8) is a function of the excitation wavelength. Both 1 a and 1 b exhibit substantial σ2 over a broad portion of the examined spectral range. The maximum value for 1 a is σ2=7140±200 GM at 940 nm and σ2=8870±150 GM at 1370 nm for 1 b. These results place 1 a,b among the highest reported 2PA cross sections for small organic chromophores (not limited to diradicals,26 exceeding those of organic dyes designed for high nonlinear absorption ( mostly below 1000 GM27 with some notable exceptions such as (twisted) metalated porphyrinic oligomers28) while we did not at all try to create NLO materials (for a more thorough discussion, see SI, Section 12).29a, 27e, 29b We attribute the stellar NLO-performance to the diradicals’ planarity and extensive π-conjugation.29a, 27e
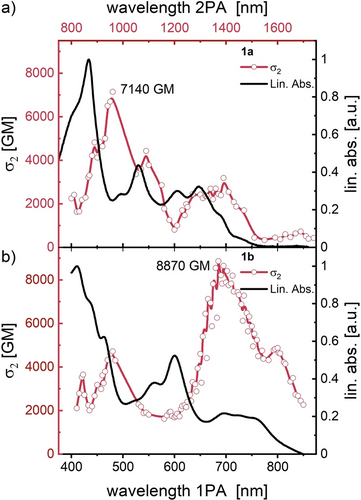
Normalized linear (one photon) absorption (1PA) spectra (black) and the corresponding two-photon absorption (2PA) cross sections (red, α2) of a) 1 a and b) of 1 b measured in benzene solution. The red lines act as guide-to-the-eye.
Conclusions
In conclusion, we prepared and investigated (meta−)bithieno- phenoxylazaacenes 1 a,b with m-QDM subunits und compared 1 b to its ortho- (o-A) and para-isomer (p-A). Meta-1 a,b show surprisingly small singlet-triplet gaps (ΔEST=0.13–0.25 kcal mol−1) according to VT-EPR measurements. The conjugation pathway over the m-QDM unit furnishes non-Kekulé structures with electronically semi-integrated systems; 1 b resembles o-A in its spectroscopic data/properties more than p-A, with its y0 of 0.88 and a vanishing S−T-gap. Surprisingly though o-A displays an even higher diradical character. Both 1 a and 1 b are exceptional 2PA materials that highlights the potential of quinoidal azaacence diradicals as a key structural element for high-performance NLO materials in the near-infrared region.
Supporting Information
The authors have cited additional references within the Supporting Information.30
Acknowledgments
We acknowledge the German Research Foundation (DFG) through the SFB 1249 (Project Number 281029004-SFB 1249, projects A1, B1, and B6) for the financial support. K.F. thanks Prof. Dr. Dr. H.-J. Himmel for access to and Dr. R. Maskey and M. Schulz for assistance with SQUID measurements and Dr. S. Maier for assistance with ACID plots. Open Access funding enabled and organized by Projekt DEAL.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
Data related to NMR, ESR, IR, MS, SQUID, UV/Vis and CV are available from heiDATA, an institutional repository for open research data from Heidelberg University, at DOI 10.11588/data/R3IMSQ. Deposition numbers CCDC 2344147 (3), 2344148 (4 a), 2344149 (6 a), 2344150 (6 b), 2344151 (1 a) and 2344152 (1 b) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.