A Light-Powered Single-Stranded DNA Molecular Motor with Colour-Selective Single-Step Control
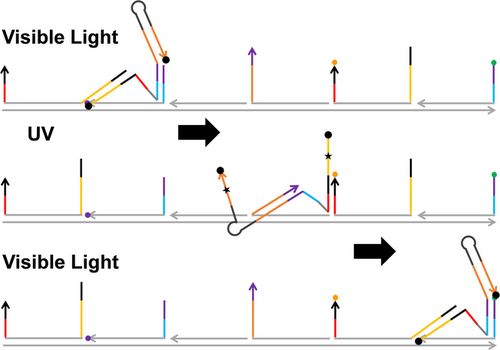
Graphical Abstract
Molecular motors access small length scale and have potential for precision technology. But the top-down control of these motors is a challenge as they are molecular objects subject to stochastic fluctuations. Now a rationally designed single-stranded DNA molecular motor demonstrates a novel nano-optomechanical driving mechanism that pushes the top-down control of molecular motors down to every single step.
Abstract
Top-down control of small motion is possible through top-down controlled molecular motors in replacement of larger actuators like MEMS or NEMS (micro- or nano-electromechanical systems) in the current precision technology. Improving top-down control of molecular motors to every single step is desirable for this purpose, and also for synchronization of motor actions for amplified effects. Here we report a designed single-stranded DNA molecular motor powered by alternated ultraviolet and visible light for processive track-walking, with the two light colours each locking the motor in a full directional step to allow saturated driving but no overstepping. This novel nano-optomechanical driving mechanism pushes the top-down control of molecular motors down to every single step, thus providing a key technical capability to advance the molecular motor-based precision technology and also motor synchronization for amplified effects.
1 Introduction
Molecular motors1 have the potential to replace the basic actuators like MEMS or NEMS (micro- or nano-electromechanical systems, both far beyond molecular scale) in Today's precision technology for top-down control of small motion, with expected gains in finer motion control and in non-intrusive operation for potentially high-throughput processing of molecular objects. The top-down motion control through molecular motors first requires top-down control of these motors, ideally down to a motor's every single step to minimize the controlled motion and best synchronize many molecular motors for collective motion and amplified mechanical outputs. In these regards, molecular motors powered by top-down administered light irradiations are particularly suitable. Such optically powered molecular motors are achieved first by Feringa et al.2 in 1999 for a small-molecule rotational motor, and later by Wang et al.3 in 2012 for a translational molecular motor made of engineered DNA (deoxyribonucleic acid). These early studies are followed by more optically powered molecular motors of different designs.3-5 However, it is still a big challenge to push the top-down optical control of molecular motors to individual steps. Ideally, an optical operation should saturate the driving for one step but no more than one step. This single-step control is missing in the present molecular motors as their optomechanical driving mechanisms cannot avoid overstepping upon saturated driving. Specifically, these reported optical motors are driven either by a single-colour light irradiation2, 4 or by a combination of two-colour irradiations3, 5 to accomplish a full directional step—both in a probabilistic way due to the stochastic nature of photon absorption and optomechanical conversion in molecular systems. Namely, applying the designed optical operation once, be it the single-colour irradiation or the combined two-colour irradiations, results in a full step or zero step by chance. Repeating the single-colour or two-colour operation saturates the driving for a step, but inevitably results in more steps by an uncontrollable number (hence no single-step control).
Here we report a rationally designed translational DNA molecular motor with a new two-colour driving mechanism that avoids the overstepping problem and enables top-down single-step control. This motor, driven by alternated ultraviolet (UV) light and visible light, makes a full step under either colour of light but avoids more than one step until the colour change. As a consequence, the motor is locked by either colour for just one step and allows the step's saturated driving by the same colour of light. This simultaneous optical locking and driving is achieved for the motor's every single step in a top-down executed, colour-selective way. This study thus pushes optical control for molecular motors down to every single step, and provides a key technical capability for the envisioned precision technology based on molecular motors, and perhaps also for top-down synchronization of molecular motors for amplified actions. Besides, the bicolour driving for two steps achieved in this study represents a more efficient optomechanical conversion mechanism for molecular motors than the bicolour-per-step driving of previous motors. Furthermore, the light-driven motor from this study is a new track-walking motor with a minimal single-molecule construction (i.e., made of a single-stranded DNA (ssDNA)), which is advantageous for future integration with DNA origami platforms1f, 6 for optomechanical nano-actuators and nano-robots.
2 Results and Discussion
2.1 Motor and Track Design and Fabrication
The ssDNA motor is a hairpin with a 15bp stem and a 34nt overhang as schematically illustrated in Figure 1a (‘bp’ for base pair, ‘nt’ for nucleotide). The motor has two 20nt leg-like segments, i.e., a-b-c segments for leg A and k-j-i for leg B, which have different nucleotide sequences for exclusive hybridization between leg A and the bi-overhang binding site A (i.e., neighbouring F1A, F2A with 21bp gap, see Figure 1b) and between leg B and the bi-overhang site B (neighbouring F1B, F2B with 21bp gap). The two types of binding sites are alternately arranged on a double-stranded backbone at a constant inter-site spacing (21bp) to form a linear periodic track (with its two polar ends marked as plus or minus end in Figure 1b). For sake of optical driving, the ssDNA motor has azobenzenes7 tethered to its nucleotide backbone, which adopt trans configuration under visible light or cis configuration under UV light to stabilize or disrupt DNA duplexes, respectively. Specifically, the motor's leg A carries 5 azobenzenes (in the 10nt c segment); leg B has none but the stem protecting this leg carries 5 azobenzenes (in i*’ segment complementary to the leg's 10nt i segment, see Figure 1a). Under the visible light, the azobenzenes are in trans configuration so that leg B is half protected by the hairpin but leg A is not. Hence leg A has full binding to site A, i.e., both its F1A and F2A overhangs, for a downhill cartwheeling migration8 from the 10nt F1A to the 15nt F2A (state 7 to state 8, Figure 1c). But leg B has partial binding to site B, i.e., its F1B only, with the cartwheeling to F2B blocked by the closed hairpin (state 1, Figure 1c). Under UV light, the motor's azobenzenes are in cis configuration so that leg B is fully exposed by the UV-induced hairpin opening but leg A partially loses hybridization ability. Leg B now has full binding to site B for a downhill cartwheeling from the 10nt F1B to the 15nt F2B (state 2 to state 5), but leg A has partial binding to site A (i.e., only F1A) with the cartwheeling to F2A blocked (state 6). Thus, the two legs have differential high or low binding affinity for the track under either UV or visible light, with the affinity order being opposite for the two light colours. This colour-dependent converse affinity control for the two legs is critical for the motor's step control as well as for its plus-end-directed motion on the track under alternating UV/Visible light irradiations.
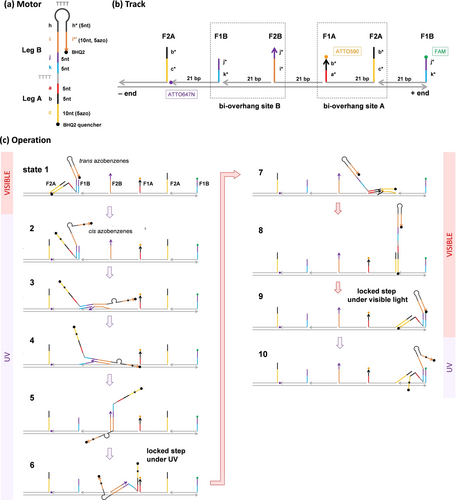
The motor-track system. (a), (b) Design of the motor and track (‘nt’ stands for nucleotides and ‘bp’ for base pairs; * marks complementary sequences, ‘ marks mutations, e.g., a point mutation in i*’ segment to help the hairpin opening under UV. (c) Schematic of the motor's operation under alternating UV and visible light. The asterisk indicates cis configuration of the motor-carried azobenzenes (in c and i*’ segments) under UV light.
Due to the converse leg affinity and size restriction, the motor under visible light forms an asymmetric but stable inter-site state, i.e., state 1 in Figure 1c, in which the motor's rear leg (leg A) hybridizes with F2A and the front leg (leg B) hybridizes with the nearest F1B. Under UV light, the motor forms another stable asymmetric inter-site state, i.e., state 6, in which the rear leg (leg B now) hybridizes with F2B and the front leg (leg A) hybridizes with the nearest F1A. The motor operation is normally started under visible light from state 1. Applying UV light switches leg A to low binding affinity for its dissociation from the F2A (i.e., selective rear leg dissociation) but fully exposes Leg B (via hairpin opening) for spontaneous downhill cartwheeling from the F1B to F2B (states 2–5). The cartwheeling, which is a directional intra-site displacement of the track-bound leg to the track's plus end, places the dissociated leg A near the F1A overhang in front for their hybridization (state 6). Both leg B's cartwheeling and the ensuing leg A-F1A binding can be completed under the UV irradiation to bring the motor to state 6. Thus, the UV irradiation alone can drive the motor from state 1 to state 6, resulting in a hand-over-hand step to displace the motor's centre of mass 42 bps (~14 nm) towards the track's plus end. This is a full step for the motor with the ending state (i.e., state 6) remaining stable under the UV irradiation. The motor is thus locked for a single step and allows its saturated driving by elongating or strengthening the UV light, until the UV is replaced by a visible light irradiation. The visible light resumes leg B's low binding affinity for its dissociation off F2B (again, selective rear leg dissociation, via toehold-mediated strand displacement upon hairpin closing), and also resumes leg A's high binding affinity for its downhill cartwheeling (from F1A to F2A) to place the dissociated leg B near the front F1B for hybridization (states 7–9). Similar to the UV irradiation, the visible light alone can drive another 14 nm full step of the motor to the track's plus end, with the ending state (state 9) identical with the initial state (state 1) that remains stable under the visible light. The motor is again locked for a single step and allows its saturated driving now by the visible light. Under a series of alternating UV and visible light, the motor will move continuously on the periodic track by the two types of full steps towards the same direction. This unidirectionality allows sustainable and scalable linear displacement if the periodic track is elongated, unlike the back-and-forth oscillating displacement within a limited range by some previous light-driven DNA walkers.4, 5f This is a processive motor as the converse affinity control for its two legs suppresses the chance of simultaneous weak binding for both legs, i.e., the channel for the motor's derailment off the track.
The UV-induced dehybridization of an azo-carrying leg strand off a DNA track has turned out to be an effective mechanism for optical driving of DNA molecular motors. Previous motors3, 5c, 5d, 5f exploiting this driving mechanism lack the converse affinity control, typically with the front leg's forward intra-site displacement halted under the UV irradiation that dissociates the rear leg from a two-legged motor-track binding state (like state 1 or 6 in this study). Hence the motor remains in a low-affinity single-legged binding state under the UV as the dissociated leg cannot access the front binding site due to the halted displacement of the track-bound front leg (like the exposed leg B in state 2 unable to access the front F1A, see Figure 1c). For a full forward step starting and ending at stable two-legged states, the motor further needs a visible light irradiation to complete the track-bound leg's displacement so that the dissociated leg can access the front site (hence bicolour driving of UV plus visible light for a full step). But the dissociated leg under the visible light has a chance to resume its hybridization ability before the track-bound leg's displacement and therefore bind the closer back site for a futile step. Thus the UV light and the visible light each produce only a half-step with a single-legged low-affinity middle state in which the motor may still reverse the first half step and is also susceptible to off-track derailment. It is thus difficult to saturate a full step towards 100 % probability by elongating/strengthening the UV or/and visible light of each bicolour driving operation since the chance for the motor's derailment from the low-affinity middle state is increased but the chance for futile steps is not necessarily minimized. Repeating the bicolour operation raises the probability for full steps but loses control of the step number as each operation produces one or none step randomly.
The nucleotide sequences for the motor's functional segments are given in Table S1; the full sequences for constituent DNA strands of motor-track systems are given in Supporting Information. Two track versions are mainly studied (see schematics in Figure S1): version 1 has its F1B overhang mutated for lower binding affinity with leg B to make its F1B→F2B cartwheeling more downhill (by removing 2 nucleotides from F1B's j* segment and further introducing a point mutation in k* segment); version 2 keeps the native F1B and has an extra F1A overhang to form a full F1A-F2A bi-overhang site at the minus end. The tracks are fabricated by an annealing procedure (Methods) and characterized using polyacrylamide gel electrophoresis (PAGE, see Figure S2). In the sequence choice for leg A, leg B and complementary binding overhangs, special cares are taken to avoid unwanted cross-binding of leg A with F1B or F2B and of leg B with F1A or F2A. The fluorescence and PAGE studies using truncated azo-free leg/overhang strands confirm that these unwanted bindings are minimal (~10 % versus >80 % for the desired bindings of leg A with F1A or F2A and leg B with F1B or F2B, see Figure 3, S3, S4 and Methods). The motor thus has fairly exclusive bindings of leg A with site A and leg B with site B to suppress undesirable motor-track binding states that compete with state 1 or state 6 (e.g., intra-site states in which the motor's leg A hybridizes with F2A and leg B hybridizes with F1A of the same bi-overhang site A under visible light, or the motor's leg B hybridizes with F2B and leg A hybridizes with F1B of the same site B under UV).
2.2 Site-Controlled Motility Experiments
The motor's operation is studied by site-controlled motility experiments in which the motor is initially bound to the track's minus end to form state 1, or to the track's middle to form state 6 under a UV treatment (Methods). To report the motor's on-track motion, the ssDNA motor is labelled with quenchers on both ends (Figure 1a) and the track is labelled with three different dyes (Figure 1b) subject to quenching by the motor when it forms state 1 at the track's minus or plus end or state 6 in the middle (Figure 1c). Following previous studies,5c, 5g the fluorescence from the site-controlled motor-track assembly under alternating UV/Visible (UV/Vis) light irradiations is divided by the fluorescence from a track-only control experiment to remove influence of dye photobleaching (negligible or below 10 % for three used dyes, see Figure S5). The control-calibrated fluorescence signal indicates relative motor population at different sites (the lower the signal, the higher the population at a site). The signal before and after the optical operation confirms the initial site-specific motor placement (by apparently higher population at the desired site, see Figure 2a, b and Figure 4a) and the motor's light-driven motion (Figure 234).

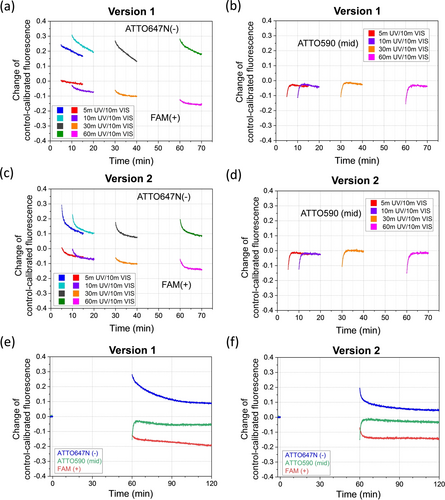
Motor operation starting from the track's minus end. (a), (b) Motor version 1 based on the track with a point mutation to accelerate the motor's F1B-to-F2B cartwheeling (see Figure S1b for the track with the mutation marked in one F1B overhang). Panel (a) shows the control-calibrated fluorescence signal free of photobleaching influence (raw operation and control data in Figure S6). Panel (b) shows the signal in (a) minus its own starting value at the first UV cycle to better display the fluorescence change from the motor's light-driven movement. (c), (d) Motor version 2 based on the track without the mutation (but with one extra F1A overhang at the minus end, see Figure S1c). All the data shown here are obtained under alternating UV/Visible light cycles with each UV and visible light irradiation lasting 5 minutes and 10 minutes, respectively. The first UV starts at time zero, with the no-data gaps along the time axis as the UV irradiations and the intermittent data collected during the visible light irradiations.
Motor operation starting from the track's minus end by a single UV/Visible light cycle. (a)–(d) Motor version 1 and version 2 for four different UV durations but all for 10 minutes visible light. Like Figure 2, the UV irradiation starts from time zero throughout the non-data gap and the shown data span the duration of the visible light irradiation. (e), (f) The two motor versions operated by a long single cycle with 1-hour UV and 1-hour visible light.
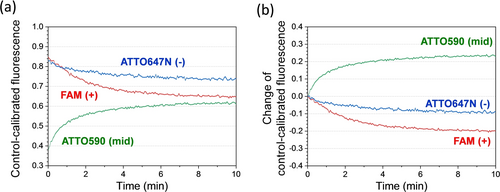
Motor Operation starting from the track's middle site. The motor-track assembly is prepared under UV light, and then subjected to a single visible light irradiation for 10 minutes over which the fluorescence data are recorded (shown here). Like Figure 2, the control-calibrated data in panel (a) minus its own initial value at time zero yields the data in panel (b).
The fluorescence motility experiments with the motor started from the minus end verify the motor's ability to make two plus-end-directed steps per UV/Visible light cycle (Figure 2). Immediately after the first UV irradiation, the fluorescence of the minus-end dye rises and the fluorescence of the mid-site dye drops, indicating a UV-induced step from the minus-end site to the middle site, i.e., state 1 to state 6 in Figure 1c. When the irradiation is switched to visible light, the mid-site fluorescence rises rapidly and the plus-end fluorescence starts to drop below its initial value prior to the first UV light, indicating a visible light-induced step from the middle site to the plus-end site, i.e., state 6 to state 9. These distinct fluorescence patterns suggest processive translocation of the motor from the minus end to the plus end through the middle site by a single UV/Vis cycle. The same fluorescence patterns for the three sites repeat for later UV/Vis cycles, with a successively increasing profile for the minus-end fluorescence, a successively decreasing profile for the plus-end fluorescence, and a slightly decreasing profile for the mid-site fluorescence. These fluorescence profiles are again consistent with a processive motor flow through the middle site (with both incoming and outcoming motor, hence a profile between the oppositely divergent profiles for minus and plus ends). The motor-track version 1 and version 2 show almost identical fluorescence patterns but with larger magnitude of minus-end fluorescence rise and plus-end fluorescence drop for version 1 than version 2. This is consistent with the expectation of better plus-end-directed motility for the motor when its intra-site cartwheeling towards the plus end is more downhill.
The motor's ability for two full steps per UV/Vis cycle is further confirmed by a single-cycle motility experiment with different UV duration (5 m, 10 m, 30 m, 60 m) but the same visible light duration (10 m) for sake of comparison. All the single-cycle data for motor-track version 1 and version2 replicate the characteristic fluorescence patterns for the motor's UV-induced step from the track's minus end to the middle site and a visible-light-induced second step from the middle site further to the plus end (Figure 3a–d). For the present 6-overhang track, a single step is indeed insufficient for the minus-end-started motor to reach the plus end, but such a long-range translocation (requiring two consecutive steps) is necessary for the gain of motor population at the plus end as signified by the net fluorescence drop there below the pre-operation level after the single UV/Vis cycle (note the smaller net drop of final mid-site fluorescence in Figure 3b, d that indicates a slight motor population gain too at the middle site; hence the plus-end gain of motor population must come from initial motor population at the minus end). Thus, the net plus-end fluorescence drop from the single-cycle experiment provides one more evidence for the motor's two consecutive steps by a single UV/Vis cycle.
With the UV elongated from 5 minutes to 10, 30, and 60 minutes, the single optical cycle produces increasingly bigger plus-end fluorescence drop for both version 1 and version 2 (Figure 3a, c). This is expected as a longer UV is more effective to dissociate the motor's leg A off the minus-end F2A overhang and open the hairpin at leg B for the motor's translocation from the minus end to the middle site (hence biggest UV-induced mid-site fluorescence drop for 60 m UV in Figure 3b for version 1 and in Figure 3d for version 2). A single cycle of 60 m UV followed by 60 m visible light results in a net plus-end fluorescence drop near that from six cycles of 10 m UV and 10 m visible light per cycle or from two cycles of 30 m UV and 30 m visible light per cycle (see Figure 3e versus Figure S6b, Figure S7a for version 1 and Figure 3f versus Figure S6d, Figure S7b for version 2).
The motor's direction towards the track's plus end is further supported by the mid-site start experiment and a control experiment with the motor started from the plus end. The plus-end start experiment, with the motor initially prepared in state 1 at the plus end (i.e., state 9 in Figure 1c), yields a virtually flat fluorescence profile for the middle site and minus end under cyclic optical operation (Figure S8), despite successively increasing fluorescence for the plus end (likely due to motor dissociation from 10nt terminal F1B under UV, see state 10 in Figure 1c). These data indicate negligible backward motion of the motor from the plus end to the middle site and the minus end. In the mid-site start experiment, the motor is initially prepared in state 6 under UV as confirmed by the apparently more quenching of the mid-site dye than the dyes at minus and plus ends (Figure 4a). A visible light irradiation triggers the motor's on-track motion, which is more towards the plus end than the minus end as indicated by double fluorescence drop at the plus end compared to the minus end (Figure 4b).
The minus-end start experiment and the plus-end start control, which both have the motor initially in state 1 but located at opposite terminal ends of the track, prove that the motor bound between the pair of neighbouring F2A and F1B overhangs always moves towards the plus end under UV regardless of location of the overhang pair. This is true even if the (F2A, F1B) pair is located in the middle of a track to be sandwiched by another pair of neighbouring overhangs F2B, F1A on both sides (see Figure 1B): then the motor under UV still moves to the (F2B, F1A) pair in front to form state 6 (as proven by the minus-end start experiment) but not to the same (F2B, F1A) pair behind (as proven by the plus-end start control). The UV-induced step from the (F2A, F1B) pair has indeed a good directionality with apparent forward motion shown in Figure 2 versus negligible backward motion shown in Figure S8. The mid-site start experiment further proves that the motor bound between the second pair of neighbouring overhangs (F2B, F1A), which is sandwiched by the (F2A, F1B) pair on both sides as shown in Figure 1b, moves preferentially towards the (F2A, F1B) pair in front under visible light. This confirms that the visible light-induced step from the (F2B, F1A) pair also has a net directionality towards the plus end (though not as good as the UV-induced step). Altogether, these experimental observations lead to the conclusion that the motor is capable of plus-end-directed motion on any long track under cyclic UV/Vis operation, since the track is essentially a periodic array of both overhang pairs (F2A, F1B), (F2B, F1A) alternately arranged on a duplex backbone. Besides, this ssDNA motor has reasonably fast motion in response to the optical driving, with a kinetic analysis of the fluorescence data yielding a time scale of ~3 minutes for the step by UV or visible light, and ~0.5 minute for hairpin closing (Supporting Information).
2.3 Translocation Probability
The motor's translocation probability to the track's plus end can be extracted from the fluorescence data following a reported method.5b The probability for a dye-labelled site to be occupied by a motor can be extracted from the control-calibrated fluorescence in Figures 2, 3, as P(t)=[1−IM(t)]/γ with IM(t) as the control-calibrated fluorescence at time t and γ as the dye's quenching efficiency by the motor-carried quencher (γ≈1 for this study due to ∼100 % effective contact quenching for the three dyes). Then the translocation probability of the motor to the plus end, Ptrans, is calculated as the change of occupation probability at the plus end divided by the sum of initial occupation probability at the minus end and the middle site where the motor starts (note that ~50 % initial occupation exists at the middle site, as shown in Figure 2a, due to imperfect motor introduction to the minus end). The control-calibrated fluorescence data in Figure 3e are reasonably stabilized at the end of a long single UV/Vis cycle, yielding the final plus-end occupation change as ~0.2. The sum of initial occupation at the minus end and middle site is available from Figure 2a as ~1.3. Hence Ptrans≈15 %. This is the chance of the motor, under a single UV/Vis cycle, to translocate to the plus end from the minus end or middle site—by two steps or one step, respectively. We can also estimate the translocation probability for multiple UV/Vis cycles from the date in Figure 2b: the plus-end fluorescence is largely stabilized at the end of 6 light cycles to yields Ptrans≈14 %. The Ptrans value by the six cycles (5 m UV & 10 m VIS per cycle) is similar to the above value by the single cycle with longer UV/Vis irradiations (60 m UV & 6 0 m VIS). For comparison, two previous light-driven DNA motors have the translocation probability available for a single step after multiple UV/Vis cycles, namely ~7.6 % after six cycles (10 m UV & 10 m VIS) for an azo-tethered motor5c and ~13 % after six cycles (20 m UV & 10 m VIS) for another motor5g driven by optically rechargeable fuels. Compared with this study, these are lower values though they are for single-step translocation only and for longer UV/Vis irradiations (by the same light source5c, 5g as for this study).
Compared to the previous motors, this new motor not only has better directional translocation under repeated UV/Vis cycles, but also is capable of equally effective accumulation of the translocation probability for a locked step by elongating a single-colour operation (UV or VIS). This implies a possibility of accumulating translocation probability to ~100 % for every single step by repeating/strengthening the single-colour optical driving (e.g., high-intensity pulsed light and repeats, higher azo-density in the motor, etc., in future study). The ~100 % accumulation of translocation probability is possible for a locked step even if each repeat of the single-colour driving is far below 100 % effectiveness. The previous DNA motors require the bicolour UV/Vis cycle as the minimum optical driving for a full step (hence no colour-selected step locking between these minimum cycles). Repeating the minimum bi-colour driving accumulates the translocation probability but over an uncontrollable number of steps, resulting in more and more spreading of the on-track motor distribution for a motor-track ensemble even if each motor-track copy has the same starting position for the motor. The motor from this study has the unique potential for optically synchronized steps to maintain the narrow motor distribution.
3 Conclusions
We have demonstrated a nano-optomechanical driving mechanism that pushes top-down optical control for molecular motors down to every single step. This novel optomechanical mechanism, together with a new light-driven ssDNA motor capable of such controlled stepwise motion along a periodic track, provides key elements in future development of top-down precise control of small motion based on molecular motors in replacement of larger MEMS/NEMES for Today's precision technology. Indeed, the small single-molecule motor from this study may be integrated with DNA origami platforms1f, 6 for top-down controlled optomechanical nano-actuators or nano-robots potentially capable of non-intrusive and high-throughput manipulation of molecular objects. The colour-selective single-step control from this study also provides an optical method to help synchronize molecular motor actions for amplified effects. Future development is anticipated towards top-down optical synchronization between multiple motor copies in a nano-actuator/robot, and ultimately between multiple copies of nano-actuator/robots that are DNA origami-based and may form an orientation-controlled array9 on a lithography-patterned substrate. These are challenging long-term targets, and this study is a major step forward in the direction.
Acknowledgments
This work was supported by Ministry of Education of Singapore and the office of deputy president (Research & Technology) of National University of Singapore under grant numbers A-8000982-00-00, A-0008378-00-00 and A-8000628-00/01 (to Z. W.). This work was also partially supported by JSPS KAKENHI grants JP21H05025 (to H. A.) and Grant-in-Aid for Transformative Research Areas “Molecular Cybernetics” 20H05968 (to K. M.).
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.