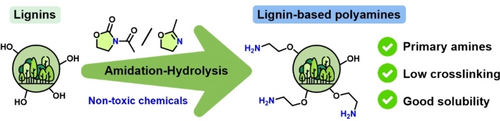
Benign and Selective Amination of Lignins towards Aromatic Biobased Building Blocks with Primary Amines
Graphical Abstract
Abstract
Lignin is a widely available second-generation biopolymer and the main potential source of renewable aromatic building blocks. Lignin-based polyamines offer great potential in applications based on chemical and materials sciences. However, common aminations techniques for lignin usually involve toxic chemicals and generate hindered and low reactivity amines.
In this study, we developed two new, simple, and benign 2-step methodologies for the elaboration of lignin-based polyamines from different technical lignins (kraft, soda and organosolv) with a selectivity towards reactive primary amines. These methods involve grafting amide groups onto lignin followed by a hydrolysis step. Non-toxic heterocyclic compounds N-acetyl-2-oxazolidinone and 2-methyl-2-oxazoline were used as amidation agents. Hydrolysis was performed in acetone-water mixtures. Reactions were studied on model compounds and optimized on lignins. Aminated lignins were fully characterized and primary amines were quantified using quantitative 19F NMR.
Our methods generated aminated lignins with low apparent molar masses and high solubility in water and solvents. Nitrogen contents of the products ranged between 2.0 and 3.5 mmol/g with reactive primary amines counts up to 1.7 mmol/g. These soluble and reactive lignin-based polyamines offer great potential as a replacement for fossil-based polyamines in e.g., the synthesis of aromatic polymer materials or as potential chelating, antibacterial agents.
Introduction
Lignin is a potent second-generation (2G) renewable biochemical feedstock for aromatic chemicals. It represents overall around 20 % of lignocellulosic biomass.1, 2 As a by-product of cellulose and hemicellulose extraction from biomass, it has great potential as a low-cost and largely available biopolymer. Around 100 Mt of lignin are produced each year in pulp and paper and 2G bioethanol industries.3-5 Despite its large availability, only around 8 % (8 Mt/y) of lignin production was isolated for commercial applications worldwide in 2021, the rest was burnt for local energy production.6
Lignin's structure results from the polymerization of 3 main propylphenol monomers: p-Coumaryl alcohol (H unit), Coniferyl alcohol (G unit) and Synapyl alcohol (S unit). The ratio of monomers and the linkages between them is related to the biomass source (hardwoods, softwoods, annual plants …), whereas the structure of isolated lignins is highly dependent on the extraction method used to separate it from cellulose and hemicelluloses. Kraft, Sulfite, Soda and Organosolv are the most common extraction processes. Compared to the other lignins, sulfonated lignins from sulfite process have specific properties with their own industrial applications. Based on sulfur or not, Kraft, Soda and Organosolv lignins show similar condensed structures. These latter are under-developed with rather low industrial valorizations and applications till now.
Lignin's skeleton is rich in functional groups such as phenols (PhOH), alcohols (AlOH), and carboxylic acids (COOH). Lignin makes an interesting platform chemical with a high hydroxyl content and aromatic backbone. It has shown to have great potential in high-value bio-based products. One field where lignin seems to be particularly useful is polymers and materials. It has been used e.g., in blends and composites in polyolefins and as macro-monomers (building blocks) or crosslinking agent in polyurethanes, polyesters and epoxy resins.7-14 As aromatic materials, on top of reaching excellent mechanical properties, lignin-based polymers display UV stability, flame resistance and antioxidant behaviors.15-17
In this context, our team has shown that performing materials could be obtained from lignin such as copolymers and composites with polyesters,9, 18, 19 polyurethanes (PUs),20, 21 clicked polymers22, 23 and vitrimers.24 These diversified products are achieved thanks to functionalization towards more reactive and homogeneous group distribution. Lignin functionalization is a large field that has been the subject of numerous reviews in the last decade.9-14
Amines are key intermediates with extensive applications in agricultural, pharmaceutical, textile, polymers and cleaning industries. For instance, amines are essential for the synthesis of polyamides, polyureas, epoxide resins and the development of polyhydroxyurethanes without isocyanates (PHUs).25, 26 Amination of lignin has great potential as it produces aromatic polyamines. Such products are lacking otherwise on the sustainable amine market.27 Lignin-based polyamines have been used in a large spectra of applications. Aminated lignins were used as curing agents for epoxide resins, PUs and PHU thermosets.28-31 They were also introduced in polymers to improve aging and fire resistance.32-34 Amination changes lignin's reactivity and can improve its water solubility. In water soluble applications, they were examined as flocculants,35-37 chelating agents,38-43 nitrogen fertilizers44 and in waterborne polymers.31, 35, 45
First reported in the 1930s, lignin amination is not really a recent topic.46 But despite the interest on the subject since, amination methods have mainly revolved around Mannich reaction (Figure 1). Applications of the products of Mannich reaction on lignin have been recently reviewed.47 The reaction involves the phenolic groups of lignin, an amine and formaldehyde. Grafting occurs at the free ortho positions of phenolic moieties. The reaction is relatively simple to be implemented and can lead to quantitative substitutions levels.29, 35, 48 However, free ortho positions can be scarce in a large number of lignins, especially from hardwoods.49 This has led several researchers to use first phenolation to increase the number of reactive sites, leading to higher nitrogen contents on lignins.34, 44, 48 However, phenolation adds a second step to the synthesis, introduces fossil carbon to the structure, and uses particularly toxic phenol. Moreover, the selectivity of Mannich reaction is limited. The formed amines are a mixture of primary, secondary and tertiary amines due to further reaction of the amine grafted with formaldehyde, generating sometimes important crosslinking.28-30 The use of formaldehyde also raises concerns about the safety and sustainability of this method.28
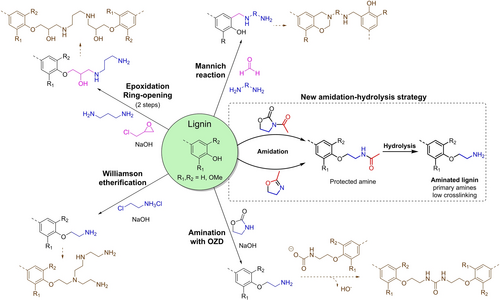
In the past decade, new amination pathways based on lignin's hydroxyl groups have been explored (Figure 1). These methods are more adapted to different types of technical lignins since they do not rely on specific lignin units. Pan et al. developed a method based on the reaction of epoxidized lignin with a polyamine to generate aminated lignin.50 Williamson etherification was used by Chen et al. to form aminated lignins using the reaction of a chloroamine with phenols.38 More recently, Liu et al. proposed a method based on the reaction of phenols with a simple cyclic urethane, 2-oxazolidinone (OZD) in solvent-less conditions.51 This reaction is somewhat similar to the oxyalkylation of lignin with cyclic carbonates developed in our group.52
Even though the different methods meet the same goal of grafting amines on lignin, the structures formed are diverse and thus not fully comparable. Moreover, they reach disparate nitrogen contents, from 1 to 6 mmol/g. As Mannich reaction, all of the current methods lead to low solubility and hindered polyamines. It is due to the reactivity of the grafted amines as nucleophiles, which leads to hyperbranching and/or crosslinking. Thus, obtained lignin-based polyamines are high molar mass products that contain a mixture of nitrogen-bearing groups, such as secondary and tertiary amines or ureas (Figure 1). This can be particularly critical in polymers synthesis.38, 50, 51
The aim of this study was then to develop two different transformation pathways towards aminated lignins that would limit crosslinks and be selective to primary amine groups. For this purpose, two non-toxic heterocyclic compounds, 2-methyl-2-oxazoline (mOZ) and N-acetyl-2-oxazolidinone (AcOZD), were used as amidation agents. The amidated lignins were then hydrolyzed to aminated lignins (Figure 1). The two routes were studied on model compounds and then optimized on lignin. They were then tested on different lignin feedstocks obtained from different processes: a hardwood organosolv lignin (HW-OL), a softwood kraft lignin (SW-KL), and a wheat straw soda lignin (WS-SL). The aminated products were fully characterized by 1H, 31P, 1H−13C, 1H−15N NMR, size-exclusion chromatography (SEC), thermogravimetric analysis (TGA), differential scanning calorimetry (DSC) and elemental analysis. Primary amines were also quantified using quantitative 19F NMR. Finally, the methods were compared to other lignin aminations and evaluated through green metrics. The different selected materials, chemicals, synthesis pathways and characterizations techniques are given in the Supporting Information (SI).
Results and Discussion
Analysis of the Amidation Reactions on Model Compounds
Two amidating compounds were selected to react on lignins and models. N-acetyl-2-oxazolidinone (AcOZD) was chosen as an acetylated derivative of 2-oxazolidinone (OZD), which was previously shown to be reacting with lignin but leading to high amounts of crosslinking.51 The second amidating agent was 2-methyl-2-oxazoline (mOZ), as it was shown to form similar amide compounds by reaction with simple phenolic compounds.54, 55
AcOZD and mOZ are simple heterocyclic derivatives of ethanolamine (Figure 2). They can potentially be bio-based and CO2-derived.56-63 Neither AcOZD nor mOZ have been reported as toxic to human health. Additional details on their synthesis are given in Supporting Information.
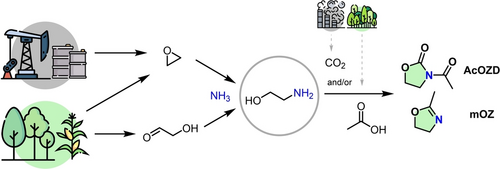
N-acetyl-2-oxazolidinone (AcOZD) and 2-methyl-2-oxazoline (mOZ) synthesis via ethanolamine from fossil resources or from biomass.
To identify the potential reactions of AcOZD and mOZ on each reactive group in lignin, the amidation was first studied on different model compounds. Benzyl alcohol, 2-methoxy-4-methylphenol and benzoic acid were chosen as model compounds to mimic the AlOH, PhOH, and COOH groups in lignins, respectively.
We decided to perform the amidation with AcOZD in DMSO as it is high boiling point, benign and potent solvent for lignin dissolution. K2CO3 was used as a catalyst as it was already shown to be a good catalyst for phenol activation.20, 52 The reaction study was performed at 120 °C with 1.1 eq. of AcOZD and 0.1 eq. of K2CO3. The progress of this reaction was followed by 1H NMR on aliquots extracted with ethyl acetate.
PhOH model (2-methoxy-4-methylphenol) reaction evolution is given in Figure 3 and Figure S5. The reaction led to a final product grafted with an acetamide group, as expected. Interestingly, AcOZD acted also as acetylating agent. Acetylated products appeared initially and disappeared after 4 h of reaction as shown by the acetate characteristic peak at 2.32 ppm (3b) in 1H NMR. New signals associated with the amide-based products, appeared more slowly at 4.00 (5a), 3.60 (4a) and 1.97 ppm (3a). Acetates could convert back to free hydroxyl groups as confirmed by performing the reaction in DMSO-d6 and following by 1H NMR (Figure S6). On the other hand, amidation was irreversible, with the formation of strong ether bonds and the release of CO2 by decarboxylation of AcOZD.
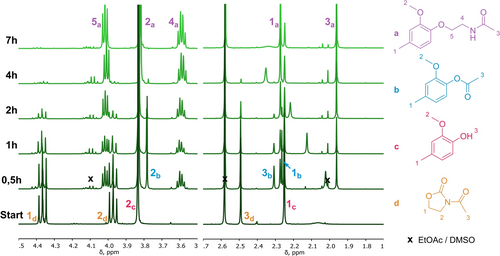
1H NMR Spectra of extracted aliquots of the reaction mixture for the reaction of 2-methoxy-4- methylphenol (PhOH model) with AcOZD. Zoom on 1.7–2.7 ppm and 3.5–4.5 ppm regions. Solvent CDCl3. Full spectrum is given in Figure S5.
Similar studies were performed on COOH and AlOH models (benzoic acid and benzyl alcohol respectively). COOH model also converted to an amide compound through a new ester bond (Figure S7). On the other hand, AlOH model did not present any significant amide grafting at the end of the reaction (Figure S8) and was almost fully converted into an acetate. Figure 4A gives a summary of the main reactions occurring on each chemical group as well as the potential side reactions.
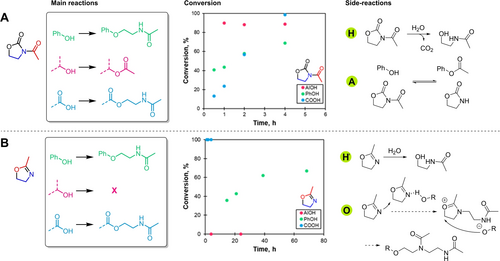
Main reactions of PhOH, AlOH and COOH models, kinetics of conversion and possible side reactions occurring when reacting with AcOZD (A) and mOZ (B).
AlOH model was the most reactive with AcOZD, with a maximum conversion into acetate of 90 % achieved in one hour (Figure 4A). COOH and PhOH models were less reactive with maximum conversions of 99 and 69 % after 4 h respectively. PhOH model did not reach full conversion due to a loss of AcOZD during the reaction and could be associated to AcOZD or OZD hydrolysis due to water traces (H in Figure 4A, and Figures S6 and S8).
A similar reaction study was performed with mOZ. Some preliminary tests showed no improvement of the reaction kinetics using basic catalysts. Acid catalysts generated ring-opening polymerization of mOZ, a already described phenomenon.64 The amidation was thus performed without solvent nor catalyst as previously reported on simple phenols.54, 55 The amidation reaction conditions were chosen to be 160 °C with an excess of mOZ acting as a reactive solvent. The reaction was performed in a closed vessel to prevent the loss of mOZ (bp=110 °C). The conversion was followed with direct 1H NMR of the reaction mixture (Figures S9 to S11).
With mOZ, PhOH and COOH models converted to amides whereas AlOH model did not react (Figure 4B). The reaction was particularly slow compared to AcOZD. COOH model reacted quantitatively within 1 h but PhOH model achieved maximum conversion of 67 % to amides only after 70 h of reaction (Figure 4B).
Some side products were evidenced in small amounts (<10 %) in the case of PhOH model due to long reaction times. We identified them as grafted dimers of mOZ. Oligomerization of mOZ could happen under the mild catalysis of hydrogen bond donor groups such as hydroxyl groups as shown (O) in Figure 4B. This phenomenon is not problematic as it would only generate linear polyamides.
From these results on different models, AcOZD and mOZ could be good candidates to form amides from lignin hydroxyl groups. New amide groups were expected to graft through ether bonds onto PhOH groups and ester bonds onto COOH groups. Side reactions evidenced were not considered as problematic as they would not generate crosslinking or impede on amidation.
Amidation and Amination Optimization on an Organosolv Lignin
Lignins and model compounds reactivities could differ. To assess the reactivity of lignin with the amidation agents, reaction studies were first performed on a hardwood organosolv lignin (HW-OL) at different temperatures. HW-OL is mainly based on PhOH and AlOH groups (Table S1 and Figure S2).
Reactions were followed by quantitative 31P NMR and IR of the processed aliquots. 31P NMR offered insight into the conversion of PhOH groups were amides would graft through ether bonds. PhOH groups transformation was accelerated with increasing temperature for both amidation methods (Figure 5A and 5B). The best conversions for the two amidation methods were reached with AcOZD at 160 °C for 4 h (90 %) and mOZ at 160 °C (closed reactor) for 16–24 h (80 %). Interestingly, with mOZ the reaction of PhOH groups was faster on lignin than the corresponding on model compound.
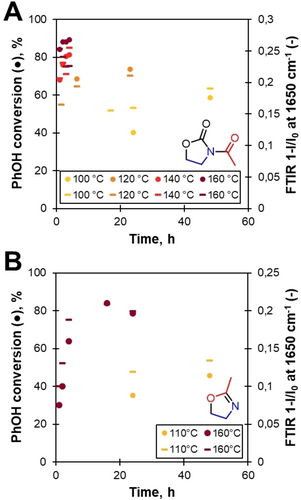
Evolutions of the conversion of PhOH groups based on 31P NMR (•) and amide formation as seen in IR (–) during amidation reactions with AcOZD (A) and mOZ (B).
IR allowed to assess semi-quantitatively the efficiency of the grafting process. Over the course of the two reactions, two new absorption bands appeared at 1650 and 1735 cm−1 (Figure 6 and Figures S12, S13). The first one was identified as the carboxyl (C=O) elongation of the amide group, the second one being acetate/ester C=O elongation. By comparing maximum band transmittance at 1650 cm−1, we established a good correlation between IR absorption in the amide region and 31P conversion data (Figure 5). Nonetheless, small variations appeared. More particularly, IR data suggested a decrease in amide content after a few hours in the case of AcOZD at 160 °C. This probably indicated limited side reactions of PhOH groups at higher temperatures with this compound. Thus 140 °C seemed to be the best temperature for amidation with AcOZD, leading to the greatest absorption in IR for amides.
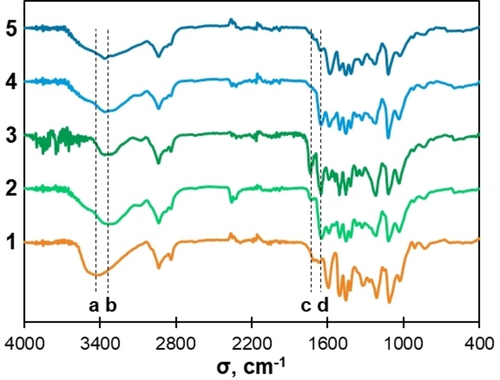
IR Spectra of HW-OL (1), amidated with mOZ (2), amidated with AcOZD (3) and the product of hydrolysis of HW-OL amidated with AcOZD after 4 h (4) and 24 h (5). a: O−H band (3400 cm−1). b: N−H band (3350 cm−1). c: C=O ester band (1730 cm−1). d: C=O amide band (1650 cm−1).
AlOH groups evolution could also be studied by 31P NMR and IR (Figure S14). In the case of AcOZD, conversions were above 60 % and were correlated to an increase in the acetate band at 1735 cm−1 in IR, confirming the transformation of AlOH groups to acetate groups. For mOZ, conversions of AlOH groups were lower (below 50 %) and did not correlate with an increased ester band in IR. However, the relatively significant decrease of AlOH groups led us to believe that some modification of lignin's backbone could also occur with the high temperatures of the reaction.
From these results on HW-OL, best-chosen conditions of amidations for lignins were (i) AcOZD (2 eq.), K2CO3 (0.1 eq.), 140 °C during 4 h and (ii) mOZ (7 eq.), 160 °C (closed vessel) during 24 h.
To obtain amine groups, amidated lignins were then hydrolyzed in a mixture of solvent and aqueous solution (1 : 1 vol/vol). The concentration of lignin was fixed at 10 % (w/v) to have good solubility of the lignin and minimize kinetic limitations by diffusion. Acetone, methanol and DMSO were tested as solvents and HCl and NaOH as catalysts for the hydrolysis. Acetone/HCl 2 M mixtures performed the best, with full solubility of the lignin at 10 % along the reaction and almost a full disappearance of the C=O amide band in IR after 24 h at 75 °C (Figure 6). The reaction also led to the cleavage of the weaker ester bonds on AlOH and COOH groups, as seen by the disappearance of the C=O ester band at 1735 cm−1. An enlargement of the N−H band could also be seen between 3600 and 3100 cm−1, indicating the formation of free amines.
Hydrolysis reaction was stopped at 24 h despite small traces of amide bonds still visible in IR. Further tests showed that after 48 h, the amide signals would completely disappear. However, the aminated lignins were less soluble in solvents. This would suggest that longer hydrolysis could further affect the lignin structure. Long hydrolysis could also lead to minor cleavage of ether bonds between phenols and amines in acidic conditions, as previously reported.54, 55
The structure of the amidated and aminated lignin products from HW-OL was assessed by 1H, 1H−13C HSQC and 1H−15N HMBC NMR in DMSO-d6 (Figure 7 and Figures S15 to S17). 1H−13C HSQC spectra in Figure 7 show the full analysis of the chemical structure of amidated and aminated products. Amidated lignins show chemical structures associated with amides Dα, Dβ and Dγ formation. After hydrolysis, amine structures D’α, D’β appear and only traces of amides remain. Distinct signals for groups grafted on carboxylic acid, H, G and S units are visible. It is possible that minor grafting onto AlOH groups would also appear as the signals associated would overlap with those of S units.
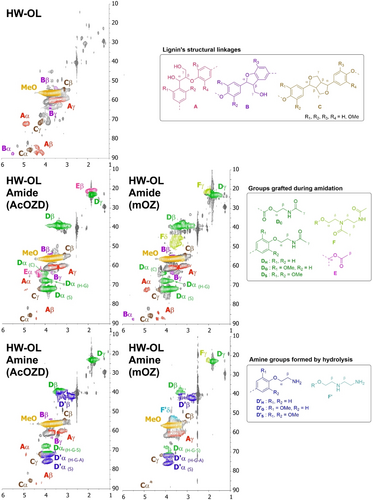
1H−13C HSQC Spectra of HW-OL, Amidated HW-OL using mOZ, Amidated HW-OL using AcOZD, and aminated HW-OL obtained by hydrolysis of both amides. Solvent: DMSO-d6.
In the case of AcOZD, acetate signals (E) are clearly evidenced on amidated lignins and disappeared completely after hydrolysis. For mOZ, some structures associated with dimer grafting could be seen (F). These structures were later hydrolyzed in aminated lignins. The signals associated to protons Fα, Fβ, F’α and F’β overlap with Dα, Dβ, D’α and D’β. HSQC also showed that HW-OL backbone structure was relatively preserved during the transformation. A, B and C signals, associated with β-O-4, β-5 and β-β linkages, decreased along the amidation and amination but could still be evidenced on final products.
1H−15N HMBC was performed to further confirm the selectivity of the reactions (Figure S17). For amidated lignins, only one signal appeared at (1H 1.8 ppm, 15N 113.2 ppm), which was characteristic of the 3J coupling of amides. This confirmed the main selectivity towards terminal acetamides. After hydrolysis, no signal could be seen in the 1H−15N HMBC spectrum indicating successful cleavage of amides. However, amine signals were not visible because of the formation of hydrogen bonds in amines leading to a probable broadening of NMR signals.
The extent of amination over the molar mass distribution of the lignin was evaluated by fractionating one aminated lignin (HW-OL amine AcOZD) by sequential solvent fractionation. First, the solubility of the aminated lignin was evaluated in different solvents (Figure S18). Then, the aminated lignin was fractionated by partial dissolution in acetone/water mixtures. In order of solubility, three fractions were obtained representing respectively 24, 13 and 56 wt % of the initial aminated lignin. The fractions A and B had similar 1H−13C HSQC Spectra and PhOH content as the full aminated lignin (Figure S19 and Table S2). The nitrogen content of the aminated lignin and its fractions was quantified (Table S2). The nitrogen content showed little variation (within 10 %) between the fractions. The most soluble fraction had the highest nitrogen content as expected from a higher initial PhOH content.65 This study shows that the amination occurred over the whole molar mass range of lignin. Our team had previously shown same results by reacting low dispersity lignin fractions with ethylene carbonate.65
Aminations of Different Types of Lignins
The optimal amidation and hydrolysis conditions were then applied to 3 different lignins: HW-OL (hardwood organosolv lignin previously used for optimization, rich in S units), SW-KL (soft-wood kraft lignin, rich in G units) and WS-SL (wheat straw soda lignin, rich in COOH groups). We obtained 6 different amidated lignins and then 6 aminated lignins. The reactions were performed and scaled up to 10 g without specific issues. Performing the selected pathways on different lignins also allowed to confirm the robustness of the global approach.
Mass yields of the process are given in Table S3. They were calculated as the ratio of the mass of product over the initial lignin mass. The first step gave yields up to 130 wt % due to excess mass from grafting and an highly performing recovery by precipitation in acidic water. Hydrolysis step yield was around 80 wt %, indicating a good recovery of aminated lignins by dialysis. Overall, the two-step methodology led to yields of around 90 wt %. Main losses came from vessel transfers.
The extent of amidation/amination on each lignin was evidenced by 31P NMR and elemental analysis. 31P NMR was performed in CDCl3/Pyridine (1/1.6 v/v) mixture for lignins and amidated lignins. Aminated lignins were not soluble in this solvent mixture. We thus adapted a procedure initially developed with lignosulfonates to perform the analysis in DMF-d7.66
All lignins showed similar behaviors after the amidation and hydrolysis (Figure 8) steps, with PhOH groups conversions in the order of 2–3 mmol/g. WS-SL showed the best conversions, up to 95 % of the initial PhOH groups content. Comparing the two amidation agents, mOZ led to higher conversion of PhOH groups. AlOH and COOH groups also reacted partially as expected from the previous results in the reaction study with HW-OL.
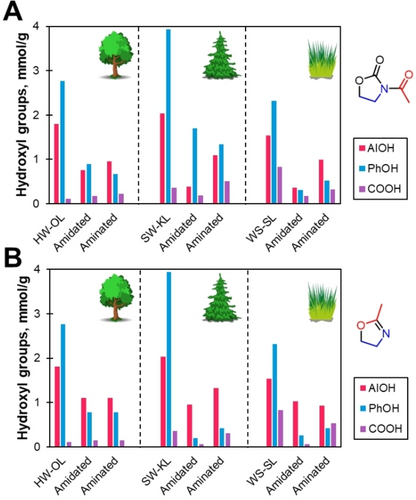
AlOH, PhOH and COOH groups evolution during the amidation-hydrolysis process as seen by 31P NMR. A) with AcOZD. B) with mOZ.
Hydrolysis of amidated lignins kept PhOH group content to similar values, indicating no significant ether hydrolysis and conservation of grafted chains. On the other hand, AlOH and COOH groups content increased due to the hydrolysis of ester groups. However, they did not come back to their original value, probably due to side reactions inside lignin structure.
The elemental analysis on amidated and aminated lignins were used to evaluate the extent of the grafting (Figure 9, Table 2 and S4). Amidated lignins nitrogen content ranged from 2.0 to 3.9 mmol/g (Figure 9) which was in the same order of magnitude as PhOH groups converted. Aminated lignins had lower nitrogen contents, between 1.5 and 3.5 mmol/g. This decrease is due to the hydrolysis of amides grafted on COOH groups.
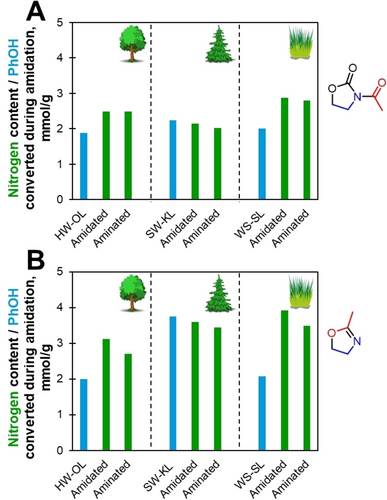
Nitrogen content of amidated and aminated lignins compared to PhOH groups content of the original lignins.
mOZ seemed to be the best amidation/amination agent as it provided higher nitrogen contents than AcOZD. Aminated lignins with mOZ had nitrogen contents from 2.7 to 3.5 mmol/g, while those with AcOZD had contents only from 1.5 to 2.8 mmol/g. This is associated to the higher PhOH groups conversions with mOZ as well as the possible grafting of mOZ oligomers, as discussed in the model compounds study.
From the initial reaction study on model compounds, aminations were expected to occur only on reacted PhOH groups. Compared to the amount of converted PhOH groups in each lignin, a correlation is difficult to assess. Aminated SW-KL had nitrogen contents slightly below PhOH groups content of initial lignin, but HW-OL and WS-SL showed greater nitrogen contents. This excess nitrogen could imply that amidation can take place on the other reactive sites, such as AlOH groups (which would not be differentiated in HSQC). Another possibility is an increase in free PhOH groups during reaction, due to e.g., some depolymerization. In the case of WS-OL, the nitrogen excess could also be partly due to initial nitrogen content of the lignin (see Table 2).
Aminated lignins were fully soluble at 20 mg/mL in water in acidic and alkaline conditions (Figure 10). This result is relatively unusual compared to aminated lignins obtained from other methods, which most often present insoluble fractions in water and/or low solubility at lower pH.33, 38, 51, 67 The solubility decreased at high ionic strengths (HCl 2 M and NaOH 10 M). The solubility was also minimal at pH around 10, at the probable isoelectric point of the aminated lignins. Furthermore, aminated lignins showed high solubilities in DMSO and DMF (Figure S22). This allowed the analysis of the products by NMR.53, 68

Solubility range for aminated HW-OL with mOZ at different pH in water. pH adjusted with HCl 2 M and NaOH 10 M. 0 corresponds to HCl 2 M and 14 to NaOH 10 M.
Size-exclusion chromatography (SEC) was used to assess the evolution of the apparent molar masses during the transformations. All lignins were acetylated prior to analysis to maximize solubility and limit heterogeneity in interactions with the solvents and columns.
The samples were first analyzed by SEC in THF (Table 1 and Figures S23 to S25). The fully soluble samples, amidated and aminated SW-KL and HW-OL do not show any increase in apparent number-average molar masses (Mn) and apparent mass-average molar masses (Mw). This implies that crosslinking is limited on these lignins. Some depolymerization could also have happen during functionalization, as seen from the partial cleavage of β-O-4 linkages evidenced by HSQC (Figure 7).
Substrate |
|
THF |
DMF/LiBr |
||||
|---|---|---|---|---|---|---|---|
|
|
Mn, kg/mol |
Mw, kg/mol |
Đ |
Mn, kg/mol |
Mw, kg/mol |
Đ |
HW-OL |
– |
1.6 |
4.4 |
2.7 |
3.6[b] |
8.1[b] |
2.3[b] |
amides |
AcOZD |
1.4 |
2.4 |
1.8 |
6.3[b] |
16.6[b] |
2.6[b] |
|
mOZ |
1.6 |
2.4 |
1.6 |
7.7[b] |
23.9[b] |
3.1[b] |
amines |
AcOZD |
1.1 |
2.4 |
2.1 |
6.1[b] |
16.4[b] |
2.7[b] |
|
mOZ |
1.7[a] |
2.8[a] |
1.7[a] |
6.9[b] |
18.0[b] |
2.6[b] |
SW-KL |
– |
1.6 |
4.7 |
3.0 |
3.9[b] |
9.9[b] |
2.5[b] |
amides |
AcOZD |
1.3 |
2.8 |
2.2 |
|
|
|
|
mOZ |
1.2[a] |
1.9[a] |
1.6[a] |
|
|
|
amines |
AcOZD |
1.3 |
3.1 |
2.3 |
5.8[b] |
15.2[b] |
2.6[b] |
|
mOZ |
1.2[a] |
1.8[a] |
1.5[a] |
9.0[b] |
25.1[b] |
2.8[b] |
WS-SL |
– |
1.0[a] |
1.8[a] |
1.9[a] |
3.1[b] |
6.0[b] |
1.9[b] |
amides |
AcOZD |
1.3[a] |
2.2[a] |
1.7[a] |
|
|
|
|
mOZ |
1.3[a] |
2.0[a] |
1.6[a] |
|
|
|
amines |
AcOZD |
1.3[a] |
2.1[a] |
1.6[a] |
10.1[b] |
66.1[b] |
6.5[b] |
|
mOZ |
1.1[a] |
1.7[a] |
1.5[a] |
5.3[b] |
11.7[b] |
2.2[b] |
- [a] Not fully soluble (only the soluble fraction was analyzed). [b] Multi-modal apparent molar mass distribution. Probable lignin aggregation, see Figures S26 to S28.
Some samples were not fully soluble in THF, making their chromatograms unreliable (Figures S26 to S28). To complement these results, we analyzed the acetylated lignins in DMF/LiBr where all the acetylated samples were fully soluble.
In DMF/LiBr, the chromatograms show a clear multimodal apparent molar mass distribution for initial lignins and more significantly for amidated and aminated lignins. It was not the case in THF for fully soluble samples (Figures S23 to S28). These results indicate aggregation of lignin in DMF/LiBr, more pronounced in the case of the modified lignins. This phenomenon has already been evidenced previously.69 This would lead to a significant overestimation of the apparent molar masses in this solvent, especially for amidated and aminated lignins. Nonetheless, the results of SEC in DMF/LiBr still show relatively low apparent Mn.
Overall, the SEC analysis suggests that the apparent molar masses of the three lignins evolved similarly during the full process and limited crosslinking occured. The obtained aminated lignins have relatively low apparent molar masses. This is an improvement over other amination methodologies which could cause important crosslinking, often leading to insolubility.30, 38, 51, 53
The thermal properties of the lignins were studied by TGA and DSC. In TGA, all aminated lignins showed onset temperature T5% (temperature at 5 % weight loss) between 220 and 240 °C (Table S5, and Figures S29 to S31). This shows that relatively high temperatures could be then used for further reactions, e.g. polymerizations. DSC confirmed the thermal stability of aminated lignins, as no significant thermal event was seen up to 200 °C (Table S5, Figures S32 to S34). Additional discussions on the decomposition temperatures can be found in Supporting Information.
In DSC, amidated lignins showed lower Tg (80 to 130 °C) than the initial lignins (120–140 °C). This was expected as aliphatic chains and lower hydrogen bonding associated with hydroxyl groups disappearance induce lignins plasticization with major gains in mobility. This effect is more important in the case of amidated lignins with AcOZD (Tg of 80 to 100 °C), that have acetylated AlOH groups. On the other hand, aminated lignins did not show a clear glass transition, probably because of free amine groups that could lead broadened glass transition.
Quantification of Primary Amines and Evaluation of Reactivity of the Aminated Lignins
For polymerization or globally for chemical reactions, primary amines are desired as they are far more reactive than secondary amines, while tertiary amines do not react.70-72 Ninhydrin test was used as a qualitative tool to evidence the presence of primary and secondary amines in the hydrolyzed products. Samples containing reactive amine groups color from yellowish to blue/purple. Figure 11 shows that the hydrolyzed products turned purple, confirming the formation of primary and/or secondary amines.

Ninhydrin test results on HW-OL products. 1: HW-OL, 2a: Amidated HW-OL with AcOZD, 2b: Amidated HW-OL with mOZ, 3a: Hydrolysis product of 2a, 3b: Hydrolysis product of 2b.
Elemental analysis accounts for all nitrogen-bearing groups. This includes primary amines, but also the remaining traces of amide groups seen by IR and NMR. In order to quantify the primary amine groups, a method based on quantitative 19F NMR was adapted from the work of Ji et al. initially developed on polyamides and aminated polystyrenes.73 In this method, the primary amines form imines with a fluorinated benzaldehyde compound (Figure 12). Using an excess of benzaldehyde and molecular sieves to capture water, the reaction is quantitative and highly selective towards primary amines. The method thus allows for the characterization of the real reactivity potential of aminated lignins in polymeric applications. 4-trifluoromethylbenzaldehyde (p-TFBA) was used as a fluorinating agent and trifluoroethanol (TFE) as an internal standard. p-TFBA and TFE appeared as sharp peaks at −62.2 and −75.7 ppm respectively. Imines formed by primary amines emerged as a broad signal between −62.0 and −61.5 ppm (Figure 12).
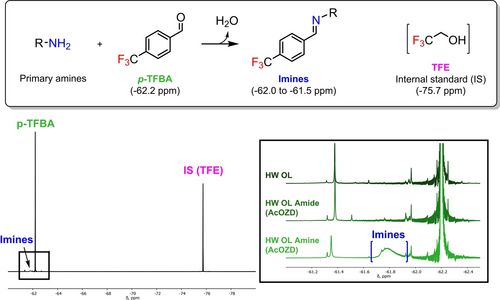
Scheme of the reaction between primary amines and p-TFBA. 19F Spectra of HW-OL Amine from AcOZD and zoom on the imine region for HW-OL, HW-OL Amide from AcOZD and HW-OL Amine from AcOZD. Solvent: DMSO-d6.
Primary amine contents were lower than nitrogen content as only accessible primary amines were effectively targeted in the structure (Table 2). Despite higher nitrogen contents measured in the case of mOZ, primary amine content was similar between both methods. This could be correlated with the formation of secondary amines with mOZ, as evidenced by HSQC.
Substrate |
|
Nitrogen content, mmol/g |
Primary amine content, mmol/g |
AHEW[a], g/eq |
|---|---|---|---|---|
HW-OL |
– |
0.00 |
0 |
|
amides |
AcOZD |
2.49 |
0 |
|
|
mOZ |
3.12 |
0 |
|
amines |
AcOZD |
2.49 |
1.1 |
450 |
|
mOZ |
2.71 |
1.5 |
330 |
|
|
|
|
|
SW-KL |
– |
0.00 |
0 |
|
amides |
AcOZD |
1.98 |
0 |
|
|
mOZ |
3.60 |
n.s. |
|
amines |
AcOZD |
2.03 |
1.1 |
470 |
|
mOZ |
3.44 |
n.s. |
n.s. |
|
|
|
|
|
WS-SL |
– |
0.64 |
0 |
|
amides |
AcOZD |
2.87 |
0 |
|
|
mOZ |
3.91 |
n.s. |
|
amines |
AcOZD |
2.80 |
1.7 |
300 |
|
mOZ |
3.48 |
1.3 |
380 |
- [a] Considering only primary amines.
As reported in Supporting Information, we also examined an acidic titration procedure to estimate primary amine content used by Liu et al. in their recent paper on lignin (Table S6 and Figure S35).51 This method was found to be unreliable for the quantification of acido-basic groups in our samples. This confirmed that fast aqueous acidic titration of lignin can be approximative or not reliable with lignin as already reported.74, 75 Further discussion on titration is provided in Supporting Information.
Comparative Evaluation of the Amination Methodologies
Compared to other amination methods, this two-step pathway shows great potential (Table 3). The method could be applied to lignins with or without sulfur and having different monomer distributions, OH group contents and impurity levels. Nitrogen contents achieved ranged between 2.0 and 3.5 mmol/g, which is close to the average values obtained by other methods. This is particularly potent compared to Mannich reaction which usually relies on the use of polyamines for grafting, leading to an overestimation of reactive amines by a factor of 2, 3 or even more. We demonstrated selectivity towards reactive primary amines that comes with the two-step amidation-hydrolysis procedure we chose. Reactive primary amine groups ranged between 1.1 and 1.7 mmol/g. The aminated lignins obtained by our method have relatively low apparent molar masses. The aminated products showed great solubility in water and polar solvents, which is also essential for some applications.
Method |
Nitrogen content, mmol/g |
Nitrogen bearing groups[a] |
Added crosslinks |
Solvent |
Health/Safety |
Energy |
AE[b] |
E factor[c] |
Ref. |
|
|---|---|---|---|---|---|---|---|---|---|---|
Mannich reaction |
1–6 |
I, II, III amines, benzoxazines |
Yes |
Aqueous or organic |
Formaldehyde and polyamine |
60–90 °C, 2–8 h |
>95 % |
1.4 to |
14 |
[33, 40, 53, 67, 77–80] |
Epoxydation-ring opening |
5 |
I, II, III amines |
Yes |
Aqueous |
Epochlorhydrin and polyamine |
50 °C, 8 h+80 °C, 6 h |
83 % |
N/A |
[50] |
|
Williamson etherification |
2–3 |
I, II, III amines |
Hyper-branching |
Aqueous |
2-chloroethylamine hydrochloride |
80 °C, 24 h |
65 % |
16 |
[38] |
|
Amination with OZD |
2–5 |
Ureas, amines |
Important |
None |
|
150 °C, 4 h |
87 % |
4.2 |
[51] |
|
Amidation-hydrolysis |
2.0–3.5 |
Amides, I amines (reactive: 1.1–1.7 mmol/g) |
Low |
DMSO |
|
140 °C, 4 h+75 °C, 24 h |
74 % |
9 |
This work (AcOZD) |
|
None |
|
160 °C, 24 h+75 °C, 24 h |
83 % |
9 |
This work (mOZ) |
|||||
- [a] I, II, III amines=primary, secondary and tertiary amines, [b] Atom Efficiency, [c] Wastes/Product mass, water, HCl and NaOH excluded for most methods (see in Supporting Information).
The environmental impact of the procedures was estimated through qualitative assessments, atom economy (AE) and overall E factor. All calculations and details are found in Figure S36 and Table S7. The comparison of the different amination methods through these metrics is difficult as they reach different degrees of amination and different structures. E factor calculations were particularly problematic due to the lack of consistent yield and mass balance found in literature on this subject. These values should also only be regarded as indicative as they could probably benefit from optimizations.
As shown in Table 3, the main drawback of Mannich reaction is the use of toxic and potentially carcinogenic chemicals in the form of formaldehyde and polyamines. However, Mannich reaction can be optimized to particularly efficient energy and mass uses. Williamson etherification and epoxidation ring-opening methods don't use formaldehyde but have similar drawbacks as Mannich reaction due to the use of particularly toxic chloroalkylamine and epichlorhydrin. Amination with OZD improves greatly on the safety and waste generated compared to other methods. However, the aminated lignins obtained with this method are highly crosslinked, making difficult their uses and valorizations.
The two new amidation-hydrolysis pathway developed have significant benefits on top of the quality of the aminated lignins obtained. The method is relatively benign as the chemicals used encompassed only DMSO, acetone and the two heterocyclic compounds with K2CO3, NaOH and HCl sole inorganic bases and acids needed. Our method was however, more energy intensive than others due to higher temperatures and longer reaction times. Considering the waste generation, our pathway was in the range of other amination methods with an E factor of around 9. This value was mainly affected by the mass of acetone in the hydrolysis step. Despite its early stage of development, the E factor already places our method in the high range of bulk chemicals.76 Reduction of waste and improvement of energy efficiency could be the topic of further potential optimization. One-pot amidation-hydrolysis is probably possible to simplify the procedure and limit water wastes. Microwave activation of the amidation and hydrolysis reactions could also be potent in limiting reaction times and temperatures.
Conclusion
In this study, we successfully developed alternative, safe and selective routes towards aminated lignins. These new routes associated amidation and hydrolysis steps, using simple and non-toxic heterocyclic compounds. We initially studied the reactions on model compounds and then applied it to three lignins obtained from different processes and botanical resources, with different architectures with and without sulfur. Aminated lignins achieved nitrogen contents up to 3.5 mmol/g. The methods are selective towards reactive primary amine groups, with contents up to 1.7 mmol/g. These functionalization methodologies seem to limit crosslinking, allowing for high solubility of these aminated lignins in water and organic solvents. Along with this study, we also established a new quantitative 19F NMR technique for the characterization of primary amines in aromatic compounds such as lignins.
This methodology clearly unlocks new potentials for lignin-based polyamines, as aromatic and biobased building blocks for a large range of applications. This global approach improves greatly the traditional methods which generated highly hindered structures without being selective to reactive primary amines. Moreover, the environmental impact of the method is limited due to the benign nature of the chemicals involved. Such lignin-based polyamines could be economically viable to develop biobased and aromatic architectures.
The polyamines formed have both reactive primary amines and good solubility making them particularly useful as precursors for epoxide resins, PUs and PHUs. Aminated lignins obtained from our methods could also be a good replacement for existing commercial fossil-based polyamines, as their AHEW are in the same range. Their aromatic backbone is an added benefit for polymer applications, as it could provide better mechanical properties, thermal stability, fire resistance, UV stability and antioxidant properties.
The use of these building blocks in safe, aromatic, biobased and performing polymers will be the topic of future investigations.
Supporting Information
The authors have cited additional references within the Supporting Information.38, 40, 51, 57-63, 66, 67, 73, 74, 79-87
Author Contributions
Nathan Wybo. Investigation, Data curation, Conceptualization, Methodology, Visualization, Writing—original draft, Writing—review & editing.
Antoine Duval. Conceptualization, Methodology, Writing—review & editing, Supervision, Funding acquisition.
Luc Avérous. Conceptualization, Writing—review & editing, Supervision, Funding acquisition.
All authors approved the final version of the manuscript.
Acknowledgments
The authors are thankful to the Agence Nationale de la Recherche (France) ANR Biopoliol (ANR-21-CE43-0026) for the founding of this project. We thank Arjan T. Smit (TNO, Petten, Netherlands) for kindly supplying the organosolv lignin used in this study. We acknowledge the AMS Service (CNRS/Université de Strasbourg FR2010) for the elemental analysis, as well as Cronenbourg NMR Core Facility (CNRS/Université de Strasbourg, UMR 7042 LIMA, Strasbourg, France) and Dr Emeric Wasielewski for his involvement in the choice, development and the execution of specific NMR techniques used in this work.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.