Functionalization of Supramolecular Polymers by Dynamic Covalent Boroxine Chemistry
Graphical Abstract
Boronic acid self-condensation provides ready access to boroxine-based, C3-symmetrical monomer architectures that can cooperatively assemble into helical supramolecular polymers. The dynamic boroxine scaffold enables fast intermolecular exchange of boronic acid between different monomers and facilitates the introduction of functionality into assembled structures by co-condensation with functional boronic acids.
Abstract
Molecular scaffolds that enable the combinatorial synthesis of new supramolecular building blocks are promising targets for the construction of functional molecular systems. Here, we report a supramolecular scaffold based on boroxine that enables the formation of chiral and ordered 1D supramolecular polymers, which can be easily functionalized for circularly polarized luminescence. The boroxine monomers are quantitatively synthesized in situ, both in bulk and in solution, from boronic acid precursors and cooperatively polymerize into 1D helical aggregates stabilized by threefold hydrogen-bonding and π–π stacking. We then demonstrate amplification of asymmetry in the co-assembly of chiral/achiral monomers and the co-condensation of chiral/achiral precursors in classical and in situ sergeant-and-soldiers experiments, respectively, showing fast boronic acid exchange reactions occurring in the system. Remarkably, co-condensation of pyrene boronic acid with a hydrogen-bonding chiral boronic acid results in chiral pyrene aggregation with circularly polarized excimer emission and g-values in the order of 10−3. Yet, the electron deficiency of boron in boroxine makes them chemically addressable by nucleophiles, but also sensitive to hydrolysis. With this sensitivity in mind, we provide first insights into the prospects offered by boroxine-based supramolecular polymers to make chemically addressable, functional, and adaptive systems.
Introduction
Inspired by nature, chemists have combined covalent and non-covalent synthesis for the construction of complex molecular systems.1-3 Indeed, covalent modifications of structures stabilized by non-covalent interactions are widespread in nature and enable complex functions, such as regulation and movement.4 By following this strategy, the combination of reversible covalent reactions occurring simultaneously with assembly processes5-12 has enabled the emergence of supramolecular systems with life-like properties.13, 14 A recent example is the assembly of self-replicating disulfide molecules that can evolve to catalyze a wide range of chemical reactions.15, 16 Another example shows how four distinct (supramolecular) products could be selectively formed only by tuning the stoichiometry of the system, which was rationalized by the coexistence of supramolecular phases.17, 18 However, incorporating chemical reactivity into supramolecular structures is not an easy task.19, 20 In fact, the lability of the non-covalent and reversible covalent interactions makes the assembly inherently dynamic and highly sensitive to changes in molecular structure and external conditions. Consequently, reactive supramolecular scaffolds that combine in situ synthesis under mild conditions with high modularity for functionalization are a recent challenging target in the field.
Significant progress has been made in the design of supramolecular scaffolds for the controlled assembly of functional molecular units in polymers, discrete and three-dimensional metal–organic complexes, and nanoparticles.21 Among supramolecular scaffolds, C3-symmetrical monomers have played a special role22 as their molecular architecture has proven beneficial for supramolecular polymerization into helical aggregates, as shown by numerous reports in literature.20-26 Therefore, a molecular scaffold that allows easy and modular synthetic access to C3-symmetrical monomers, while being chemically addressable, would be ideal to accelerate research on functional and life-like supramolecular polymeric systems.
For this purpose, an attractive class of compounds are boroxines, the self-condensation product of boronic acids. Boroxine formation from the respective boronic acid is a dynamic and reversible process that is high-yielding under dehydrating conditions,27 or by ligand-assisted synthesis28-31 in bulk and solution. Furthermore, boronic acids play a central role in organic synthesis,32-35 and in the construction of COFs,36 macrocycles,37, 38 cages,39, 40 and dynamic covalent polymer networks,28, 41, 42 giving a wide range of commercially accessible boronic acid precursors. As a result, the combination of chemical addressability, orthogonality to supramolecular interactions,43, 44 C3-symmetrical architecture, and commercial availability of precursors, the boroxine scaffold offers new opportunities to explore boroxine-based monomers as reactive motifs in supramolecular polymers. It is therefore unexpected that, despite supramolecular polymers derived from dative boron-nitrogen bonds,45 no boroxine-based supramolecular polymers have been reported to date.
Herein, we report the design, synthesis, and characterization of boroxine-based supramolecular polymers, which serve as a combinatorial platform, amenable to facile systematic study with tools from supramolecular chemistry. By combining experimental and computational methods, we demonstrate how self-condensation of a benzamide-substituted boronic acid gives easy access to a C3-symmetrical monomer, enabling supramolecular polymerization in both bulk and solution. By employing co-condensation, we investigate the dynamic nature of the hydrogen-bonding boroxine scaffold and its potential to induce aggregation of a functional chromophore that would not assemble otherwise.
Results and Discussion
Design and Synthesis
We first explored the supramolecular monomer S-BOR, with para-benzamide units on the boroxine ring (Figure 1b). Molecular modelling of S-BOR indicated supramolecular polymerization into one-dimensional helical fibres via threefold hydrogen-bonding of the peripheral amides and π–π stacking of the aromatic benzene units (Figure S1). We further introduced homochiral solubilizing side chains to bias the helicity of the supramolecular polymer and thereby enable assembly studies with circular dichroism (CD) spectroscopy.
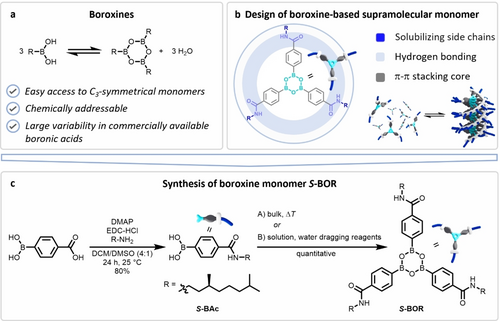
a) Condensation reaction giving access to the boroxine scaffold starting from boronic acid precursors. B) Supramolecular monomer design including π–π stacking and hydrogen-bonding benzamide units. C) Synthesis of S-BOR starting from commercially available 4-carboxyphenylboronic acid.
The respective boronic acid precursor S-BAc was synthesized in one step from the commercially available 4-boronobenzoic acid and (S)-3,7-dimethyloctan-1-amine under Steglich amidation conditions in 80 % yield. Upon self-condensation of S-BAc, the boroxine can be obtained after the elimination of three equivalents of water. This process is entropically favoured, and the equilibrium is shifted towards the boroxine at elevated temperatures. Following the principle of Le Chatelier, the removal of water further shifts the equilibrium towards the boroxine.
We investigated two different strategies to synthesize S-BOR from the boronic acid S-BAc, in bulk as well as in solution. Heating the bulk boronic acid in a vacuum oven at 110 °C overnight resulted in the quantitative conversion to the boroxine as evidenced by the 1H NMR displayed in Figure 2a (see Figure S2 for full spectrum). The disappearing boronic acid signal at 8.17 ppm as well as the slight downfield shift of the aromatic protons from 7.77 to 7.82 ppm and 7.84 to 7.93 ppm indicates boroxine formation. Additional DOSY experiments showed an increase of the hydrodynamic radius (RH) by a factor ~1.8 (Figure S3), which matches the expected increase in size upon trimerization. Further, in agreement with FT-IR spectroscopy of boroxine compounds,27 the characteristic boronic acid vibrations at 1115, 1006 and 803 cm−1 disappeared upon condensation and the diagnostic boroxine vibrations at 705 and 678 cm−1 appeared (Figure 2c). Using variable temperature IR, the sudden increase in intensity of the boroxine vibration at 705 cm−1 above 80 °C indicates that conversion to boroxine occurred around this temperature (Figure 2d, full spectra shown in Figure S4). In contrast to the work of Aida and co-workers on solvent-free autocatalytic supramolecular polymerizations on phthalocyanines,46 we could not identify any autocatalytic properties in our system.
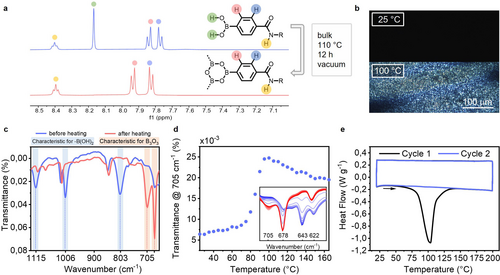
Bulk synthesis of S-BOR. A) 1H NMR (DMSO-d6, 10 mM, 25 °C) and c) FT-IR spectra of S-Bac (blue) and S-BOR (red). B) Changes in POM (scalebar 100 μm) upon heating S-BAc from 25 °C to 100 °C (10 °C min−1). The POM sample was kept for 1 h at 100 °C before capturing the displayed picture.D) Temperature-dependent FT-IR monitored at 705 cm−1 and FT-IR spectra in the region between 700 cm−1 and 730 cm−1, showing the disappearance of S-Bac and the appearance S-BOR (30–165 °C, 10 °C min−1). E) DSC traces (closed conditions, 10 °C min−1) monitoring the conversion of S-Bac to S-BOR.
The boroxine formation is further supported by differential scanning calorimetry (DSC) characterization, where the DSC pan was not hermetically sealed to allow the forming water to evaporate (Figure 2e). The large endothermic transition visible from 75 °C onwards can be attributed to the boroxine formation. Upon heating under closed conditions, the pressure build-up in the DSC pan shifted the endothermic transition of the boroxine formation to 141 °C (Figure S5). Under the polarized optical microscope (POM), boronic acid S-BAc showed no birefringent domains, indicating the absence of ordered structures prior to trimerization (Figure 2b). Upon driving self-condensation by heating the sample to 100 °C under a constant nitrogen flow, birefringent domains became evident, stipulating the presence of ordered microstructures. We reason that upon formation of the C3-symmetrical boroxine, supramolecular assembly becomes feasible via hydrogen-bonding and π–π stacking, causing the occurrence of birefringent domains. This is further supported by VT-IR measurements, where a shift from 1635 to 1632 cm−1 of the amide I vibration indicates supramolecular aggregation via hydrogen-bonding (Figure S6). The high dynamicity of the boronic acid-boroxine equilibrium resulted in fast hydrolysis (within seconds) under ambient conditions, thus making further sample preparation challenging.
We therefore aimed to optimize the boroxine synthesis in solution, where the boroxine hydrolysis can be limited by using drying reagents. Initial solution trimerizations were attempted in polar protic DMSO-d6 (30 mM) over 3 Å molsieves and were monitored by 1H NMR. No conversion from S-Bac to S-BOR could be observed under these conditions, even at elevated temperatures (Figure S7). We reason that the trimerization in hydrogen-bonding solvents might be energetically less favourable as the boronic acid and the open forms, dimer and trimer, are stabilized by strong hydrogen-bonding with the respective solvent. DFT calculations of the trimerization in DMSO and apolar cyclohexane confirm this hypothesis as the trimer formation appears to be less favoured in polar protic solvents (Figure 3a).
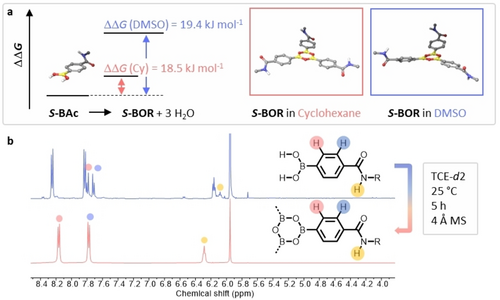
a) DFT calculations of ΔΔG of the boroxine formation from the respective boronic acid with minimized boroxine structure in cyclohexane and DMSO. The aliphatic side chains were substituted by methyl groups to reduce computational costs. B) Changes in 1H NMR spectra (TCE-d2, 10 mM, room temperature) upon boroxine formation.
We thus changed the solvent system to apolar decalin-d18. Upon stirring S-Bac in decalin-d18 for 2 h at room temperature over 4 Å molsieves, the complete dissolution of S-Bac indicated conversion to the less polar boroxine, but aggregation resulted in the absence of any traceable 1H NMR peak (Figure S8). Therefore, we switched to the polar aprotic solvent TCE-d2. Upon dispersing S-Bac in TCE-d2 (30 mM) over 4 Å molsieves for 2 min, the 1H NMR spectrum showed the presence of S-Bac and S-BOR (Figure 3b). After leaving the sample for 5 h at room temperature, full conversion was obtained. Similar to the trimerization in bulk, DOSY showed an increase in RH from 0.55 nm to 0.93 nm, stipulating S-Bac trimerization in solution, which was further confirmed using mass spectrometry (Figures S9–S10). Under inert conditions, no degradation of the boronic acid could be observed over months (Figure S11), prompting us to perform further sample preparation in the glovebox.
Assembly of S-BOR Into 1D Supramolecular Polymers
To study the aggregation of S-BOR, the concentrated stock solution in TCE-d2 (10 mM) was diluted with decalin (DEC, 25–100 μM), equilibrated for 5 min and monitored by CD and UV/Vis spectroscopy (Figure 4). In 1 vol % TCE-DEC, a positive bisignate Cotton effect with a maximum at 257 nm was observed (Figure 4b), suggesting the formation of chiral supramolecular aggregates. The zero crossing of the CD signal coincides with the absorption maximum, indicating exciton coupled chromophores in the aggregate (Figure S12).
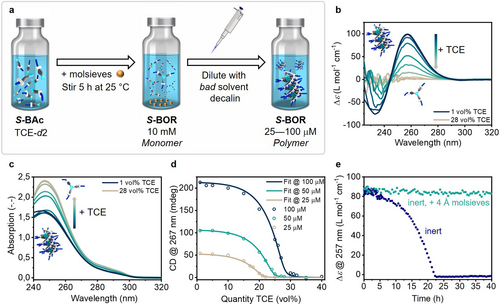
a) Schematic representation of the sample preparation starting from a mixture of S-Bac in TCE-d2, by addition of 4 Å molsieves, and subsequent dilution with decalin to obtain S-BOR supramolecular polymers. B) CD and c) UV/Vis spectra of S-BOR (c=50 μM, l=1 cm) in the denaturation experiment by gradual addition of TCE. D) CD intensity at 267 nm at various solvent composition of S-BOR and concentrations. The open circles indicate experimental data, and the lines depict the fits obtained from the denaturation model. e) Hydrolysis kinetics with and without the presence of 4 Å molsieves (c=50 μM, l=1 cm, 5 vol % TCE/DEC) monitored at 257 nm.
To assess the stability of the supramolecular polymers, we then performed denaturation experiments by preparing multiple samples with increasing amount of TCE while keeping the concentration constant (50 μM), following the sample preparation protocol described above. Increasing the amount of TCE resulted in an equilibrium shift from supramolecular polymer to monomer, as visible in UV/Vis and CD spectroscopy (Figure 4b and c). Upon TCE addition, the absorbance intensity increased and a slight red shift from 245 nm to 247 nm was observed, indicative for the disassembly of H-aggregates (Figure 4c). A thermodynamic mass-balance model was fitted to these denaturation experiments at three individual concentrations (25, 50 and 100 μM, Figure 4d) to determine the thermodynamic parameters of the supramolecular polymerization. The Gibbs free energy change for elongation was ΔGe=−35.4 kJ mol−1 with a nucleation penalty (NP) of 12.9 kJ mol−1, suggesting a cooperative supramolecular polymerization mechanism.47 Further, the linear dependency of ΔGe on the fraction of TCE is described by mTCE and was determined as 47.7 kJ mol−1 (Section 7 in Supporting Information).
We further attempted to study temperature-induced disassembly. During the first heating run, we observed the expected decrease in CD intensity upon thermal dissociation of the supramolecular polymer (Figure S13c). However, the subsequent cooling run did not show a complete recovery of the initial CD intensity, stipulating possible decomposition of the boroxine. Interestingly, the absorbance showed a slight blue shift after the initial heating-cooling run, suggesting the formation of a less conjugated system, like the boronic acid (Figure S13a). In addition, the used cuvettes appeared to show a stain after removal of the solution, which could not be easily cleaned with common organic solvents (Figure S13d). This indicates that the free hydroxy groups of the quartz-glass might react with boroxine to form boronic ester-functionalized quartz-glass, impeding variable temperature measurements. At room temperature however, the CD intensity remained constant over the course of multiple hours when the samples were kept over molsieves (c=100 μM, 5 vol % TCE/DEC, Figure 4e), pointing towards high boroxine stability in conditions where water is constantly removed from the system. In the absence of molsieves, disappearance of the CD signal was observed over the course of 17 h alongside precipitation. This suggests that the few ppm (≈10 ppm) of water remaining in the pre-dried solvents is enough to hydrolyze the boroxine and cause disassembly. Considering a water saturation of 10 ppm in pre-dried decalin and a sample concentration of 100 μM, the ratio of water to boroxine is ≈1. Thus, even when working under dry conditions, water is approximately equimolar to boroxine, which explains the observed hydrolysis.
Copolymerization Studies and Amplification of Asymmetry
In the past, mixing of chiral and achiral monomers in sergeant-and-soldiers experiments has significantly contributed to the understanding of the amplification of asymmetry in supramolecular co-polymerizations.50 Without a stereocentre in the monomer, discotic supramolecular monomers typically polymerize into equal amounts of left- and right-handed helices with no detectable CD signal, as shown by the achiral derivative n-BOR (Figure S14). We were intrigued to investigate the amplification of asymmetry in boroxine-based supramolecular polymers and compare mixtures of chiral and achiral boronic acid precursors with chiral and achiral boroxine trimers. In the in situ sergeant-and-soldiers experiment, both sergeants and soldiers were prepared in situ by co-condensation of chiral S-Bac with achiral n-Bac (Figure 5a), while in the classical approach, chiral S-BOR and achiral n-BOR monomers were co-polymerized. First, the chiral and achiral boronic acids were mixed in ratios ranging from 0 to 100 mol % of S-Bac to n-Bac in TCE-d2 (30 mM) and were co-reacted as previously described for the homo-condensation. We employed MALDI mass spectrometry to analyse these mixtures by comparing the intensity of the [M+Na]+ peaks, assuming that all four derivatives have similar ionizability (Figure S15b–g). Four different hetero-boroxine monomers in a 1 : 3 : 3 : 1 distribution of S-BOR : S1-n2-BOR:S2-n1-BOR : n-BOR, respectively, were obtained from a 1 : 1 mixture of S-Bac and n-Bac (Figure S15a,g). The statistical distribution indicates similar reactivities for the chiral and achiral boronic acids (Table S1). 1H NMR analysis did not show any traceable difference between the different species (Figure S16), limiting our analysis to MALDI mass spectrometry.

Illustration of a) the in situ sergeant-and-soldiers experiment (S&S). b) Plot of the CD maximum (circles) as a function of the percentage (mol%) of S-BAc added to n-BAc in the in situ and the classical S&S experiments of S-BOR and n-BOR (with in situ monomer exchange) with thermodynamic modelling (solid and dashed lines). CD experiments were conducted in 5 vol % TCE/DEC (l=1 cm, c=50 μM) at room temperature.
We diluted the obtained TCE-d2 stock solutions of the co-condensates with decalin to trigger assembly. Amplification of asymmetry was observed in the mixed samples, with about 20 mol % of chiral S-Bac necessary to reach full helical bias (Figure 5b, beige circles). MALDI experiments indicated that n-BOR and S1-n2-BOR are the major fractions at mixing ratios below 20 mol % of S-Bac (Figure S15b–f). This stipulates that the amplification of asymmetry in the system depends solely on the presence and quantity of point chirality in the side chains, irrespective of the composition of the boroxine monomer.51 We further performed the classical sergeant-and-soldiers experiment by mixing chiral S-BOR with achiral n-BOR. The amplification of asymmetry seemed to be nearly identical (Figure 5b, blue circles), prompting us to investigate the chemical composition after mixing of S-BOR and n-BOR. MALDI experiments show the presence of the hetero-boroxines within seconds after sample preparation (Figure S17). This suggests fast intermolecular boronic acid exchange between the boroxines,28 yielding similar statistical mixtures of hetero-boroxines as obtained after in situ co-condensation and formation of supramolecular polymers of variable composition.
Co-Condensation with Pyrene Boronic Acid for Circularly Polarized Luminescence (CPL)
The ease of boroxine formation and fast exchange kinetics between boronic acids and boroxines described in the previous section prompted us to elucidate the possibility to co-condense functional boronic acids with S-Bac. We anticipated that co-condensation of S-Bac with functional boronic acids might allow to construct hetero-boroxines that are still able to assemble into ordered aggregates by intermolecular hydrogen-bonding of S-Bac. This would open the possibility to use S-Bac as supramolecular scaffold to arrange functional molecules in space.21 The large number of commercially available boronic acid derivatives bearing functional units allowed us to freely choose a co-reactant with the desired properties. Pyrene is well known to exhibit a strong excimer emission upon aggregation, which provides a useful readout of potential aggregation using fluorescence spectroscopy (Figure 6a).52 Thus, as a proof of concept, we studied the co-condensation of pyrene boronic acid (Py-Bac) with S-Bac. Upon mixing of S-Bac and Py-Bac in a 5 : 1 ratio in 10 mM TCE-d2 solution, three different boroxine species were observed in 1H NMR spectroscopy (Figure S18a), that were identified as S-BOR (53 %), S2-Py1-BOR (37 %) and S1-Py2-BOR (10 %). The homo-condensate Py-BOR was not detectable by 1H NMR and only traces appeared in MALDI experiments (Figure S18b). Isolating the different co-condensates was impeded by the high hydrolysis sensitivity of the boroxine. We thus decided to use the mixture as obtained to conduct studies into the chiroptical properties of the system.
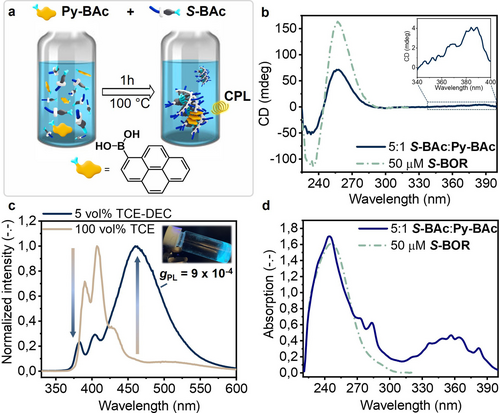
a) Schematic representation of the co-condensation of S-BAc and Py-BAc. b) CD spectra of the 5 : 1 S-BAc : Py-BAc mixture and pure S-BOR (c=50 μM, 5 vol % TCE/DEC). c) fluorescence spectra (c=10 mM, TCE and 5 vol % TCE/DEC). d) UV/Vis spectra of the 5 : 1 S-BAc : Py-BAc mixture and pure S-BOR (c=50 μM, 5 vol % TCE/DEC, l=1 cm).
After dilution of the 10 mM TCE-d2 stock solution with decalin (50 μM, 5 vol % TCE/DEC), a positive bisignate CD signal was observed similar in shape to the CD signal of S-BOR homopolymers (Figure 6b). Additionally, the presence of a weak CD signal between 340 nm and 400 nm, coinciding with the new absorption feature arising from pyrene (Figure 6d), indicated the chiral assembly of pyrene in solution. This is surprising, as the presence of the pyrene disrupts hydrogen-bonding in the supramolecular polymer. This indicates that the mixed Py-Bac/S-Bac monomers are still able to form ordered supramolecular aggregates despite the reduced amounts of stabilizing intermolecular hydrogen bonds. Moreover, fluorescence measurements at 10 mM concentrations showed strong pyrene-excimer emission around 460 nm with only negligible contributions from pyrene-monomer emission around 410 nm (Figure 6c). The high concentration resulted in gelation of the mixture, as shown in the inset of Figure 6c. In pure TCE (10 mM), only weak excimer emission could be detected, strongly suggesting that the excimer emission obtained at 10 mM in 5 vol % TCE/DEC is intermolecular in nature, and does not arise from intramolecular excimer formation in Py-BOR or S1-Py2-BOR monomers. At lower concentrations (50 μM) in 5 vol % TCE/DEC, the pyrene-monomer emission is more pronounced, but the pyrene-excimer emission remains visible, confirming the presence of pyrene-pyrene interactions in the supramolecular polymer, even at low concentrations (Figure S19). Additional CPL measurements of the mixture in 10 mM decalin showed a dissymmetry in emission in the order of gPL ~9×10−4 (Figure S20), stipulating that the pyrene is aggregated in a chiral fashion53 as also indicated by the CD response at lower concentrations. The presence of the intermolecular and circularly polarized pyrene-excimer emission stipulates stacked pyrene-pyrene moieties in a chiral orientation, indicating possible incorporation of pyrene into the 1D supramolecular polymers of S-BOR. Unfortunately, we were unable to determine a precise molecular picture of the aggregate microstructure, but the chiroptical results confirm the use of boroxine as supramolecular scaffold for CPL-active supramolecular polymers.
Conclusion
In this work, we report the synthesis of a boroxine-based monomer and study its cooperative assembly into chiral 1D supramolecular polymers. Starting from boronic acid precursors, the easy synthesis provides fast and efficient access to C3-symmetrical architectures for the design of chemically addressable monomers that assemble into supramolecular polymers. Mixing of chiral and achiral boroxine monomers in a classical sergeant-and-soldiers experiment show fast intermolecular exchange between boroxine monomers, highlighting the dynamic nature of the boroxine scaffold. Furthermore, by co-condensation of a chiral boronic acid with pyrene boronic acid, we drive the assembly of pyrene into chiral aggregates, as evidenced by circularly polarized pyrene-excimer emission.
Despite the intriguing potential of the boroxine platform as a reactive supramolecular scaffold, we propose that openly pointing out the pitfalls of the system will accelerate future research.20 The boroxine scaffold is sensitive to nucleophilic attack, making it on the one hand chemically addressable, but on the other hand sensitive to hydrolysis. Consequently, careful control of the reaction conditions and handling of the system under inert conditions are required to achieve reproducible results. With this sensitivity in mind, the boroxine supramolecular scaffold provides new opportunities for the construction of complex molecular systems.
Supporting Information
Details on synthesis, characterization of the synthesized molecules, sample preparation and additional measurements are provided in the Supporting Information.
Author Contributions
M.D.P., E.W.M. and G.V. conceived and designed the overall project. T.S. provided the DFT calculations. S.A.H.J. contributed the mass balance modelling. T.H.R.K helped synthesizing the boronic acids. S. C. J. M. performed the CPL measurements. X. L. contributed the MALDI analysis.
Acknowledgments
This project received funding from the European Commission Research Executive Agency (Grant Agreement Number: 859752 HEL4CHIROLED H2020-MSCA-ITN-2019), the Dutch Research Council (OCENW.M20.256) and the Dutch Ministry of Education, Culture and Science (Gravitation Program 024.005.020). The authors thank the ICMS Animation Studio (Eindhoven University of Technology) for providing the illustrations. We thank Marcin L. Ślęczkowski for the synthesis of (S)-3,7-dimethyloctan-1-amine used for the preparation of S-Bac, and Joost van der Tol for help with measurements.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.